间歇运动和粒细胞集落刺激因子促进心梗大鼠干细胞动员与内源性心肌细胞增殖的激光共聚焦/流式细胞术观察分析
史秀超,蔡梦昕,田振军
间歇运动和粒细胞集落刺激因子促进心梗大鼠干细胞动员与内源性心肌细胞增殖的激光共聚焦/流式细胞术观察分析
史秀超1,2,蔡梦昕1,田振军1
目的:探讨跑台间歇运动结合粒细胞集落刺激因子对心肌梗死大鼠干细胞动员的效果和在内源性心肌细胞增殖中的作用及其对心功能的影响。方法:90只成体雄性SD大鼠随机分为假手术组(Sham)、心梗组(MI)、心梗+间歇运动组(ME)、心梗+粒细胞集落刺激因子(G-CSF)组(MG)和心梗+G-CSF+间歇运动组(MGE)。结扎大鼠左冠状动脉前降支制备心肌梗死模型。ME和MGE组大鼠在心肌梗死手术结束1周后进行3周跑台间歇运动。MG和MGE组大鼠术后1 h皮下注射人源重组粒细胞集落刺激因子(rhG-CSF),10 μg/kg/d×5 d,其他各组大鼠给予同剂量生理盐水。免疫荧光法检测并计算梗死边缘区心肌细胞增殖百分率;流式细胞术检测外周血单个核细胞中c-kit+和CD29+细胞百分率;Western Blot方法检测心肌组织中c-kit和CD29蛋白表达水平。TTC染色检测心肌梗死面积百分率;彩色多普勒超声和血流动力学方法检测心功能。结果:与Sham组相比,MI组PCNA+心肌细胞百分率、外周血单个核细胞中c-kit+和CD29+细胞百分率、心肌组织中c-kit和CD29蛋白表达和心肌梗死面积显著增加,心功能显著降低;与MI组比较,ME、MG和MGE组大鼠梗死边缘区心肌组织中PCNA+心肌细胞百分率、外周血单个核细胞中c-kit+和CD29+细胞百分率、心肌组织中c-kit和CD29蛋白表达均显著增加,梗死面积显著减小,心功能显著增强,且MGE组变化最为显著。结论:间歇运动或粒细胞集落刺激因子可显著促进干细胞动员归巢,诱导心肌细胞增殖,缩小梗死面积,提升心功能,且二者联合效果优于单一因素作用。
心肌梗死;间歇运动; 粒细胞集落刺激因子;干细胞动员;心肌细胞增殖;鼠;动物实验
心肌梗死(myocardial infarction,MI)发生后,心肌细胞大量坏死或凋亡,心室发生恶性重塑,导致心力衰竭[43]。增加有收缩功能的心肌细胞数目是基础和临床研究的热点。一般认为,成体哺乳动物心肌细胞缺乏增殖能力。近年研究证实,成体哺乳动物心肌细胞存在更新现象,其更新率随年龄增加而降低[6,21,35]。研究表明,心肌细胞具有增殖现象[28],但其机制与方法研究需要不断丰富,寻求有效促进内源性心肌细胞增殖的细胞来源、方法和手段,对促进心肌损伤修复意义重大。针对心脏损伤修复的干细胞疗法已有大量研究报道[30,49],目前认为,可参与心肌再生的细胞来源包括部分有收缩功能的单个核心肌细胞、心肌固有的干/祖细胞(Cardiac stem/progenitor cells,CSCs/CPCs)[24,26,53]以及外周成体干细胞,如骨髓干细胞(Bone marrow stem cells,BMSCs)和诱导型多功能干细胞(Induced pluripotent stem cells,iPSCs)等[49,50,51]。MI发生后,梗死区微环境发生改变,可在一定程度上动员CSCs/CPCs和外周成体干细胞,如BMSCs等趋化至损伤部位进行增殖与分化,参与内源性心肌修复[13,14,42,52,58]。
间歇运动是指高强度与低强度锻炼或自由活动等方式交替进行的运动形式。文献表明,高强度间歇运动较中强度有氧运动对心脏结构与功能的影响更为显著[12,17,31,56,59]。 MI患者进行运动康复锻炼可有效改善心脏恶性重塑现象,提升心功能和改善生活质量[15,41,46]。动物实验发现,运动可促进正常生理状态下心肌细胞增殖[7,57]。本实验室前期研究发现,8周持续有氧运动可促进正常和MI大鼠心肌组织增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)和细胞增殖标记物Ki67蛋白表达[1],有效提高心功能。粒细胞集落刺激因子(granulocyte colony-stimulating factor,G-CSF)可促进骨髓间充质干细胞和造血干细胞等趋化至损伤部位,参与心肌损伤后的修复[27,45]。运动可促进血液循环中干细胞数目增多[8,9,22],但间歇运动结合干细胞动员剂G-CSF能否更有效促进自体干细胞动员和心脏内源性心肌细胞增殖,缺乏直接证据。文献表明,心梗或心梗后注射G-CSF动员BMSCs入血均存在时间窗[44,55]。根据G-CSF动员BMSCs时间窗和心脏运动负荷产生效应的最短时间,本研究采用心梗1 h后给予G-CSF动员,手术后1周进行3周间歇运动干预,采用激光共聚焦显微术和流式细胞术及Western Blot方法,观察与分析间歇运动和粒细胞集落刺激因子对心梗大鼠内源性心肌细胞增殖和干细胞动员的效果。
1 材料与方法
1.1 主要仪器和试剂
主要仪器:ALC V8 动物呼吸机、BM Ⅱ型病理组织包埋机、LEICA RM 2126切片机、尼康Nikon C2 Plus激光共聚焦显微镜(laser scanning confocal microscope,LSCM)、PowerLab/8S生理信号采集系统、美国BD公司FACS Aria流式细胞仪、BioTek epoch超微量微孔板分光光度计、GE Logiq 7彩色多普勒超声诊断仪、Bio Rad电泳仪和转移槽等。
主要试剂:甘氨酸、牛血清白蛋白、Tween20、甲叉双丙烯酰胺、丙烯酰胺、DAPI(罗氏公司)、重组人粒细胞集落刺激因子(recombinant human granulocyte colony-stimulating factor,rhG-CSF 山东齐鲁制药有限公司)、小鼠抗大鼠PCNA(Cell Signaling Technology公司)、2,3,5-氯化三苯基四氮唑(2,3,5-Triphenyltetrazolium chloride,TTC Amresco公司)、心肌型兔抗大鼠肌钙蛋白T(cardiac troponin T,cTnT)、兔抗大鼠CD29和c-kit(北京博奥森公司)、FITC标记的山羊抗兔抗体和TRITC标记的山羊抗小鼠抗体(武汉博士德生物工程有限公司)、动物外周血和脏器组织单个核细胞分离试剂盒(灏洋生物公司)。
1.2 动物分组与MI模型制备
动物分组:清洁级3月龄Sprague Dawley雄性大鼠90只,体重180~220 g,购于西安交通大学实验动物管理中心(动物质量合格证号:SCXK2013-003号)。随机分为假心梗组(Sham)、心梗组(MI)、心梗+间歇运动组(ME)、心梗+G-CSF组(MG)和心梗+G-CSF+间歇运动组(MGE),每组18只。大鼠饲养室温度保持20~29℃,相对湿度50%~60%,采用国家标准啮齿类动物干燥饲料喂养,自由饮食。
MI大鼠模型制备:采用左冠状动脉前降支结扎法[1],同时监测心电图并观察结扎点下方至心尖部位心肌颜色变化。以心电图ST段弓背抬高、结扎线以下心肌发白,或出现病理性Q波或T波倒置为结扎成功标志,之后逐层缝合关胸。Sham组只开胸穿线,不结扎。
1.3 间歇运动和G-CSF给药方案

1.4 心功能检测
心动超声检测:实验造模结束后,常规腹腔麻醉并称重,8%Na2S脱毛净胸,取仰卧位将头部及四肢固定在手术台上。使用GE Logiq7彩色多普勒超声诊断仪,i12L宽带,术中探头频率为12 MHz,超声检测中图像深度调至2 cm,增益固定为60 dB。将探头置于左前胸,与前正中线呈30°左右夹角,显示胸骨旁左室长轴切面,探头顺时针旋转90°显示左室短轴切面图像,于胸骨旁左室长轴切面二维测量左室室壁厚度及梗死区范围。在二维导引下,将取样线置于左室健索水平,取M型超声心动图,速度为200 mm/s。测量左室舒张末期内径(left ventricle diastolic diameter,LVDd)及左室收缩末期内径(left ventricle systolic diameter,LVDs)。仪器自动计算左室短轴缩短率(left ventricular fractional shortening,LVFS)=(LVDd-LVDs)/LVDd ×100%和左室射血分数(Left ventricle ejection fraction,LVEF)=[(LVDd3-LVDs3)/LVDd3]×100%。测量指标均取3个连续心动周期的平均值。
血流动力学检测:经右颈总动脉逆行插管至大鼠左心室,以多导生理记录仪记录最大左室收缩压(Left ventricle systolic pressure,LVSP)、左室舒张末压(left ventricle end diastolic pressure,LVEDP)、左室压力最大上升速率(The maximum ascending velocity of left ventricle pressure,LV+dp/dtmax)和最大下降速率(The maximum descending velocity of left ventricle pressure,LV-dp/dtmax)。
1.5 取材与样本制备
大鼠心功能测试结束后,立刻断头取血,肝素钠抗凝,分离外周血单个核细胞。摘取心脏,每组随机取6个心脏中性甲醛固定,常规石蜡包埋,连续切片(厚度5 μm),用于免疫荧光实验;另取6个心脏液氮速冻,置-80℃冰箱,用于western blot 实验。剩余6只大鼠常规腹腔麻醉,开胸摘取心脏,5% TTC溶液主动脉逆向灌流染色,液氮速冻,10 min后取出,手工切片(厚约2 mm),数码相机拍照,统计梗死区(乳白色)与非梗死区(红色)面积,并计算梗死面积百分率。
1.6 外周血单个核细胞分离和流式细胞仪检测
大鼠外周血单个核细胞(peripheral blood mononuclear cell,PBMC)分离严格按照灏洋生物公司的“各种动物外周血和脏器组织单个核细胞分离试剂盒操作说明”进行。在15 ml玻璃管中依次加入A和D液各2 ml,取1 ml新鲜抗凝血按1∶1比例与全血及组织稀释液混匀叠加于D液的液面上,400倍重力离心15 min,收集离心管中由上至下第二层单个核细胞悬液,注入5 ml细胞洗涤液试管中,以500倍重力离心20 min,弃上清,沉淀的细胞用PBS重悬2次即得单个核细胞悬液,500倍重力离心20 min ,沉淀的单个核细胞用2.5%戊二醛固定30 min,PBS洗涤3次,离心弃上清,3% BSA 37℃封闭1 h,离心去上清,加入兔抗大鼠c-kit或CD29抗体,37℃孵育2 h,PBS洗涤3次,FITC标记的山羊抗兔c-kit或CD29抗体37℃孵育2 h,PBS洗涤5次。不加一抗,只加FITC标记的山羊抗兔二抗的PBMCs作为阴性对照,FACS Aria 流式细胞仪进行检测。
1.7 免疫荧光检测
石蜡切片脱蜡至水,pH6.0枸橼酸缓冲液抗原修复,切片于修复液中降至室温,PBS清洗,湿盒中正常山羊血清37℃封闭1 h,滴加兔抗大鼠心肌型肌钙蛋白T(cTnT,1∶50)和小鼠抗大鼠细胞增殖核抗原(PCNA,1∶100),湿盒中4℃过夜。室温复温45 min,PBS清洗,滴加FITC标记的山羊抗兔抗体(1∶50)、TRITC标记的山羊抗小鼠抗体(1∶50)和DAPI(1 μg/ml),湿盒中37℃孵育1 h,PBS清洗,甘油缓冲液封片。设置空白对照和阴性对照。尼康Nikon C2 Plus激光共聚焦显微镜观察拍照。
1.8 Western Blot实验
取心梗边缘区心肌组织提取蛋白,10% Tris-甘氨酸SDS聚丙烯酰胺凝胶电泳分离蛋白,转膜,封闭后孵育干细胞表面抗原c-kit和CD29(浓度均为1∶500),4℃过夜。室温孵育二抗30 min(浓度为1∶2 000),洗膜后ECL发光,内参照为GAPDH。
1.9 图像和数据采集与分析
免疫荧光显微图片经Image-Pro Plus 5.1软件采集并分析;Western Blot胶片经扫描后采用Quantity One 4.6软件进行分析;GraphPad Prism 5.0软件作图。所有数据均用SPSS 17.0软件进行处理,采用One-Way ANOVA进行统计学分析,组间显著性差异水平为P<0.05和P<0.01。
2 实验结果
2.1 间歇运动和/或G-CSF可促进心梗大鼠骨髓干细胞动员增加
c-kit+和CD29+均为骨髓干细胞标记物[29,53]。通过分离大鼠外周血单个核细胞,用FITC标记c-kit和CD29抗原,流式细胞仪计数被标记的和未标记的细胞,计算免疫标记的单个核细胞百分率,反映骨髓干细胞动员效果。结果表明,与Sham组比较,MI组外周血单个核细胞中c-kit+和CD29+细胞的百分率增加显著(P<0.01)。与MI组比较,ME组和MG组外周血c-kit+和CD29+干细胞百分率显著增加(P<0.05),MGE组更为显著(P<0.01,图1)。

图1 本研究大鼠外周血c-kit+和CD29+细胞的流式细胞仪检测结果与统计图Figure 1.The Result of c-kit and CD29 Positive PBMCs Detected by Flow Cytometer and Statistical Graph
2.2 间歇运动和/或G-CSF可促进大鼠心梗边缘区c-kit和CD29蛋白表达增加
Western Blot检测结果显示,与Sham组比较,MI组心梗边缘区心肌c-kit和CD29蛋白表达均显著增加(P<0.01)。与MI组比较,ME组和MG组心梗边缘区心肌c-kit和CD29蛋白表达显著增加(P<0.05),且MGE组增加效果显著优于单一因素(P<0.01,图2)。
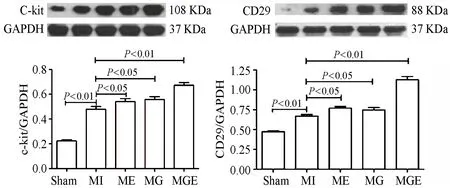
图2 本研究大鼠心梗边缘区c-kit和CD29蛋白表达结果与统计图Figure 2.Expression of c-kit and CD29 Protein in Peri-infarct Area of Rat Heart and Statistical Graph
2.3 间歇运动和/或G-CSF干预可促进心梗大鼠心肌细胞增殖
PCNA为定位于增殖细胞核的抗原,cTnT为定位于心肌细胞质的特异性结构蛋白。DAPI为DNA强力结合染料。PCNA+和cTnT+双阳性细胞可反映新生心肌细胞;新生心肌细胞百分率=新生心肌细胞数/所有心肌细胞数×100%。免疫荧光显示,Sham组PCNA+和cTnT+双阳性细胞极少。MI组、ME组、MG组和MGE组均可见PCNA+和cTnT+双阳性细胞。与Sham组比较,MI组PCNA+和cTnT+双阳性细胞数显著增加(P<0.01);与MI组比较,ME组、MG组和MGE组心肌细胞增生百分率显著增加,且MGE组PCNA+和cTnT+双阳性细胞数最多(P<0.05,P<0.01,图3)。
2.4 间歇运动和/或G-CSF干预可缩小心梗面积,改善心功能
TTC染色结果显示,与Sham组比较,MI组梗死面积显著增加(P<0.01);与MI组比较,ME组和MG组大鼠心梗面积显著缩小(P<0.05),且MGE组梗死面积缩小更加明显(P<0.01,图4)。

图3 本研究心梗边缘区大鼠心肌细胞增殖的激光共聚焦扫描显微镜观察结果与统计图Figure 3.The Immunofluorescent Results of Cardiomyocyte Proliferation Detected by LSCM in Peri-infarct Area of Rats and Statistical Graph

图4 本研究心梗大鼠心脏TTC染色结果与统计图Figure 4.The Results of TTC Staining of Rat Hearts with Myocardial Infarction and Statistical Graph
血流动力学和超声心动检测结果显示,与Sham组比较,MI组大鼠LVEF、LVFS、LVSP、LV+dp/dtmax和LV-dp/dtmax显著降低(P<0.01),LVEDP显著升高(P<0.01),心功能严重受损;与MI组比较,ME组和MG组大鼠LVEF、LVFS、LVSP、LV+dp/dtmax和LV-dp/dtmax显著升高(P<0.05),LVEDP显著下降(P<0.05); MGE组大鼠LVEF、LVFS、LVSP、LV +dp/dtmax和LV-dp/dtmax升高更显著(P<0.01),LVEDP下降更显著(P<0.01),心梗大鼠心功能改善明显(图5、图6)。

图5 本研究心梗大鼠心动超声结果与统计图Figure 5.Echocardiography of Myocardial Infarction Rats and Statistical Graph

图6 本研究心梗大鼠心脏血流动力学结果与统计图Figure 6.Hemodynamic Statistical Results of Myocardial Infarction Rats and Statistical Graph
3 分析与讨论
3.1 间歇运动和/或G-CSF均可促进心梗大鼠动员干细胞归巢与分化
近年的研究发现,干细胞在心肌再生过程中扮演着重要角色[29,34,42],心脏干细胞归巢行为的发现为MI后心脏损伤修复的研究带来新希望。eCSCs/CPCs作为心肌细胞增殖的最直接来源细胞,在损伤发生后可被炎性因子诱导激活,进一步分化为心肌细胞并修复受损心肌组织[16,38]。研究发现,注入c-kit+CSCs可促进心肌修复,降低29%的梗死面积和左室重塑,提升心功能[11]。因此,促进心肌c-kit+细胞数量增加具有重要意义。Orlic等研究证实,BMSCs可被诱导分化为心肌细胞、内皮细胞和血管平滑肌细胞,促进心肌细胞新生,改善血供和心功能,降低个体死亡率[39,40]。此外,近期研究发现,干细胞同样可通过旁分泌效应,促使存活的心肌细胞再生[18,32]。CD29为BMSCs的主要标记物之一,来自大鼠股骨和胫骨的BMSCs与新生大鼠心室肌细胞共培养可分化为心肌细胞,提升心功能[29]。因此认为,促进干细胞动员和提高损伤部位干细胞水平对心肌再生具有积极作用。外源性注射G-CSF可通过SDF-1/CXCR4信号轴诱导BMSCs趋化,参与组织再生[37,52]。G-CSF作为强有力的干细胞动员剂,可有效促进骨髓干细胞进入循环或直接趋化至心肌损伤部位,促进心肌组织再生。有研究报道,运动可促进CSCs和循环中干细胞的激活、动员、归巢和分化[14,54],诱导MI患者循环内皮祖细胞水平增加[25]。
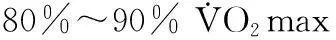
3.2 间歇运动和/或G-CSF促进心梗大鼠心肌细胞增殖
心肌梗死后有功能的心肌细胞大量丢失是造成心肌组织恶性重塑的主要原因之一。如何有效促进内源性心肌增殖是近年来临床医学和基础研究的热点问题。有文献报道,正常生理和病理状态下,心肌细胞存在增殖现象。Kajstura等发现,缺血心肌中处于有丝分裂期的心肌细胞数量比正常生理状态下增加了近10倍[20]。Bostrom等研究发现,游泳可促进心肌PCNA蛋白表达,诱导心肌细胞发生有丝分裂,其机制可能与C/EBP beta信号有关[7]。Waring等报道,有氧运动可上调NRG1、FGF2和Periostin等多种细胞生长因子表达,增加Brdu+和Ki67+心肌细胞数目[57]。以上研究表明,运动可促进正常生理状态下心肌细胞的增殖。本研究采用PCNA和cTnT双阳性表达来反映心肌细胞增殖水平。结果发现,MI后的第4周,梗死边缘区心肌组织中检测到PCNA+和cTNT+双阳性细胞水平显著增加,证实了MI后心肌细胞增殖被激活。ME组和MG组PCNA+心肌细胞比MI组大鼠显著增加,说明间歇运动和G-CSF均可使梗死边缘区心肌细胞产生较强的增殖效应。MGE组梗死边缘区PCNA+心肌细胞比ME组和MG组显著,提示,间歇运动协同G-CSF能产生比单一因素干预更强的心肌细胞增殖效应。
3.3 间歇运动和/或G-CSF缩小心肌梗死面积,提升心功能
研究报道,心肌梗死后的运动康复或给予G-CSF可改善心肌损伤后心功能[4,16]。本研究显示,间歇运动和G-CSF均可缩小心梗面积,二者协同作用效果更为显著,说明间歇运动和G-CSF在心肌梗死后心肌组织再重塑方面发挥积极作用。本研究进一步发现,心肌梗死后大鼠心脏LVEF、LVFS、LVSP、LV+dp/dtmax和LV-dp/dtmax显著降低,LVEDP显著升高,心功能严重恶化。心梗后进行间歇运动或G-CSF干细胞动员,均可以显著升高LVEF、LVFS、LVSP、LV+dp/dtmax和LV-dp/dtmax和降低LVEDP,心功能得到有效改善,间歇运动和G-CSF联合作用效果优于单一因素。
间歇运动或G-CSF均可动员心梗大鼠骨髓干细胞进入外周血,有效增加循环血中c-kit+和CD29+单个核细胞比率和增加心肌梗死边缘区c-kit和CD29蛋白表达,提示,进入外周血的干细胞可能在趋化因子SDF-1作用下沿SDF-1/CXCR4信号轴向心肌梗死及其边缘区归巢、分裂和分化,生成新的心肌细胞或者通过干细胞旁分泌作用,促进心肌细胞数目增加,缩小梗死面积,提升心功能。间歇运动联合G-CSF干预可更有效动员干细胞归巢,增加梗死边缘区新生心肌细胞比率,其心功能改善效果优于单一因素的作用。
4 结论
间歇运动协同G-CSF干预可显著促进内源性干细胞动员归巢,诱导心肌细胞增殖,缩小梗死面积和提升心功能,且二者联合效果优于单一因素的作用。
[1]蔡梦昕,张娟娟,史秀超,等.有氧运动和G-CSF干预对心梗大鼠心肌细胞再生的影响及其机制探讨[J].体育科学,2013,33(5):50-58.
[2]陈运贤,欧瑞明,钟雪云,等.粒细胞集落刺激因子动员骨髓干细胞治疗大鼠急性心肌梗塞[J].中国病理生理杂志,2002,18(1):1-3.
[3]田振军,贺志雄,刘智炜,等.持续和间歇有氧运动对心梗大鼠心肌Myostatin及其受体表达的影响[J].体育科学,2013,33(11):66-74.
[4]ACHILLI F,MALAFRONTE C,LENATTI L,etal.Granulocyte colony-stimulating factor attenuates left ventricular remodelling after acute anterior STEMI:Results of the single-blind,randomized,placebo- controlled multicentre stem cell mobilization in acute myocardial infarction (STEM-AMI) trial[J].Eur J Heart Fail,2010,12(10):1111-1121.
[5]ANDJIC M,SPIROSKI D,ILIC S O,etal.Effects of short-term exercise training in patients following acute myocardial infarction treated with primary percutaneous coronary intervention[J].Eur J Phys Rehabil Med.2015,Nov 19.[Epub ahead of print].
[6]BERGMANN O,BHARDWAJ R D,BERNARD S,etal.Evidence for cardiomyocyte renewal in humans[J].Sci,2009,324(5923):98-102.
[7]BOSTROM P,MANN N,WU J,etal.C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling[J].Cell,2010,143(7):1072-1083.
[8]BREHM M,PICARD F,EBNER P,etal.Effects of exercise training on mobilization and functional activity of blood-derived progenitor cells in patients with acute myocardial infarction[J].Eur J Med Res,2009,14(9):393-405.
[9]CHENG F C,SHEU M L,SU H L,etal.The effect of exercise on mobilization of hematopoietic progenitor cells involved in the repair of sciatic nerve crush injury[J].J Neurosurg,2013,118(3):594-605.
[10]CIOLAC E G,BOCCHI E A,BORTOLOTTO L A,etal.Effects of high-intensity aerobic interval training vs.moderate exercise on hemodynamic,metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension[J].Hypertens Res,2010,33(8):836-843.
[11]DAWN B,STEIN A B,URBANEK K,etal.Cardiac stem cells delivered intravascularly traverse the vessel barrier,regenerate infarcted myocardium,and improve cardiac function[J].PNAS,2005,102(10):3766-3771.
[12]ELLIOTT A D,RAJOPADHYAYA K,BENTLEY D J,etal.Interval training versus continuous exercise in patients with coronary artery disease:A meta-analysis[J].Heart Lung Circ,2015,24(2):149-157.
[13]ELLISON G M,NADAL-GINARD B,TORELLA D.Optimizing cardiac repair and regeneration through activation of the endogenous cardiac stem cell compartment[J].J Cardiovasc Transl Res,2012,5(5):667-677.
[14]FIGUEIREDO P A,APPELL C H,DUARTE J A.Cardiac regeneration and cellular therapy:Is there a benefit of exercise?[J].Int J Sports Med,2014,35(3):181-190.
[15]FONTES-CARVALHO R,SAMPAIO F,TEIXEIRA M,etal.The role of a structured exercise training program on cardiac structure and function after acute myocardial infarction:Study protocol for a randomized controlled trial[J].Trials,2015,16:90.
[16]FUKUHARA S,TOMITA S,NAKATANI T,etal.G-CSF promotes bone marrow cells to migrate into infarcted mice heart,and differentiate into cardiomyocytes[J].Cell Transplant,2004,13(7-8):741-748.
[17]GODFREY R,THEOLOGOU T,DELLEGROTTAGLIE S,etal.The effect of high-intensity aerobic interval training on postinfarction left ventricular remodelling[J].BMJ Case Rep,2013,DOI:10.1136/bcr-2012-007668.
[18]GNECCHI M,ZHANG Z,NI A,etal.Paracrine mechanisms in adult stem cell signaling and therapy[J].Circ Res,2008,103(11):1204-1219.
[19]HARAM P M,KEMI O J,LEE S J,etal.Aerobic interval training vs.continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity[J].Cardiovasc Res.2009,81(4):723-732.
[20]KAJSTURA J,LERI A,FINATO N,etal.Myocyte proliferation in end-stage cardiac failure in humans[J].PNAS,1998,95(15):8801-8805.
[21]KAJSTURA J,ROTA M,CAPPETTA D,etal.Cardiomyogenesis in the aging and failing human heart[J].Circulation,2012,126(15):1869-1881.
[22]KESER I,SUYANI E,AKI S Z,etal.The positive impact of regular exercise program on stem cell mobilization prior to autologous stem cell transplantation[J].Transfus Apher Sci,2013,49(2):302-306.
[23]KIM C,CHOI H E,LIM M H.Effect of high interval training in acute myocardial infarction patients with drug-eluting stent[J].Am J Phys Med Rehabil.2015,94(10 Suppl 1):879-886.
[24]LAFLAMME M A,MURRY C E.Heart regeneration[J].Nature,2011,473(7347):326-335.
[25]LAUFS U,WERNER N,LINK A,etal.Physical training increases endothelial progenitor cells,inhibits neointima formation,and enhances angiogenesis[J].Circulation,2004,109(2):220-226.
[26]LAUGWITZ K L,MORETTI A,LAM J,etal.Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages[J].Nature,2005,433(7026):647-653.
[27]LEONE A M,RUTELLA S,BONANNO G,etal.Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function[J].Eur Heart J,2005,26(12):1196-1204.
[28]LI M, IZPISUA B J C.Mending a Faltering Heart[J].Circ Res,2016,118(2):344-351.
[29]LI X,YU X,LIN Q,etal.Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment[J].J Mol Cell Cardiol,2007,42(2):295- 303.
[30]LIU J,SLUIJTER J P,GOUMANS M J,etal.Cell therapy for myocardial regeneration[J].Curr Mol Med,2009,9(3):287-298.
[31]LU K,WANG L,WANG C,etal.Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis,oxidative stress and metabolism of the infarcted myocardium in a rat model[J].Mol Med Rep,2015,12(2):2374-2382.
[32]MALTAIS S,TREMBLAY J P,PERRAULT L P,etal.The paracrine effect:Pivotal mechanism in cell-based cardiac repair[J].J Cardiovasc Transl Res,2010,3(6):652-662.
[33]MCGREGOR G,GAZE D,OXBOROUGH D,etal.Reverse left ventricular remodelling- effect of cardiac rehabilitation exercise training in myocardial infarction patients with preserved ejection fraction[J].Eur J Phys Rehabil Med,2015,Nov 4.[Epub ahead of print].
[34]MERCOLA M,RUIZ-LOZANO P,SCHNEIDER M D.Cardiac muscle regeneration:Lessons from development [J].Genes Dev,2011,25(4):299-309.
[35]MOLLOVA M,BERSELL K,WALSH S,etal.Cardiomyocyte proliferation contributes to heart growth in young humans[J].PNAS,2013,110(4):1446-1451.
[36]MOTOHIRO M,YUASA F,HATTORI T,etal.Cardiovascular adaptations to exercise training after uncomplicated acute myocardial infarction[J].Am J Phys Med Rehabil.2005,84(9):684-691.
[37]NIENABER C A,PETZSCH M,KLEINE H D,etal.Effects of granulocyte-colony-stimulating factor on mobilization of bone marrow-derived stem cells after myocardial infarction in humans[J].Nat Clin Pract Cardiovasc Med,2006,3(Suppl 1):S73-S77.
[38]OH H,BRADFUTE S B,GALLARDO T D,etal.Cardiac progenitor cells from adult myocardium:Homing,differentiation,and fusion after infarction[J].PNAS,2003,100(21):12313-12318.
[39]ORLIC D,KAJSTURA J,CHIMENTI S,etal.Bone marrow stem cells regenerate infarcted myocardium[J].Pediatr Transplant,2003,7(Suppl 3):86-88.
[40]ORLIC D,KAJSTURA J,CHIMENTI S,etal.Mobilized bone marrow cells repair the infarcted heart,improving function and survival[J].PNAS,2001,98(18):10344-10349.
[41]PEIXOTO T C,BEGOT I,BOLZAN D W,etal.Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction:A randomized controlled trial[J].Can J Cardiol,2015,31(3):308-313.
[42]PFEFFER M A,BRAUNWALD E.Ventricular remodeling after myocardial infarction.Experimental observations and clinical implications[J].Circulation,1990,81(4):1161- 1172.
[43]REDDY K,KHALIQ A,HENNING R J.Recent advances in the diagnosis and treatment of acute myocardial infarction[J].World J Cardiol,2015,7(5):243-276.
[44]RIPA R S,HAACK-SORENSEN M,WANG Y,etal.Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction:Results from the Stem Cells in Myocardial Infarction (STEMMI) trial[J].Circulation.2007,116(11 Suppl):124-130.
[45]RIPA R S,JORGENSEN E,WANG Y,etal.Stem cell mobilization induced by subcutaneous granulocyte- colony stimulating factor to improve cardiac regeneration after acute ST- elevation myocardial infarction:Result of the double-blind,randomized,placebo-controlled stem cells in myocardial infarction (STEMMI) trial[J].Circulation,2006,113(16):1983-1992.
[46]RIVAS-ESTANY E,SIXTO-FERNANDEZ S,BARRERA-SARDUY J,etal.Effects of long-term exercise training on left ventricular function and remodeling in patients with anterior wall myocardial infarction[J].Arch Cardiol Mex,2013,83(3):167-173.
[47]ROGNMO O,HETLAND E,HELGERUD J,etal.High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease[J].Eur J Cardiovasc Prev Rehabil,2004,11(3):216-222.
[48]ROLIM N,SKARDAL K,HOYDAL M,etal.Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction[J].Basic Res Cardiol,2015,110(4):44.
[49]RUSSO V,YOUNG S,HAMILTON A,etal.Mesenchymal stem cell delivery strategies to promote cardiac regeneration following ischemic injury[J].Biomaterials,2014,35(13):3956-3974.
[50]SEGERS V F,LEE R T.Stem-cell therapy for cardiac disease[J].Nature,2008,451(7181):937-942.
[51]SINGLA D K,LONG X,GLASS C,etal.Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium[J].Mol Pharm,2011,8(5):1573-1581.
[52]TAGHAVI S,GEORGE J C.Homing of stem cells to ischemic myocardium[J].Am J Transl Res,2013,5(4):404-411.
[53]THEISS H D,VALLASTER M,RISCHPLER C,etal.Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis[J].Stem Cell Res,2011,7(3):244-255.
[54]WAHL P,BRIXIUS K,BLOCH W.Exercise-induced stem cell activation and its implication for cardiovascular and skeletal muscle regeneration[J].Minim Invasive Ther Allied Technol,2008,17(2):91-99.
[55]WANG Y,JOHNSEN H E,MORTENSEN S,etal.Changes in circulating mesenchymal stem cells,stem cell homing factor,and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention[J].Heart.2006,92(6):768-774.
[56]WARBURTON D E,MCKENZIE D C,HAYKOWSKY M J,etal.Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease[J].Am J Cardiol,2005,95(9):1080-1084.
[57]WARING C D,HENNING B J,SMITH A J,etal.Cardiac adaptations from 4 weeks of intensity-controlled vigorous exercise are lost after a similar period of detraining[J].Physiol Rep,2015,3(2):e12302.
[58]WEN Z,MAI Z,ZHANG H,etal.Local activation of cardiac stem cells for post-myocardial infarction cardiac repair[J].J Cell Mol Med,2012,16(11):2549-2563.
[59]WISLØFF U,ELLINGSEN Ø,KEMI O J.High-intensity interval training to maximize cardiac benefits of exercise training?[J].Exe Sport Sci Rev,2009,37(3):139-146.
[60]WISLØFF U,LOENNECHEN J P,CURRIE S,etal.Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility,Ca2+sensitivity and SERCA-2 in rat after myocardial infarction[J].Cardiovasc Res,2002,54(1):162-174.
[61]WISLØFF U,STØYLEN A,LOENNECHEN J P,etal.Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients:A randomized study[J].Circulation,2007,115(24):3086-3094.
The Observation and Analysis of Stem Cell Mobilization and Endogenous Cardiomyocyte Proliferation Promoted by Interval Exercise and Granulocyte Colony-stimulating Factor Using Confocal Microscopy and Flow Cytometry in Myocardial Infarction Rats
SHI Xiu-chao1,2,CAI Meng-xin1,TIAN Zhen-jun1
Objectives:To discuss the effects of treadmill interval exercise and granulocyte colony-stimulating factor(G-CSF) on stem cell mobilization,endogenous cardiomyocyte proliferation and cardiac function in rats with myocardial infarction(MI).Methods:90 Adult male sprague-dawley rats were randomly divided into:Sham-operated group(Sham),MI group(MI),MI with interval exercise group(ME),MI with G-CSF treatment group(MG)and MI with G-CSF treatment plus interval exercise group(MGE).The MI model of rats was established by ligation of the left anterior descending (LAD) coronary artery.Rats in ME and MGE groups were subjected to 3-week treadmill interval exercise seven days after myocardial infarction.Rats in MG and MGE groups were injected with rhG-CSF subcutaneously 1h after myocardial infarction,10 μg/kg/d for 5 days.Cardiomyocyte proliferation ratio was detected by immunofluorescence and calculated in the peri-infarct region.C-kit and CD29 positive peripheral blood mononuclear cells (PBMCs) were counted by flow cytometer.C-kit and CD29 expression in peri-infarct area was measured by western blot.Hearts were picked for TTC dyeing to decide the myocardial infarction area of each group.Hemodynamic measurement and echocardiography were performed to evaluate cardiac function.Results:Compared to Sham group,the ratio of PCNA and cTnT dual positive cardiomyocytes,c-kit+and CD29+cells ratio in the PBMCs,c-kit and CD29 expression in the peri-infarct region,myocardial infarction area were significantly increased and cardiac performance decreased significantly in MI rats;compared to MI rats in peri-infarct region,percentage of PCNA and cTnT dual positive cardiomyocytes,c-kit and CD29 positive PBMCs ratio,the expression of c-kit and CD29 protein in the peri-infarct region,cardiac performance were increased significantly,and myocardial infarction area decreased significantly in ME,MG and MGE group.The combined treatment with G-CSF and interval exercise had a better effect than either of them.Conclusions:Interval exercise or G-CSF could promote stem cell mobilization and homing,cardiomyocyte proliferation,cardiac performance and decrease myocardial infarction area significantly in MI rats.The better effects were shown with the combined treatment.
myocardialinfarction;intervalexercise;granulocytecolony-stimulatingfactor;stemcellmobilization;cardiomyocyteproliferation;rat;animalexperiment
2015-10-23;
2016-02-01
国家自然科学基金资助项目(31171141)。
史秀超(1973-),男,陕西城固人,讲师,在读博士研究生,主要研究方向为运动心血管生物学,E-mail:tianzhj2015@hotmail.com;蔡梦昕(1987-),女,河南商丘人,在读博士研究生,主要研究方向为运动心血管生物学;田振军(1965-),男,陕西绥德人,教授,博士研究生导师,主要研究方向为运动心血管生物学,E-mail:tianzj611@hotmail.com。
1.陕西师范大学 体育学院暨运动生物学研究所,陕西 西安 710119;2.渭南师范学院 化学与环境学院,陕西 渭南 714000 1.Shaanxi Normal University,Xi’an 710119,China;2.Weinan Normal University,Weinan 714000,China.
G804.7
A
10.16469/j.css.201604008

