外源性硫化氢对吗啡成瘾大鼠海马区cAMP信号通路和学习记忆能力的影响
魏 英,田 虹,秦 伟,陈远寿
(遵义医学院 生理学教研室, 贵州 遵义 563099)
基础医学研究
外源性硫化氢对吗啡成瘾大鼠海马区cAMP信号通路和学习记忆能力的影响
魏英,田虹,秦伟,陈远寿
(遵义医学院 生理学教研室, 贵州 遵义563099)
[摘要]目的 证实硫化氢能否调节吗啡成瘾大鼠海马区内腺苷酸环化酶通路,改善吗啡成瘾所导致的学习记忆相关的神经行为学影响。方法 本实验中, 应用Morris水迷宫来评估吗啡成瘾大鼠的学习记忆能力,酶联免疫试剂盒检测海马组织内腺苷酸环化酶、环磷酸腺苷及蛋白激酶A水平, 免疫印迹法检测海马内磷酸化的环磷酸腺苷反应元件结合蛋白水平。结果 Morris水迷宫测试结果表明,外源性硫化氢能提高吗啡成瘾大鼠学习记忆能力。酶联免疫及免疫印迹检测结果表明,外源性硫化氢能够明显提高吗啡成瘾大鼠海马组织内腺苷酸环化酶、环磷酸腺苷、蛋白激酶A及磷酸化的环磷酸腺苷反应元件结合蛋白水平。此外,使用胱硫醚β合成酶抑制剂盐酸羥胺的吗啡成瘾大鼠海马组织内腺苷酸环化酶、环磷酸腺苷、蛋白激酶A及磷酸化的环磷酸腺苷反应原件结合蛋白水平与吗啡组大鼠无明显差异。结论 外源性硫化氢提高吗啡成瘾大鼠海马组织内腺苷酸环化酶、环磷酸腺苷、蛋白激酶A及磷酸化的环磷酸腺苷反应元件结合蛋白水平,并改善吗啡成瘾大鼠学习记忆能力。
[关键词]硫化氢;吗啡依赖;Morris水迷宫;cAMP信号通路;海马体;大鼠
As a gaseous mediator and a novel type of endogenous neuron regulatory factor, H2S plays an important role in the central nervous system. It is clear that H2S can serve as a new biological gaseous transmitter that mediates a broad range of physiological processes in the nervous system.
Opioid is known to produce analgesia via opioid receptor activation in the brain and the spinal cord. On the other hand, repeated exposure to opioids results in the development of tolerance and dependence. Opioid withdrawal syndrome, a main cause to elicit intense craving for opioids, can be observed after abrupt cessation of drug use. Of course, withdrawal syndrome can be elicited by a competitive opioid antagonist naloxone after long periods of use[1]. Chronic opioid exposure induces persistent activation of opioid receptors, which results in the supersensitivity of the adenylate cyclase (AC) signaling system[2]. Some previous studies suggest that the up-regulation of cAMP pathway is the best established mechanism involved in the development of opioid dependence and the precipitation of its withdrawal syndrome[3].
H2S is endogenously synthesized from L-cysteine, a product of food-derived methionine, by cystathionone β-synthase (CBS) and cyctathione-γ-lyase (CSE). Some recent studies indicate that H2S is important in the regulation of opioid dependence and withdrawal and this effect is mediated by the inhibition of AC/cAMP/CREB pathway. Hence, after administration of exogenous H2S donor, behavioral and neuro-chemical effects of heroin can be significantly attenuated in rats[4-5].
Previous study has shown that opioid addiction can lead to the decline in learning and memory performance in rats[6-8]. In the present study, we examined the effect of sodium hydrosulfide (NaHS, the exogenous H2S donor) on the expression of cAMP/phosphorylate CREB in hippocampus of morphine-dependent rats. At the same time, the changes of learning and memory ability were assessed by Morris water maze.
1Materials and methods
1.1AnimalsMale Sprague-Dawley rats (200±20 g) were obtained from the Medical Experimental Animal Center of Daping Hospital of Chongqing Third Military Medical University in China. They were maintained in standard laboratory conditions (20-22 ℃, 65%-70% relative humidity and a 12-hour light/dark cycle) with food and water available. All procedures were implemented in the light of institutional guidelines. The animals were treated according to the guidelines of Laboratory Animals and the Animal Ethics of China, and approved by the Committee of the Use and Care of Animals of Zumyi Medical College.
1.2Animal group and treatmentAfter two days of adaption, rats were randomly distributed into four experiment groups: Saline+Saline group (n=6), Saline+morphine group (n=6), sodium hydrosulfide+morphine group (n=6), hydroxylamine hydrochloride+morphine group (n=6). NaHS and hydroxylamine hydrochloride dissolved in saline was administered intraperitoneally 30 min prior to morphine. An equal volume of saline was intraperitoneally injected in the saline group.
Water maze experiment was performed first before establishing the chronic morphine dependence model. Morris water maze is a diameter of 150 cm, height of 70 cm round pool, depth of 47 cm, water temperature is (23±2) ℃, water with titanium dioxide, a platform (12 cm high, 45 cm in diameter) was placed in the pool,under the water 2 cm, the room temperature is 15 ℃. The pool was divided into four quadrants, the platform in the third quadrant. Adaptive training: putting each rat into the pool, in turn, for 120 seconds at 09∶00 am on the first day (at any quadrant without the platform); navigation experiment: Start at 9:00 from the second day to the fifth day, putting rats into the pool from first to fourth quadrant in turn, recording the escape latency time, in 120 seconds, that is the time from the entry point to climbing up the platform. If the rats find the platform in the 120 seconds, recording the time, if the opposite, guiding the rats to the platform to stay for 10 seconds, the escape latency time is 120 seconds. The average of four escape latency time was recorded as the escape latency time in the day. In the fourth consecutive day, finish the test by the same method. Space exploration experiment: Taking the platform away in advance, putting each rat in the pool, at the third quadrant for 120 seconds, recording the frequency of passing the platform (FPP).
The following procedures were conducted in order to establish a chronic morphine dependence model (morphine hydrochloride Injection was purchased from Zunyi Medical Company of Guizhou Province, China, at 10 mg/ml). The rats were injected morphine subcutaneously by increasing dosage from 5 mg/kg for the first day to 50 mg/kg for the tenth day (increased by 5 mg/kg each day, twice a day), and then maintained two days for 50 mg/kg. Morphine withdrawal syndrome was precipitated by administration of naloxone injection (5 mg/kg, i.p.) (Chengdu Tiantai pharmaceutical Co. Ltd., China). After completion of the second injection in the tenth day, the animals were placed in a plexiglass square box for habituation to the test environment one hour prior to the naloxone injection. Naloxone was injected intraperitoneally to precipitate withdrawal syndrome. Signs of withdrawal were scored for thirty minutes. Ten previously identified behavioral characteristics of the rat morphine abstinence syndrome were assessed. The absolute frequency of five episodic behaviors was recorded and scored based on multiples of five incidents (0=no incidents; 1=1-5 incidents; 2=6-10 incidents and 3≥10 incidents). Behaviors scored in this manner included jumping, teeth chatter, writhing, wet-dog shakes, and rearing. Four withdrawal behaviors could not be defined in discrete episodes (ptosis, salivation, sneezing and diarrhea), and the severity of these behaviors was assessed using a four-point scale: 0=absent; 1=mild; 2=moderate; 3=severe. The amount of body weight loss was measured at the end of the rating period (i.e.,1h after the administration of naloxone) and a score was calculated based on multiples of 5 g (0=no loss; 1=1-5 g; 2=6-10 g; 3=11-15 g, etc.) The scores for each time period were then added together. The people measuring behavior were blinded to the experimental groups.
After finishing a chronic morphine dependence model, there is a need to proceed the second water maze experiment. The experiment content and method of recording results were the same as above , but deleting the adaptive training.
1.3Sample preparationThe rat was narcotized 5 min before beheading with chloral hydrate (content is 10%), the brain tissues were quickly removed on the ice box, Isolating the hippocampus on both sides, loading them into separate eppendorf tubes, and then, putting them into liquid nitrogen container, after finishing all specimen, putting them in the refrigerator (-80 ℃) .
1.4Methods
1.4.1A double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) was used to assay the levels of AC, PKA, cAMP in hippocampusSamples were homogenized in 0.01 mol/l PBS on ice, and the tissue homogenate was centrifuged at 10 000 g for 30 min at 4 ℃. The supernatant was aspirated and stored at -80 ℃ until use. Protein concentration in the supernatant was detected with the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology). Finishing each step of the experiments according to the ELISA Kit strictly, and getting the levels of cAMP (pmol/ml), AC (results are presented as ng/ml) and PKA (results are presented as ng/ml) with Multiskan Spectrum.
1.4.2Western blot assay p-CREB protein level in hippocampusProtein expression in tissue was quantified using Western blot. Briefly, samples were homogenized in RIPA Lysis Buffer containing 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS) on ice. The tissue homogenate was centrifuged at 14 000 g for 5 min at 4 ℃. The supernatant was aspirated and stored at -80 ℃ until use. Protein concentration in the supernatant was detected with the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology). SDS-PAGE (polyacrylamide gel electrophoresis) was performed on a 10% gel, on which 30 μg of total protein per well was loaded after the SDS-PAGE, the proteins were transferred from the gels onto polyvinylidene difluoride membrance. After being blocked in 5% non-fat milk in TBST (tris buffered saline, with Tween-20) at room temperature for 60 min, membranes were blocked in 5% nonfat milk and probed with antibody against phosphorylated cAMP response element-binding protein (1∶500; Abcam’s RabMAb® technology, U.S.) and antibody against β-actin Rabbit ployclonal antibody (1∶3 000, Beijing TDY Biotech CO., Ltd, China) respectively. β-actin was used for the normalization of protein load. The blots were incubated with IRDye 800CW goat anti-rabbit IgG(H+L) (1∶6 000, LI-COR CO, USA) for 1 h at room temperature and visualized with Odyseey Infrared Imaging (LI-COR CO, USA).
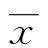
2Results
2.1Exogenous H2S alleviates the damage of learning-memory abilityAs shown in Table1, there was no statistically significant difference of the escape latency time among these normal groups of rats (P>0.05). Compared with normal group, Morphine-administered rat exhibited significant learning and memory damage (P<0.05). Compared with morphine-administered rats, the exogenous H2S improved the learning and memory damage significantly (P<0.05).After hydroxylamine hydrochloride administration, it is even worse in rats addicted to morphine.

GroupTheescapelatencytime(s)ThefirstdayTheseconddayThethirddayThefourthdayFPPThefifthdayCon5.48±2.715.58±2.497.45±5.206.09±4.024.17±1.72Mor36.05±8.77*15.48±8.75*17.97±10.49*15.91±4.02*1.60±0.55*Mor+S7.85±7.47*#4.51±1.74*#5.06±3.07*#8.70±7.24*#4.00±1.41*#Mor+HA36.49±8.17*28.71±10.20*16.26±9.74*16.01±5.00*1.25±0.96*
FPP: the frequency of passing the platform; Con is equal to saline+ saline group; Mor is equal to saline+morphine group; Mor+S is equal to sodium hydrosulfide + morphine group; Mor+HA is equal to hydroxylamine hydrochloride + morphine group; * is equal to the significant difference between the treatment group with control group,P<0.05; # is equal to the significant difference between Mor+S group with Mor group or Mor+HA group,P<0.05.
2.2Effect of Exogenous H2S on the activity of AC in hippocampusThe result is shown in Figure 1, exogenous H2S increased the activity of AC caused by morphine addiction. While the activity of AC of Mor+HA group was depressed like Mor group, hydroxylamine hydrochloride is a inhibitor of cystathionine-synthase. And it is interesting that there is no difference between Con group and Mor+S group (P>0.05), and it is the same for Mor group and Mor+HA group (P>0.05).

±s, n=4; **: P<0.01, vs Con; ##: P<0.01, vs Mor or Mor+HA. Con is equal to saline + saline group; Mor is equal to saline+morphine group; Mor+S is equal to sodium hydrosulfide + morphine group; Mor+HA is equal to hydroxylamine hydrochloride + morphine group. Fig 1 Effect of NaHS/HA pretreatment on the activity of AC and PKA in the hippocampus of morphine-treated rat
2.3Effect of exogenous H2S on the levels of cAMP in hippocampusAs showed in Figure 2, when Con group was compared with medication group, the level of cAMP in hippocampus showed difference, the content was significantly decreased in medication group (P<0.01). Exogenous H2S increased the level of cAMP in Mor+S group to some extents, and the statistics analysis is meaningful (P<0.01). While there was no difference between Mor group and Mor+HA group (P>0.05).
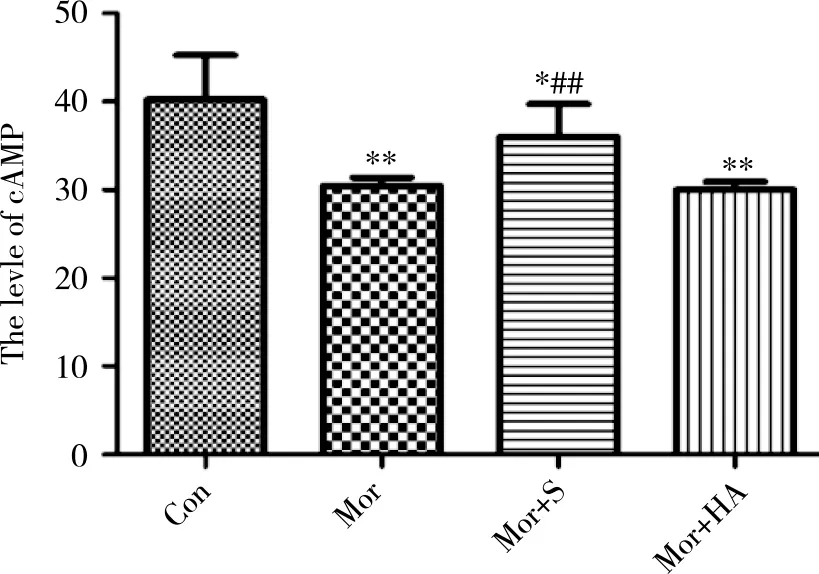
±s, n=4; *: P<0.05, vs Con; **: P<0.01, vs Con; ##: P<0.01, vs Mor or Mor+HA. Con is equal to saline + saline group; Mor is equal to saline + morphine group; Mor+S is equal to sodium hydrosulfide + morphine group; Mor+HA is equal to hydroxylamine hydrochloride + morphine group. Fig 2 Effect of NaHS/HA pretreatment on the level of cAMP in the hippocampus of morphine-treated rat
2.4Effect of Exogenous H2S on the activity of PKA in hippocampusAs showed in Figure 1, with regard to the activity of PKA in hippocampus,Mor group and Mor+HA group was significantly decreased, and exogenous H2S increased the activity of PKA caused by morphine addiction in Mor+S group (P<0.01). Likewise, there was no difference between Mor group and Mor+HA group on the activity of PKA (P>0.05).
2.5Effect of exogenous H2S on the levels of p-CREB in hippocampuscAMP signal pathway in the rat hippocampus is the important link of the learning and memorial function. Phosphorylation and activation of CREB are the hinge-mediator for the center of learning and memory to function well. To confirm it, western blot analysis was used to assay phosphorylated CREB.
Figure 3 showed that in Mor group and Mor+HA group, the level of p-CREB was significantly decreased compared with Saline+Saline group and Mor+S group (P<0.01). Exogenous H2S increased the level of p-CREB caused by morphine addiction, and there is no difference between Con group and Mor+S group and similar to the detection of the activity AC, there was no difference between Mor group and Mor+HA group (P>0.05).

±s, n=4; **: P<0.01, vs Con; ##: P<0.01, vs Mor or Mor+HA. Con is equal to saline + saline group; Mor is equal to saline + morphine group; Mor+S is equal to sodium hydrosulfide + morphine group; Mor+HA is equal to hydroxylamine hydrochloride + morphine group. Fig 3 Western blotting analysis showing the effect of NaHS/HA on the level of CREB phosphorylation in morphine-treated rat
3Discussion
Hydrogen sulfide (H2S), an important gaseous signaling molecule, is essential for the proper function of the nervous system in mammals[9]. In some pathophysiological events, exogenous application of H2S donor could improve the learning and memory impairment in some diseases of neuropathy, such as Alzheimer’s disease, brain infarction and cerebral ischemia-reperfusion injury[10-14]. The improvement effects may be through adjusting the H2S signaling pathway. Opioid dependence is a chronic relapsing brain disease, our experiment aims at exploring whether H2S could alleviate the morphine-induced neurobehavioral defects of learning and memory and how H2S adjusting the AC/cAMP/PKA/CREB signaling pathway in hippocampus of morphine-dependent rats.
The ability of learning and memory is an innate talent. Along with the further researches, we realize the base for a scheme involving at least three stages of information storage from the numerous behaviors experiments, and then, synapses was proved to be the most suitable location for memory elements, especially the ACh synapses[15].
It has been proved that cAMP signal pathway is important for the learning and memory function in the central nervous system, especially in hippocampal neurons[16-18]. An extensive body of research has demonstrated that H2S attenuated the learning and memory damage significantly through cAMP signal pathway[19]. In our experiment, different from other researches, chronic morphine addiction rat’s cAMP signal pathway in the hippocampus was inhibited, but the same is that H2S adjusted the cAMP signal pathway and maintain the corresponding protein in normal level. It may confirm that different model establishment methods produce different effects on the brain activity, furthermore, after morphine treatment, brain activity perhaps assume a variable progress at different time points.Opioid dependence is a chronic relapsing disease and characterized by compulsive drug seeking and use, it also produces excessive degrees of tolerance and physical dependence, which are also important factors in the emergence of withdrawal syndrome[20-21].
In the study of the mechanism of opioid addiction, it has been confirmed that opioid addiction involves the miraculous memory function and the reward centre of the brain, and there was correlation between the miraculous memory function and the reward centre[22-23]. The treatment of opioid addiction just like alcohol and other drug addictions, the principle of treatment is analogical. And the therapeutic outcome is widely divergent[24]. Treatment of opioid addiction is taking some alternative medicine right now, such as methadone, buprenorphine, naltrexone. However, they are not always ideal for the disease[25-26]. Recent research revealed that the evidence for antagonist therapies is weak, oral naltrexone demonstrates poor adherence and increased mortality rates[27]. Besides, it has been proved that treatment aimed at common drug mechanisms has not yet been fully validated[28]. The supersensitivity of AC that catabolizes ATP to cAMP is the most robust adaptation to repeated opioid exposure[29-30].
There has been considerable interest in potential interactions between exposure to stressors and addiction to drugs. Recently, GABA receptors were investigated for possible involvement in the resumption of drug use after a drug-free period[31-32]. Anyhow, opioid dependence would lead to serious damage in the CNS and result in rock-ribbed depress on learning and memory ability. Meanwhile, the change of learning and memory ability in opioid addiction is associated with the physical dependence of addicts. So making a deeply study on the mechanism of memory in addicts isnecessary and meaningful for understanding the unknown territory of addiction.
In the present study, we demonstrated that administration of H2S donors such as NaHS can effectively upregulate the AC-cAMP-PKA-CREB signaling pathway, which is the most important for the learning and memory. It may contribute significantly to improving the learning and memory function of rats addicted to morphine. Our finding suggests that H2S improves the ability of learning and memory maybe mediated through the two-way adjustment of H2S for AC-cAMP-PKA-CREB signaling pathway in rats addicted to morphine.
Opioids act on the CNS,play to relive pain and induce irrational euphoria[19]. Opioid medicine was not only used to minimize the pain of illness, but also used for euthanasia clinically in the Netherlands[33]. So the socio-psychological factor of those vulnerable people maybe a worthful research orientation for control of the addiction. Future research may provide insights into the prevention and treatment of opioid addiction.
4Conclusion
Under the same conditions of water maze test, the results showed that sodium hydrosulfide can improve morphine dependent rat’s ability of learning and memory. The ELISA and Western Blot assay revealed that exogenous H2S significantly improved the levels of AC, PKA, cAMP and p-CREB in morphine dependent rats. These results suggest that exogenous H2S improves learning and memory ability of morphine dependent rat, possibly by adjusting the AC/cAMP/PKA/CREB signaling pathway.
[References]
[1] Maldonado R, Koob G F. Destruction of the locus coeruleus decreases physical signs of opiate withdrawl[J]. Brain Research, 1993, 605(1): 128-138.
[2] Yu V C, Eiger S, Duan D S, et al. Regulation of cyclic AMP by the mu-opioid receptor in human neuroblastoma SH-SY5Y cells[J]. Neurochem, 1990, 55(4): 1390-1396.
[3] Nestler E J, Aghaianian G K. Molecular and cellular basis of addiction[J]. Science, 1997, 278(5335): 58-63.
[4] Yang H Y, Wu Z Y, Wood M, et al. Hydrogen sulfide attenuates opioid dependence by suppression of adenylate cycase/cAMP pathway[J]. Antioxidants & Redox Signaling, 2014, 20(1): 31-41.
[5] Jiang L H, Wang J, Wei X L, et al. Exogenous sodium hydrosulfide can attenuate naloxone-precipitated withdrawal syndromes and affect cAMP signaing pathway in heroin-dependent rat’s nucleus accumbens[J]. European Review for Medical and Pharma Cological Sciences, 2012, 16 (14): 1974-1982.
[6] 罗孝美,潘贵书,陈远寿.外源性硫化氢对海洛因成瘾大鼠学习记忆能力的影响[J].遵义医学院学报,2009,32(2):108-110.
[7] 秦伟,金寰,罗孝美,等.硫化氢对吗啡依赖大鼠空间学习记忆及海马NMDA受体表达的影响[J].遵义医学院学报,2011,34(5):453-456.
[8] 罗孝美,陈远寿,彭昌.外源性硫化氢对海洛因依赖大鼠海马长时程增强的影响[J].神经解剖学杂志,2013,29(3):316-320.
[9] Beata O. Gasomediators (NO, CO, and H2S) and their role in hemostasis[J]. Clinica Chimica Acta, 2015, 20(445): 115-121.
[10] Johansen D, Ytrehus K, Baxter G F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury Evidence for a role of KATP channels[J]. Basic Research in Cardiology, 2006, 101(1): 53-60.
[11] Minamishima S, Bougaki M, Sips P Y, et al. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice[J]. Circulation, 2009, 120(10): 888-896.
[12] Zhang X Z, Bian J S. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system[J]. ACS Chem Neurosci, 2014, 5(10): 876-883.
[13] Xuan A G, Long D H, Li J H, et al. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer’s disease[J]. Journal of Neuroinflammation, 2012, 9: 202-213.
[14] Xu X X, Liu C H, Li Z Y, et al. Effects of hydrogen sulfide on modulation of theta-gamma coupling in hippocampus in vascular dementia rats[J]. Brain Topogr, 2015, 28(6):879-894.
[15] Edward M K. A molecular basis for learning and memory[J]. PNAS, 1972, 69(11): 3292-3296.
[16] Marissa I B, Jason P W,Rachel D G, et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein[J]. The Journal of Neuroscience, 2005, 25(20): 5066-5078.
[17] Jennifer N G,Jessica L B, Peter V N, et al. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus[J]. Learning & Memory, 2008, 15(6): 403-411.
[18] Benno R, Gustav S, James L M. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation[J]. J Neurosci, 2008, 28(26): 6642-6651.
[19] Partlo L A, Sainsbury R S, Roth S H. Effects of repeated hydrogen sulphide (H2S) exposure on learning and memory in the adult rat[J]. Neuro Toxicology, 2001, 22(2): 177-189.
[20] Eric J N. Molecular basis of long-term plasticity underlying addiction[J]. Nature Reviews Neuroscience, 2001, 2(3): 119-128.
[21] Hyman S E,Malenka R C. Addiction and the brain: the neurobiology of compulsion and its persistence[J]. Nature Reiview Neuroscience, 2001, 2(10): 695-703.
[22] Williams J T, Christie M D J, Manzoni O. Cellular and synaptic adaptions mediating opioid dependence[J]. Physiological Reviews, 2001, 81(1): 299-343.
[23] Hyman S E, Malenka R C, Nestler E J. Neural mechanisms of addiction: the role of reward-related learning and memory[J]. Annual, Review of Neuroscience, 2006, 29(1): 565-598.
[24] Gastfriend D R. A pharmaceutical industry perspective on the economics of treatments for alcohol and opioid use disorders[J]. Annals of the New York Academy of Sciences, 2014,1327: 112-130.
[25] Cherny N I. The management of cancer pain[J]. Ca A cancer Journal for Clinicians, 2000, 50(2):70-116.
[26] Volkow N D, Frieden T R, Hyde P S, et al. Medication-assisted therapies-tackling the opioid-overdose epidemic[J]. New England Journal of Medicine, 2014, 370(22): 2063-2066.
[27] Connery H S. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions[J]. Harvard Review of Psychiatry, 2015, 23(2): 63-75.
[28] Nestler E J. Is there a common molecular pathway for addiction?[J]. Nature Neuroscience, 2005, 8(11): 1445-1449.
[29] Sharma S K, Klee W A, Nirenberg M. Opiate-dependent modulation of adenylate cyclase[J]. Proc Natl Acad Sci USA,1977, 74(8): 3365-3369.
[30] Josselyn S A, Shi C, Carlezon W A, et al. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala[J]. Journal of Neuroscience, 2001, 21(7): 2404-2412.
[31] Fátima M, Cristina N, Teresa M M, et al. Involvement of noradrenergic transmission in the PVN on CREB activation, TORC1 levels, and pituitary-adrenal axis activity during morphine withdrawal[J]. Plos One, 2012, 7(2): e31119.
[32] Ren X H, Lutfy K, Mangubat M, et al. Alterations in phosphorylated CREB expression in different brain regions following short- and long-term morphine exposure: relationship to food intake[J]. Journal of Obesity, 2013: http://dx.doi.org/10.1155/2013/764742.
[33] Agnes V D H, Onwuteaka-Philipsen B D, Rurup M L, et al. End-of-life practices in the netherlands under the euthanasia act[J]. New England Journal of Medicine, 2007, 356(19): 1957-1965.
[收稿2016-03-14;修回2016-04-20]
(编辑:王静)
[基金项目]贵州省卫生厅科学技术基金资助项目(NO:gzwkj2013-1-013);贵州省科学技术基金资助项目(NO:J20122351);贵州省科技厅资助项目(NO:黔科合LH字[2015]7524);遵义医学院重点学科建设项目(NO:XZXK-20120702)。
[通信作者]陈远寿,男,教授,硕士生导师,研究方向:神经疾病生理学,E-mail:jcbshengli@163.com。
[中图法分类号]R338.2
[文献标志码]A
[文章编号]1000-2715(2016)03-0233-08
Effects of exogenous hydrogen sulfide on morphine dependent rats’ learning and memory capacity and the cAMP signaling pathway in hippocampus
WeiYing,TianHong,QinWei,ChenYuanshou
(Department of Physiology, Zunyi Medical University, Zunyi Guizhou 563099, China)
[Abstract]Objective To identify whether hydrogen sulfide (H2S) alleviates the morphine-induced neurobehavioral defects of learning and memory by inhibiting the AC/cAMP/PKA/CREB signaling pathway in hippocampus of morphine-dependent rats.Methods Morris water maze was used to evaluate learning and memory ability in morphine-dependent rats. The levels of AC, cAMP, PKA in hippocampus were detected by ELISA. The level of p-CREB protein in hippocampus was detected by western blot.Results Morris water maze test showed that exogenous H2S improved the ability of learning and memory in morphine-dependent rats. ELISA and western blot revealed that exogenous H2S significantly improved the levels of AC, PKA, cAMP and p-CREB. Moreover, after cystathionine-γ-lyase inhibitor hydroxylamine hydrochloride administration, the levels of AC, PKA, cAMP and p-CREB were also significantly suppressed in morphine-dependent rats.Conclusion We speculate that exogenous H2S improves the learning and memory ability by up-regulating AC/cAMP/PKA/CREB signaling pathway in hippocampus of morphine-dependent rats.
[Key words]hydrogen sulfide; morphine-dependent; Morris water maze; cAMP pathway; hippocampus; rat

