施硫对不结球白菜硝酸盐累积及氮硫同化关键基因表达的影响
徐 瑶,牟建梅,张国芹,马佳佳,徐 君,李 军,刘凤军,佘旭东
(江苏省太湖地区农业科学研究所,江苏苏州 215155)
施硫对不结球白菜硝酸盐累积及氮硫同化关键基因表达的影响
徐瑶,牟建梅,张国芹,马佳佳,徐君,李军,刘凤军,佘旭东
(江苏省太湖地区农业科学研究所,江苏苏州 215155)
摘要:【目的】确定降低不结球白菜硝酸盐累积效果最佳的硫素形态,从转录水平筛选影响不结球白菜硝酸盐累积的关键基因,为完善不结球白菜科学施硫技术及进一步揭示硝酸盐累积分子调控机制、指导分子育种奠定基础。【方法】选取4种硫素形态及3个施用浓度处理不结球白菜,测定其对植株叶片及叶柄中硝酸盐含量的影响;利用半定量PCR技术从转录水平分析施硫对氮及硫代谢同化网络中30个基因表达的影响。【结果】不同形态硫处理均显著增加了不结球白菜的地上生物量,其中 30 mg·kg-1Na2SO4处理的增幅最大,比对照增加 49.76%,以 Na2SO4处理的增幅最大。在降低小白菜硝酸盐含量中,以Na2SO4、Na2S2O3处理效果相对显著,其中Na2SO4降低叶片中硝酸盐12.23%—23.55%,叶柄中33.08%—41.98%,降幅与浓度呈正相关,30 mg·kg-1Na2SO4处理的降幅最大;Na2S2O3处理降低植株叶片中硝酸盐15.34%—33.08%,叶柄中11.95%—19.68%。硫处理在一定程度上促进氮同化,对照叶片中NR-1、NADH-GOGAT-1、NADH-GOGAT-2、Cytoplasm-GS-4、Cytoplasm-GS-5、GDH-3表达量低于其他处理,对照叶柄中NR-1、NADH-GOGAT-2、Cytoplasm-GS-1、GDH-2表达量低于其他处理,其中,各处理NADH-GOGAT-2表达量与叶片及叶柄中硝酸盐含量变化呈现一定规律性。硫处理对植株硫同化基因也产生一定影响,对照叶片中ATPS-2、ATPS-3、ATPS-4、APSR-3、SIR、SAT1.1、SAT2.1表达量较低,而对照叶柄中仅SIR、OASTL-A表达量明显低于其他处理。【结论】Na2SO4是降低不结球白菜硝酸盐效果较为显著的硫素,且能够显著提高产量,30 mg·kg-1的Na2SO4为较优处理。NADH-GOGAT-2表达量与不结球白菜内硝酸盐含量呈负相关,推测其可能是影响氮同化的关键基因。
关键词:不结球白菜;硫;硝酸盐;氮硫同化;基因表达
联系方式:徐瑶,E-mail:xuchenyao@163.com。牟建梅,E-mail:thmjm@163.com。徐瑶和牟建梅为同等贡献作者
0 引言
【研究意义】不结球白菜(Brassica campestris ssp. chinensis Makino)是中国重要的叶菜作物,但极易富集硝酸盐,安全品质堪忧。硝酸盐是亚硝胺的合成前体,过量摄入易引发消化系统的癌变,很多国家对叶菜的硝酸盐都有严格限定,中国规定叶菜类硝酸盐含量≤3 000 mg·kg-1,但大部分地区的不结球白菜硝酸盐含量严重超标,上海、南京等地市场抽样检测结果最高可达6 293.91 mg·kg-1[1-2]。因此,加强降低不结球白菜硝酸盐含量的研究,对于保障绿色食品供应、改善人民健康状况有重大意义。【前人研究进展】植物氮同化由硝酸还原酶(nitrate reductase,NR)、亚硝酸还原酶(nitrite reductase,NiR)、谷氨酸合酶(glutamate synthase,GOGAT)、谷氨酰胺合成酶(glutamine synthetase,GS)、谷氨酸脱氢酶(glutamate dehydrogenase,GDH)等关键酶催化完成;硫同化网络则包括ATP硫酸化酶(ATP sulfurylases,ATPS)、APS还原酶(APS reductase,APSR)、亚硫酸盐还原酶(sulfite reductase,SiR)、丝氨酸乙酰转移酶(serine acetyltransferase,SAT)、O-乙酰丝氨酸硫裂解酶(O-acetylserine(thio)lyase,OASTL)等关键酶[3-4]。大量研究表明,植物体内氮硫同化存在高度相关性[3-7],这种同化相关性使植物稳定在一定的氮硫比例以保证蛋白质生物合成[8]。氮硫同化的相关性更表现在其同化酶结构的相似性,NiR与SiR的结构相似性众所周知[4],在藻青菌中SiR甚至能够在NiR缺失突变体中充当NiR发生催化作用[9]。适当施硫能够有效促进氮的吸收同化,提高氮素利用率,降低植株硝酸盐累积,同时在氮代谢的转录组与蛋白组上均有所影
响[8,10]。在油菜上的研究表明,施硫能显著降低植株中的硝酸盐含量,并导致NR及GS表达量显著增加;反之,缺硫导致其叶片与根中硝酸盐大量累积,NR 及GS的表达量急剧下降[11]。同时,增施硫也能在作物光合作用、蛋白质合成、激素代谢以及抗重金属毒害方面有明显积极作用[12-14]。【本研究切入点】通过硫素调节氮同化,降低蔬菜硝酸盐累积,成为行之有效且成本低廉的安全栽培方法。韭菜[15]、大葱[16]等作物中均有报道,施硫显著降低植株硝酸盐含量。不结球白菜也开展了相关研究[17-18],但是仅限表观水平,并未涉及分子调控机理;且国内外关于氮硫代谢网络中,分析不结球白菜硝酸盐累积的关键步骤及关键基因的研究也近乎空白,氮硫互作分子机制尚不明晰。【拟解决的关键问题】本研究比较不同形态硫对不结球白菜硝酸盐累积的影响,从转录水平分析施硫对氮及硫代谢同化网络中30个基因表达影响,筛选影响不结球白菜硝酸盐累积的关键步骤及关键基因,为完善不结球白菜科学施硫技术,进一步揭示硝酸盐累积分子调控机制奠定基础。
1 材料与方法
1.1 材料与处理
试验材料为不结球白菜品种‘华王’,试验为盆栽试验,于2014年10—11月在苏州市农业科学院试验基地进行。塑料盆43 cm×20 cm×14 cm,每盆装土4.5 kg。供试土壤为黄泥土,基本理化性质为有机质35.82 g·kg-1、碱解氮111.21 mg·kg-1、速效磷41.51 mg·kg-1、速效钾287.98 mg·kg-1、有效硫12.16 mg·kg-1、pH 7.39。以分析纯尿素、磷酸氢二氨、氯化钾为基肥,施用量为氮 80 mg·kg-1,P2O540 mg·kg-1,K2O 80mg·kg-1。每盆种植不结球白菜 25株,全生育期严格控制水分,保持绝对含水量在34%,其他均常规管理。
试验选取4种硫素形态及3个施用浓度,共设置13个处理,详见表1。每个处理设置3个重复,随机排列。硫肥于植株长至三叶期时施入土中,至五叶期时选取晴朗天气的早晨9:00—10:00取样,测定各处理地上部生物量、叶柄及叶片硝酸盐含量,分别采集叶片及叶柄样品于液氮速冻,-80℃保存,用于硝酸盐及硫代谢相关基因表达试验。
1.2 地上生物量及硝酸盐含量
取不结球白菜植株地上部分,称取鲜重,每个重复均取15株,地上部生物量为其平均值。分别测定植株叶片、叶柄硝酸盐含量,测定方法参照李合生[19]的紫外分光光度计法。
1.3 总RNA提取及引物设计
总RNA提取纯化步骤参照植物总RNA抽提纯化试剂盒(生工生物工程股份有限公司,上海)。cDNA合成参照M-MuLV第一链cDNA合成试剂盒(生工生物工程股份有限公司,上海)。根据 NCBI (http://www.ncbi.nlm.nih.gov/)上公布的油菜氮硫同化关键基因序列,由Primer 5.0软件根据其开放阅读框设计引物,其中每个基因的同源基因与多拷贝基因均分开设计引物。选择 Actin为内参基因[20]。引物合成由生工生物工程(上海)股份有限公司完成。引物序列如表2所示。
1.4 半定量基因表达分析
利用RT-PCR引物(表2)对不同处理下,不结球白菜的两个部位的样品cDNA进行扩增。反应体系参照PCR扩增试剂盒(Taq)(生工生物工程股份有限公司,上海),模板为20 ng cDNA,反应体系均覆盖已灭菌的石蜡油。反应程序为:94℃预变性2 min;94℃变性40 s,58℃退火40 s,72℃延伸70 s,35个循环;72℃充分延伸10 min。扩增产物用1%琼脂糖凝胶电泳检测,并测序比对验实扩增序列。
1.5 数据分析
试验数据统计分析采用DPS 7.05软件,作图采用Excel 2007。基因表达分析通过对比半定量 RT-PCR扩增条带的亮度强弱,比较其表达量高低。
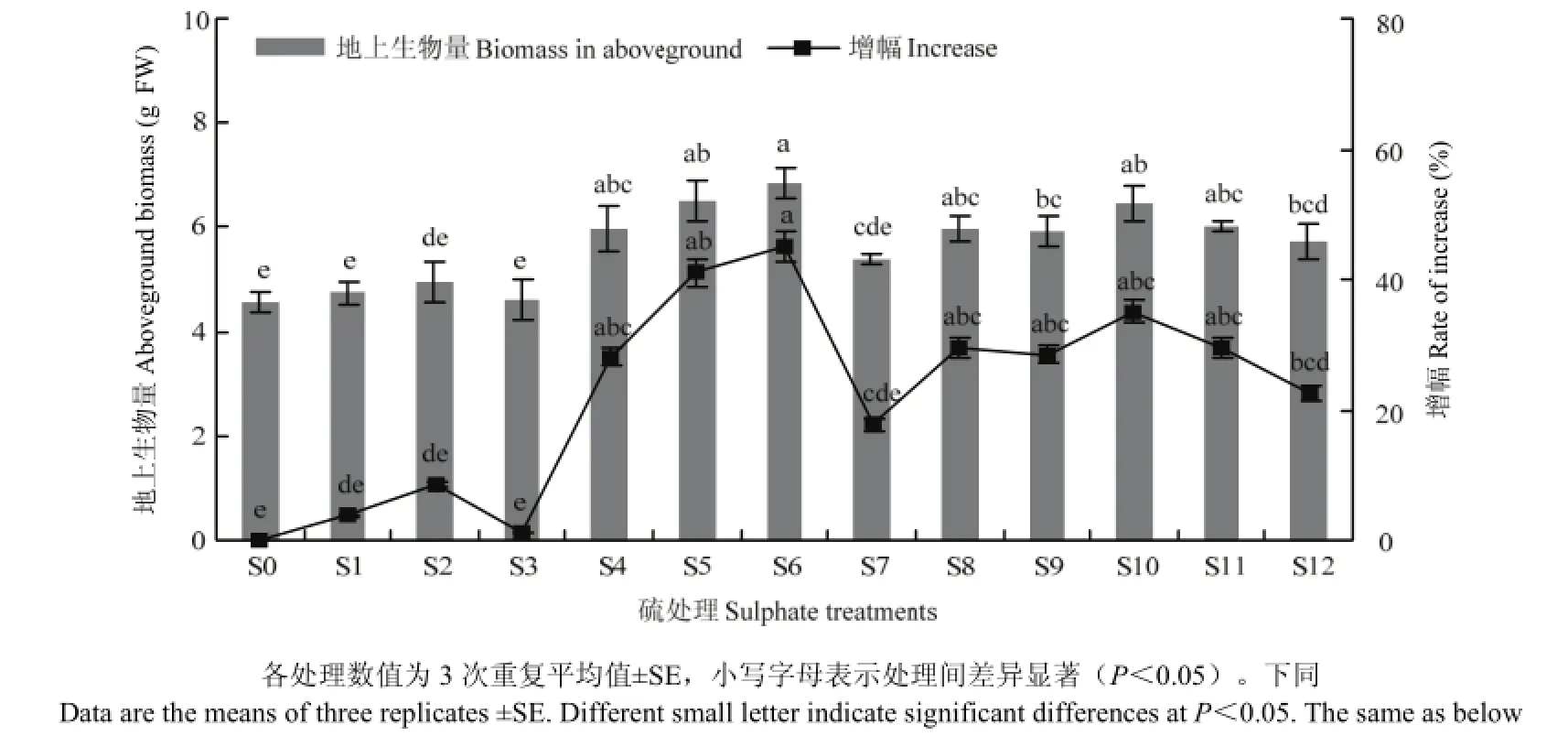
图1 硫处理对不结球白菜地上部生物量的影响Fig. 1 Changes in aboveground biomass of non-heading Chinese cabbage for sulphate treatments
2 结果
2.1 不结球白菜地上生物量
不同形态硫处理均显著增加了不结球白菜的地上生物量,其中S6的增幅最大,相对S0增加49.76%(图1)。各处理间,以Na2SO4处理的S4—S6的增幅最大,其次依次为 Na2S2O3、NaHSO3、硫磺。硫磺处理的S1—S3呈现先增加后降低的趋势,与对照相比,总体增幅为1.46%—8.78%,S2处理地上部生物量增幅最大。S4—S6处理随着 Na2SO4的浓度增加呈现上升趋势,与对照相比,增幅为 31.22%—49.76%。NaHSO3处理的S7—S9地上生物量先上升后下降,与对照相比,增幅为18.54%—30.73%。S9—S12处理随着Na2S2O3浓度增加而下降,与对照相比,增幅为25.85%—41.95%。以上结果证明施硫能有效提高不结球白菜产量。
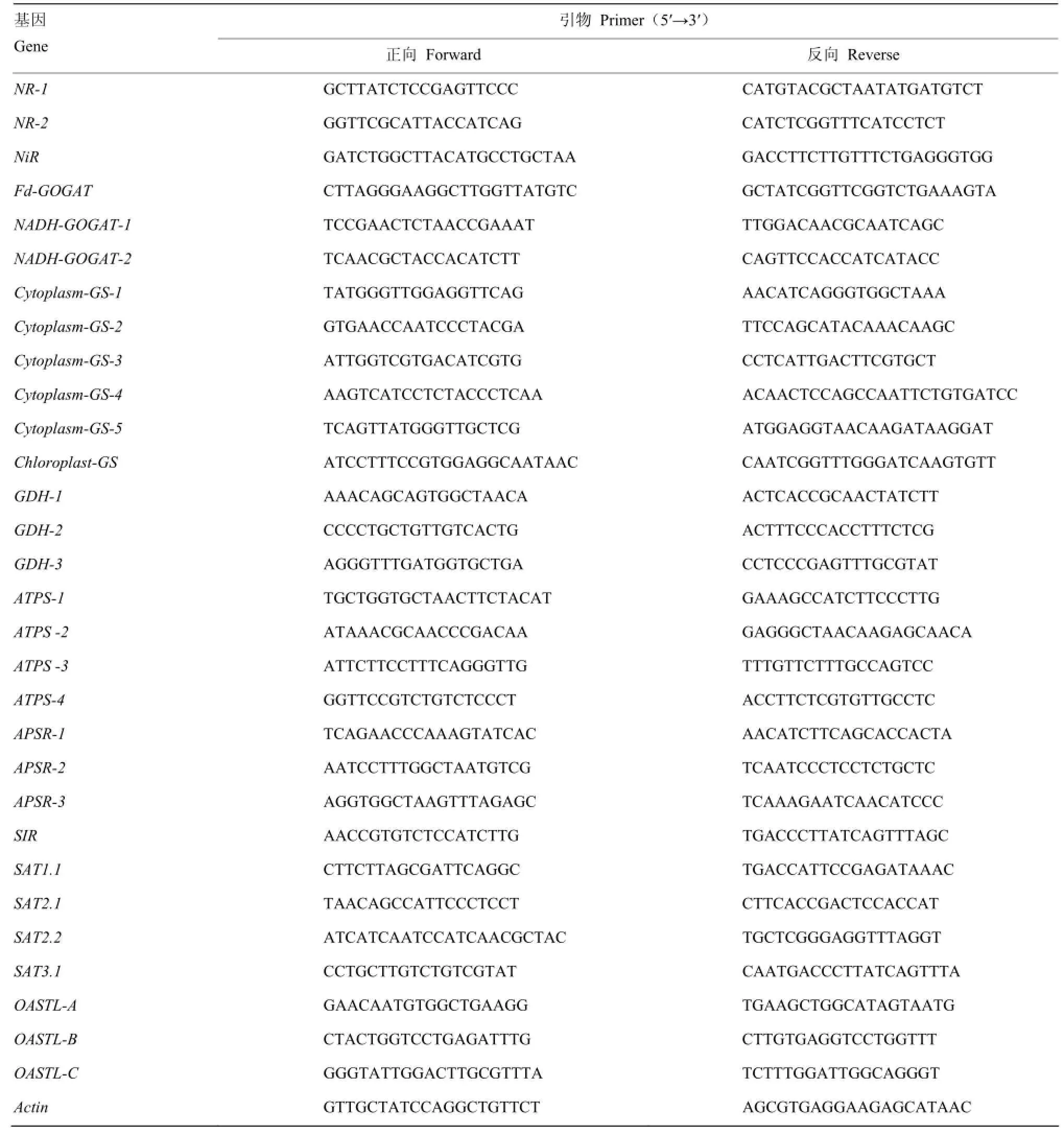
表2 氮及硫同化相关基因表达引物Table 2 The primers for the expression of nitrogen and sulphate assimilation related genes
2.2 叶片硝酸盐含量
不结球白菜叶片中硝酸盐累积随着硫素形态差异呈现不同的变化(图2)。S6、S10,以及S12均显著降低了叶片中硝酸盐含量,相较S0分别降低33.08%、23.55%、17.47%。硫素形态中Na2S2O3、Na2SO4处理效果相对显著,其中Na2S2O3效果最好,不结球白菜对低量的Na2S2O3最为敏感,S10—S12处理降低硝酸盐含量15.34%—33.08%;Na2SO4处理与 Na2S2O3相反,高浓度的Na2SO4对硝酸盐降幅最大,S4—S6处理降幅为12.23%—23.55%。硫磺对小白菜叶片中硝酸盐含量随着硫磺施用量的增加而降低。NaHSO3处理引起不结球白菜叶片中硝酸盐含量的增加,随着浓度的增加而增加,不利于降低其硝酸盐累积。
2.3 叶柄硝酸盐含量
叶柄中硝酸盐含量明显高于叶片,硫处理对降低不结球白菜叶柄中硝酸盐有着显著效果(图 3),处理效果最佳的为 Na2SO4处理,降幅为 33.08%—41.98%,降幅与浓度呈现正相关,高浓度降幅最大。Na2S2O3处理也取得较好效果,降幅为 11.95%—19.68%,低浓度处理效果较好。NaHSO3处理对叶柄硝酸盐的影响与叶片相似,硝酸盐含量与浓度正相关,低浓度处理显著降低硝酸盐累积,高浓度 S9处理与S0中硝酸盐含量没有显著差异。硫磺与NaHSO3处理效果相反,高浓度处理 S2、S3能降低叶柄中硝酸盐含量。
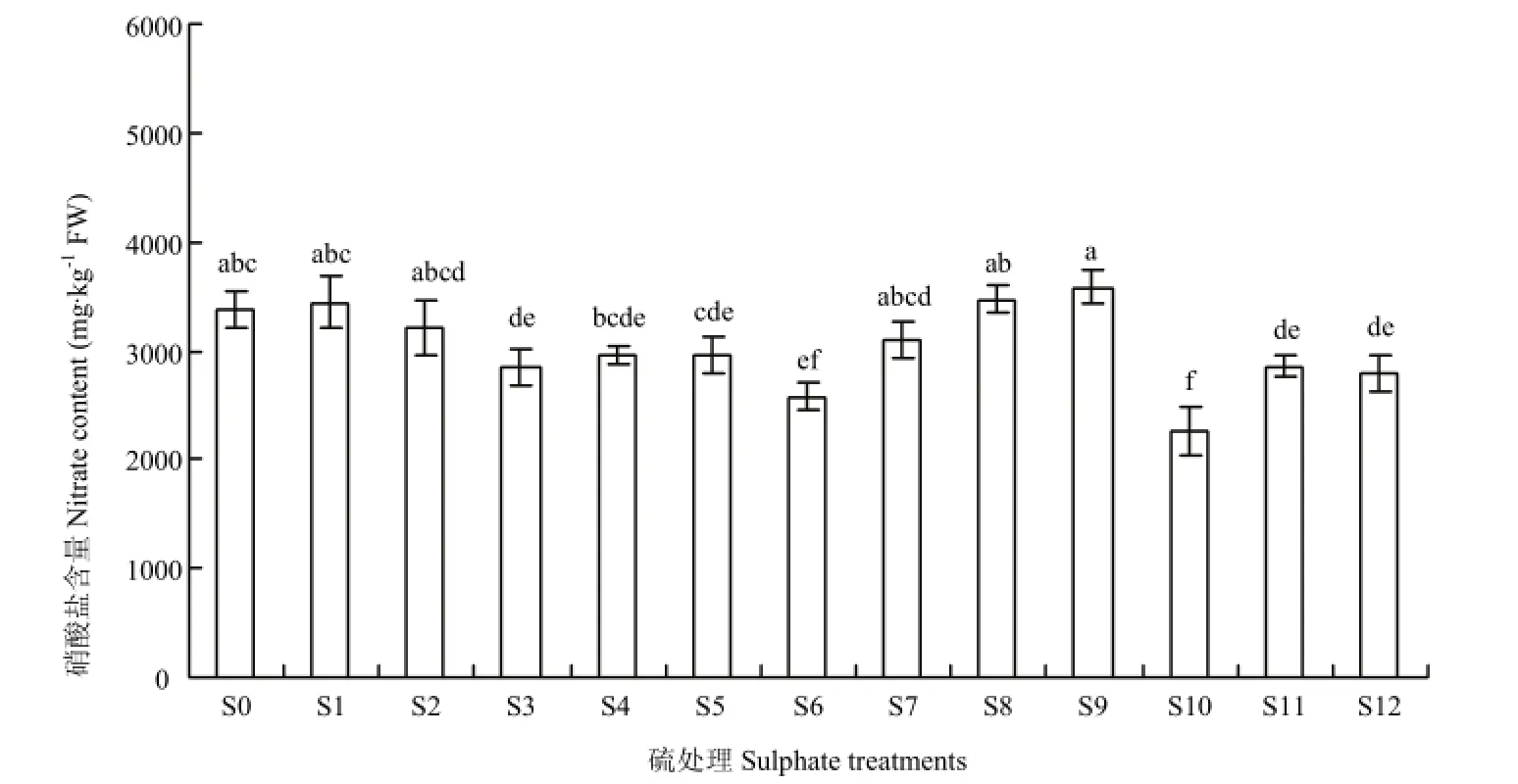
图2 硫处理对不结球白菜叶片硝酸盐含量的影响Fig. 2 Changes in nitrate content of non-heading Chinese cabbage leaves for sulphate treatments
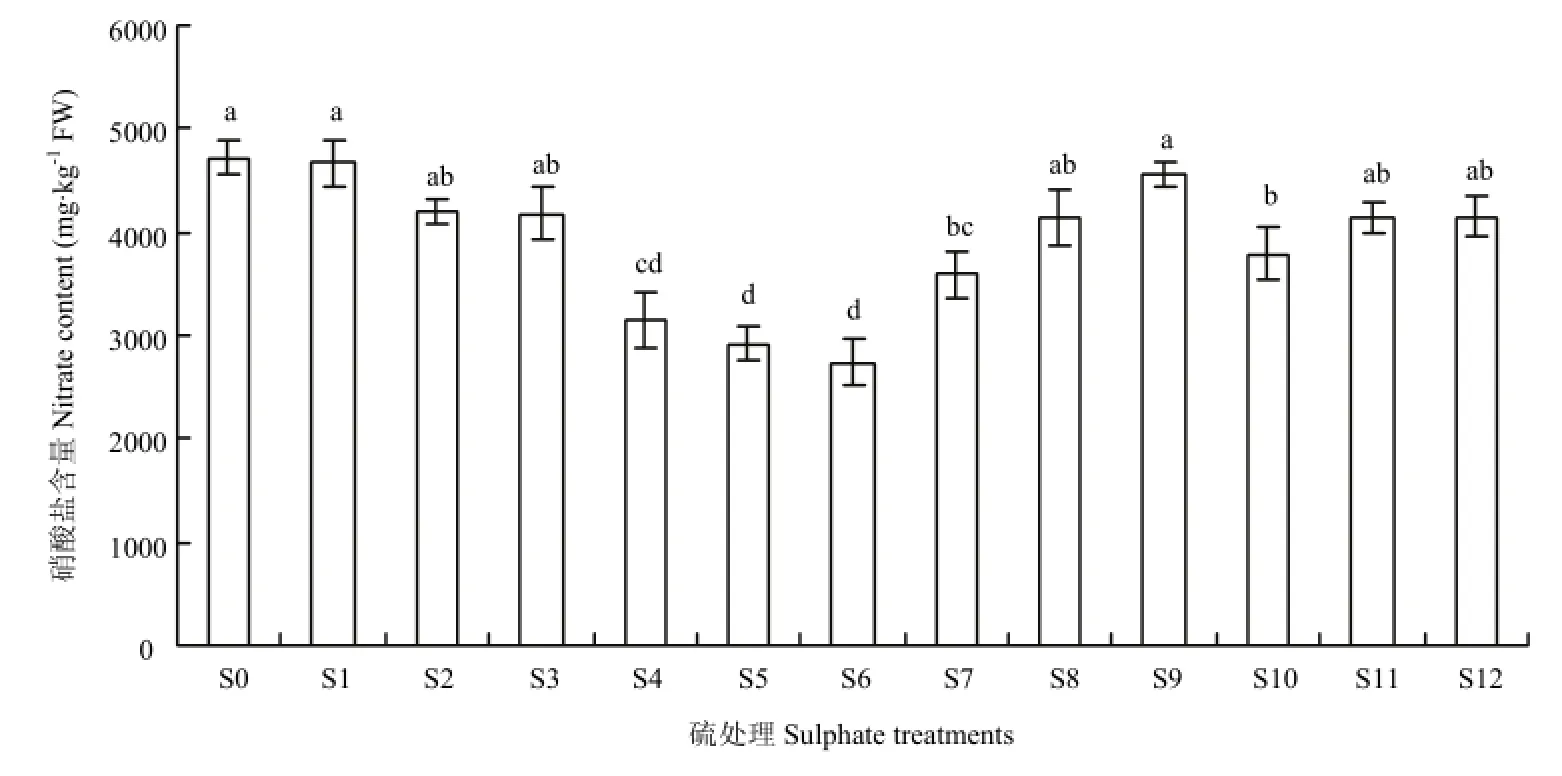
图3 硫处理对不结球白菜叶柄硝酸盐含量的影响Fig. 3 Changes in nitrate content of non-heading Chinese cabbage petioles for sulphate treatments
2.4 氮同化相关基因的表达
叶片及叶柄中硝酸盐代谢 GS/GOGAT途径及GDH途径中的基因表达随着硫处理均发生变化(图4)。叶片中NR-1、NADH-GOGAT-1、NADH-GOGAT-2、Cytoplasm-GS-4、Cytoplasm-GS-5、GDH-3的表达量表现为 S0弱于其他处理,硫处理均一定程度促进氮代谢。硫磺处理的S1—S3中,NADH-GOGAT-1的表达量均要弱于其他处理。各处理NADH-GOGAT-2表达量与叶片中硝酸盐含量变化呈现一定规律性,S10表达量最高,S6、S7、S11、S12表达量次之,而S0、S1、S9的表达量相对较低。Na2SO4处理的S4—S6中Cytoplasm-GS-1的表达量显著高于其他处理及对照。
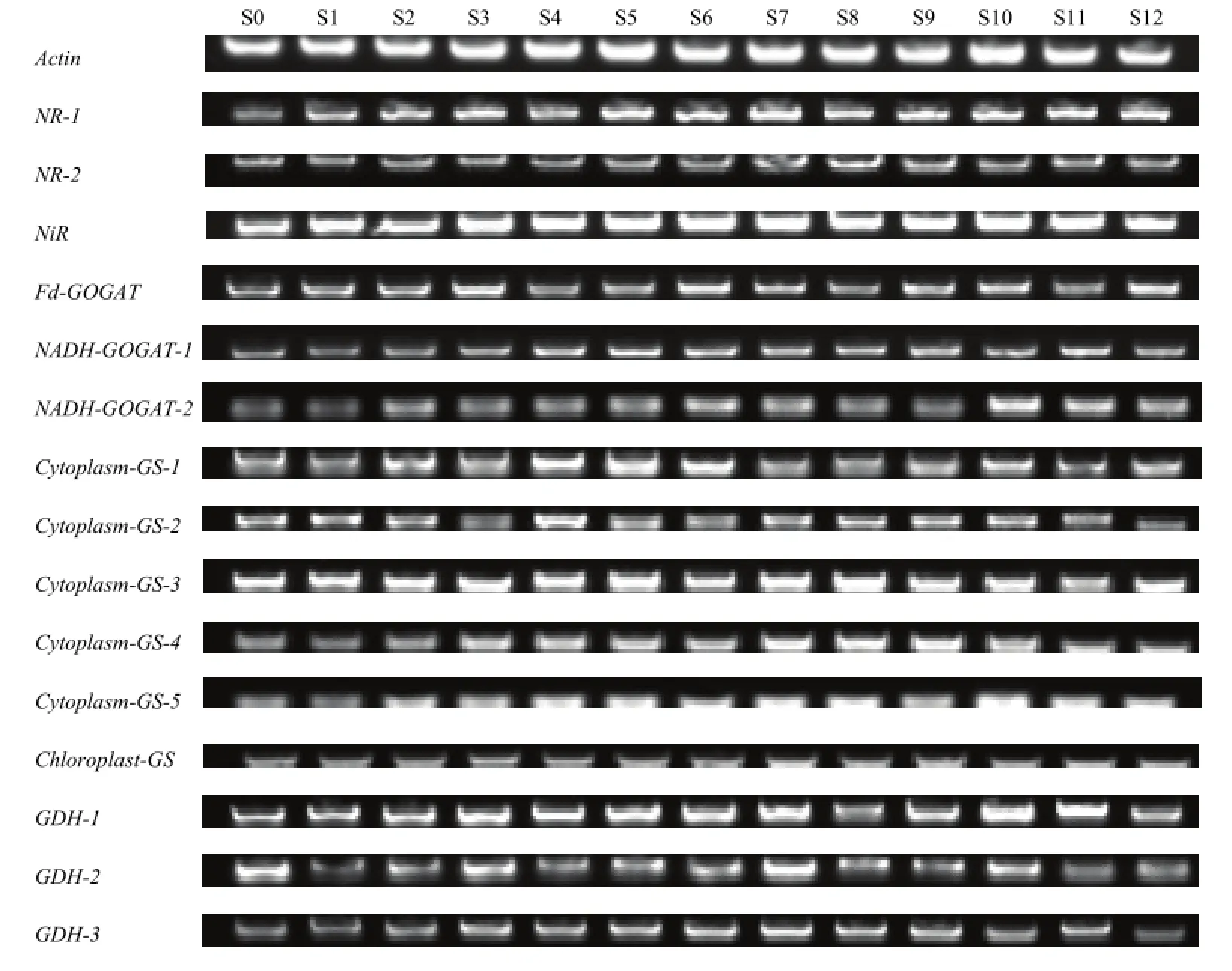
图4 硫处理对不结球白菜叶片中氮同化相关基因表达的影响Fig. 4 Sulphate affects the expression of nitrogen assimilation related genes in the leaves of non-heading Chinese cabbage
叶柄的硝酸盐代谢基因对硫处理的响应表现出一定规律,与叶片中的基因表达变化有所差异(图5)。NR-1、NADH-GOGAT-2、Cytoplasm-GS-1、GDH-2表达量 S0均弱于其他处理,硫处理均一定程度促进不结球白菜叶柄氮代谢。S6处理在较多基因中表达量均较高或最高,NR-1、NADH-GOGAT-1、NADH-GOGAT-2、Cytoplasm- GS-1、Cytoplasm-GS-2、Cytoplasm- GS-3、Cytoplasm- GS-4、Cytoplasm-GS-5、GDH-1、GDH-2、GDH-3在S6处理中的表达量较高,其中NR-1、NADH-GOGAT-2、Cytoplasm-GS-2、Cytoplasm- GS-5中 S6表达量为各处理中最高。NADH-GOGAT-2在Na2SO4处理的S4—S6中表达量最大,其次为Na2S2O3处理的S9—S12,与硫处理降低叶柄硝酸盐的含量的表现吻合。Cytoplasm-GS-2在NaHSO3处理的S7—S9中表达量弱于其他处理。
2.5 硫同化相关基因表达
硫处理对不结球白菜硫同化相关基因表达也表现出一定的规律性(图6)。在叶片中对照S0处理中很多基因的表达量较低,如ATPS-2、ATPS-3、ATPS-4、APSR-3、SIR、SAT1.1、SAT2.1,其中ATPS-3、ATPS-4、APSR-3、SAT2.1的表达量S0中为最低。另外,ATPS-1、ATPS-2、ATPS-3在硫磺处理的S1—S3中表达也弱于其他硫形态处理,且ATPS-3、SAT1.1的表达与硫磺施用量呈现正相关。Na2SO4处理的S4—S6中很多基因表现出规律性变化,ATPS-2、ATPS-3、SAT2.1、OASTL-C表达量与Na2SO4处施用量呈现正相关,而APSR-3、OASTL-A表达量与Na2SO4处施用量呈现负相关。
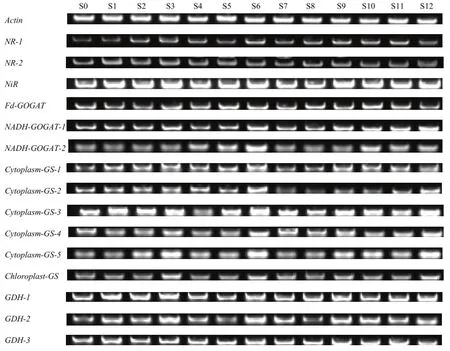
图5 硫处理对不结球白菜叶柄中氮同化相关基因表达的影响Fig. 5 Sulphate affects the expression of nitrogen assimilation related genes in the petioles of non-heading Chinese cabbage
在不结球白菜叶柄中,硫同化基因对处理的响应没有叶片明显,仅SIR、OASTL-A表达量在S0处理中明显低于其他处理(图7)。S6处理中大多数基因均表现出高表达量,包括 ATPS-1、ATPS-2、ATPS-3、ATPS-4、APSR-1、APSR-3、SIR、SAT1.1、SAT2.1、OASTL-B、OASTL-C,其中ATPS-1、SAT2.1、OASTL-C的表达量超过其他所有处理。SAT2.1、SAT2.2在Na2SO4处理的S4—S6中表达量均超过其他处理,且随着浓度增加,表达量增大。
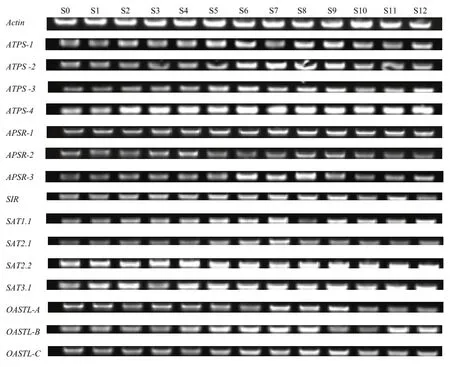
图7 硫处理对不结球白菜叶柄中硫同化相关基因表达的影响Fig. 7 Sulphate affects the expression of sulphate assimilation related genes in the petioles of non-heading Chinese cabbage
3 讨论
硫为植物生长的重要的中量元素,是生物合成半胱氨酸与蛋氨酸的主要原料[21],十字花科作物对于硫的需求尤其敏感,在生育进程中需要更为大量的硫,其风味物质形成更是与硫有着密切关系,糖苷油、芥子油均为硫脂化合物[12-13,22-23]。
植物从外界吸收硫元素,除少量从空气中吸收的SO2、H2S,大部分是从土壤中获取[24-25],其中,硫酸盐是主要的硫源,这在大量试验中得到证明[26-28],同样在本研究中,硫酸钠是最有利于不结球白菜生长及降低硝酸盐最为有效的硫素。总体而言,参试的硫素形态在降低硝酸盐累积效用上呈现 SO42->S2O32->HSO3->S的趋势(图 2、3)。其中,S2O32-、HSO3-对于植物作用差异有可能与其改变土壤 pH而影响植株吸收效率有关,S2O32-使土壤呈弱碱性,而 HSO3-则会酸化土壤。Na2S2O3对不结球白菜有显著的增产效应(图 1),同时在降低植株硝酸盐含量,尤其是叶片中硝酸盐含量有最佳的效果(图 2),但值得注意的是,小白菜对低浓度的 Na2S2O3较为敏感,但是随着Na2S2O3浓度增加,其无论在增产还是降低硝酸盐上的效果都有所降低,这可能也与其改变土壤 pH有关。
施硫能够显著提高作物的生物量,30 mg·kg-1的Na2SO4处理使不结球白菜显著增产49.76%(图1),大田试验证实相同浓度的Na2SO4确实能够有效增产,使不结球白菜增产21%(数据未公布),同样的结果在油菜、大麦等作物中得到应证[13,22,29]。硫对于促进植物叶片生长有显著作用,尤其是新叶的生长,主要原因被认为是其加速植株内源氮、硫的同化速率,同时,硫在作物光合作用、蛋白质合成、激素代谢以及抗重金属毒害方面也有明显作用[12-14]。
硫对于植株硝酸盐的改变有着显著作用,在本研究中,施硫最高降低不结球白菜叶片中硝酸含量33.08%(图2),降低叶柄中41.98%(图3),其中叶柄中降幅更大,其原因一方面是由于施硫对疏导组织的作用更为显著;另一方面,小白菜叶柄肥厚,为硝酸盐的贮藏器官,硝酸盐含量高于叶片中,最高可达到叶片中的两倍(图2、3),为硝酸盐的储藏器官,可见硫对硝酸盐储藏器官的影响大于其同化器官。与此同时,其他研究检测到硫对于植株地上部硝酸盐含量影响大于其地下部[10],并且通过15NO3-定位发现在缺硫植株中新吸收的硝酸盐含量也相应降低[30]。
目前,关于影响硝酸盐累积的研究很多,但是其分子机制尚不明晰,关键基因尚无定论。通过分析表观含量与其代谢基因相关性,以确定其关键基因的研究方法提供了新的思路[31]。硝酸还原酶是植株体内硝酸盐同化第一步的酶,催化硝酸盐转变为亚硝酸盐,在本研究中,NR表达量对施硫反应敏感,在不结球白菜的叶片及叶柄中均检测到施硫处理NR的表达量均明显高于CK,但是其对施硫的种类及浓度并不敏感,尤其是在叶片中(图4、5)。同样,其他试验也报道缺硫会引起NR活性降低及氨基酸累积[11,32-33]。
谷氨酰胺合成酶与谷氨酸合酶是 GS/GOGAT循环中重要的酶,在高等植物中,GS/GOGAT循环是正常条件下氮同化的主要途径[34-36],GDH途径则是起一种补充及缓解氨胁迫的作用[37]。在植物体内含有两种GS,分别为Cytoplasm-GS与Chloroplast-GS,在基因组中 Cytoplasm-GS是以基因群的形式存在,而Chloroplast-GS是以单一基因的形式[37-38]。研究显示,补充硫能使油菜中的GS活性增加,而减少硫则恰恰相反[11],GS可能是硫促进氮同化的关键基因。本试验中,叶片Cytoplasm-GS-1的表达量在Na2SO4处理的 S4—S6中显著高于其他处理及对照,这可能是因为Na2SO4处理降低硝酸盐比例较大(图4);同时,叶柄中Cytoplasm-GS-2在NaHSO3处理的S7—S9中表达量弱于其他处理(图5),这也可能是由于NaHSO3处理造成了硝酸盐的累积。
高等植物体内,根据电子载体的不同有两种GOGAT,分别为NADH-GOGAT与Fd-GOGAT。NADH-GOGAT大量存在植物根系中,参与初级氮同化以及分解氨基酸产生铵的再同化;Fd-GOGAT的主要作用是参与叶片中光呼吸分解铵离子的再同化[39]。NADH-GOGAT被认为是影响氮同化的重要基因,在抑制NADH-GOGAT表达的转基因水稻株系中,发现叶片中谷氨酸及其他氨基酸,与多数含氮化合物如叶绿素、吡啶核苷酸等有显著下降,地上部分的全氮含量也有所降低[40],相似的现象也出现在拟南芥突变体中[41]。本研究中,叶片及叶柄中Fd-GOGAT的表达量较高,但在施硫处理与不施硫处理间并无显著变化(图4、5);NADH-GOGAT-1仅CK叶片中表达量较低,但NADH-GOGAT-2在不结球白菜叶片及叶柄中均表现出规律变化(图4、5),叶片中NADH-GOGAT-2在硝酸盐含量最低的S10处理中表达量最高,硝酸盐含量较低的S6、S7、S11、S12中表达量次之,而硝酸盐累积的 S0、S1、S9的表达量相对较低;同样在叶柄中,NADH-GOGAT-2在Na2SO4处理的S4—S6中表达量最大,其次为Na2S2O3处理的S9—S12,与硫处理降低叶柄硝酸盐含量的表现吻合。由此可见,在不结球白菜中,NADH-GOGAT-2表达量与硝酸盐含量呈负相关,因此,推测NADH-GOGAT-2在硫素影响不结球白菜硝酸盐累积中发挥重要的作用,可能是影响氮同化的关键基因,其具体功能有待进一步验证。
硫处理会对植株硫代谢相关基因产生一定影响,ATPS是硫同化途经第一步的酶,miR395被认为是通过调控ATPS表达而影响硫同化[42]。在拟南芥中发现4个基因编码 ATPS(ATPS-1、ATPS-2、ATPS-3、ATPS-4),其中,通过数量性状遗传标记(quantitative trait locus,QTL)分析,得出ATPS-1是参与硫同化最主要的基因,拟南芥atps1突变体中ATPS-1表达也是最大贡献ATP酶活的同位基因,同样验证了以上结论[14]。在本研究中,叶片中的硫处理以 ATPS-2、ATPS-3、ATPS-4表达量显著高于对照,叶柄中却无显著变化(图6、7)。APSR在植物基因组中有3个同位基因(APSR-1、APSR-2、APSR-3),在生菜的研究中发现 APSR为调控植株硫醇含量的关键基因[10]。OASTL在高等植物中主要存在胞液中,半胱氨酸生物合成的氮前体修饰需要精准的氮、硫调控,OASLT被认为在这方面发挥重要作用[29]。本研究中,叶柄中硫同化基因响应不如叶片明显,且尚无足够证据发现氮硫调控关键基因。
4 结论
通过比较4种硫素形态对不结球白菜体内硝酸盐含量的影响,确定Na2SO4是降低不结球白菜硝酸盐效果较为显著的硫素,且能够显著提高其产量,30 mg·kg-1的Na2SO4是较优处理。NADH-GOGAT-2表达量与不结球白菜内硝酸盐含量呈负相关,推测NADHGOGAT-2在硫素影响不结球白菜硝酸盐累积中发挥重要的作用,可能是影响氮同化的关键基因。
References
[1] 谢国祥, 郭宝福, 赵士权, 王艳莉, 陈辉. 南京市市售蔬菜硝酸盐含量及居民暴露量评估. 现代预防医学, 2013, 40(7): 1236-1238. XIU G Q, GUO F B, ZHAO S Q, WANG Y L, CHEN H. The nitrate contents in commercial vegetables and assessment of nitrate exposure in Nanjing residents. Modern Preventive Medicine, 2013, 40(7):1236-1238. (in Chinese)
[2] 郭开秀, 姚春霞, 陈亦, 杨业凤, 陆利民. 上海市秋季蔬菜硝酸盐含量及风险摄入评估. 环境科学, 2011, 32(4): 1177-1181. GUO K X, YAO C X, CHEN Y, YANG Y F, LU L M. Nitrate contents in autumn vegetables and assessment of nitrate intake in Shanghai. Environmental Science, 2011, 32(4): 1177-1181. (in Chinese)
[3] KOPRIVOVA A, SUTER M, OPDEN C R, BRUNOLD C, KOPRIVA S. Regulation of sulfate assimilation by nitrogen in Arabidopsis. Plant Physiology, 2000, 122: 737-746.
[4] MARIO G, JOHN A R. Nitrogen and sulfur assimilation in plants and algae. Aquatic Botany, 2014, 118: 45-61.
[5] HOEFGEN R, NIKIFOROVA V. Metabolomics integrated with transcriptomics: assessing systems response to sulfur-deficiency stress. Plant Physiology, 2008, 132: 190-198.
[6] CARFAGNA S, VONA V, DI MARTINO V, ESPOSITO S, RIGANO C. Nitrogen assimilation and cysteine biosynthesis in barley: evidence for root sulphur assimilation upon recovery from N deprivation. Environmental and Experimental Botany, 2011, 71: 18-24.
[7] DONNA M K, JOSHUA N, NANCY L E, TIMOTHY J T, DAVID E G. Nitrogen and sulfur requirements for Clostridium thermocellum and Caldicellulosiruptor bescii on cellulosic substrates in minimal nutrient media. Bioresource Technology, 2013, 130: 125-135.
[8] HESSE H, NIKIFOROVA V, GAKIERE B, HOEFGE R. Molecular analysis and control of cysteine biosynthesis, integration of nitrogen and sulphur metabolism. Journal of Experimental Botany, 2004, 55:1283-1292.
[9] IMAMURA S, TERASHITA M, OHNUMA M, MARUYAMA S,MINODA A, WEBER A P M, INOUYE T, SEKINE Y, FUJITA Y,OMATA T, TANAKA K. Nitrate assimilatory genes and their transcriptional regulation in a unicellular red alga Cyanidioschyzon merolae: genetic evidence for nitrite reduction by a sulfite reductaselike enzyme. Plant Cell Physiology, 2010, 51(5): 707-717.
[10] ALEKSANDRA K, PETER B, ELISABETH E S, FREEK S P,STANISLAV K, MALCOLM J H, LUIT J D K. Expression and activity of sulfate transporters and APS reductase in curly kale in response to sulfate deprivation and re-supply. Journal of Plant Physiology, 2009, 166: 168-179.
[11] ZHANG Q, BOK-RYE L, SANG-HYUN P, RASHED Z,JEAN-CHRISTOPHE A, ALAIN O, TAE-HWAN K. Sulfate resupply accentuates protein synthesis in coordination with nitrogen metabolism in sulfur deprived Brassica napus. Plant Physiology and Biochemistry, 2015, 87: 1-8.
[12] Muhammad S, Mei H T, Elisabeth E S, Aleksandra K, Freek S,Posthumus, Jan H V, Saroj P, Henk S, Malcolm J H, Luit J D K. Copper exposure interferes with the regulation of the uptake,distribution and metabolism of sulfate in Chinese cabbage. Journal of Plant Physiology, 2010, 167: 438-446.
[13] ABDALLAHA M, ETIENNE P, OURRY A, MEURIOT F. Do initial S reserves and mineral S availability alter leaf S-N mobilization and leaf senescence in oilseed rape? Plant Science, 2011, 180: 511-520.
[14] ANNE H, MIKIKO K, RICHARD H, WOLFGANG F, HITOSHI S,CORNELIA H, HEINZ R. Sulphur limitation and early sulphur deficiency responses in poplar: significance of gene expression,metabolites, and plant hormones. Journal of Experimental Botany,2012, 63(5): 1873-1893.
[15] 李晓峰, 王俊玲, 李林妍, 谢鑫, 高志奎. 硫磺与水杨酸配施对韭菜硝酸盐累积及氮代谢的影响. 植物营养与肥料学报, 2013,19(5): 1264 -1271. LI X F, WANG J L, LI L Y, XIE X, GAO Z K. Effects of sulfur and salicylic acid on nitrate accumulation and nitrogen metabolism in leaves of Chinese chive. Journal of Plant Nutrition and Fertilizer,2013, 19(5): 1264-1271. (in Chinese)
[16] 孔灵君, 徐坤, 张永征, 何平. 硫对大葱生长及氮硫同化关键酶活性的影响. 园艺学报, 2013, 40(12): 2505-2512. KONG L J, XU K, ZHANG Y Z, HE P. Effects of sulfur on growth and key enzyme activities involved in nitrogen and sulfur assimilation in Chinese spring onion. Acta Horticulturae Sinica, 2013, 40(12):2505-2512. (in Chinese)
[17] 霍捷, 王俊玲, 薛占军, 王梅, 高志奎. 亚硫酸氢钠对白菜叶片硝酸盐还原及光合能力的影响. 园艺学报, 2012, 39(4): 669-676. HUO J, WANG J L, XUE Z J, WANG M, GAO Z K. Effects of sodium bisulfite on nitrate reduction and photosynthetic capacity in the leaves of non-heading Chinese cabbage. Acta Horticulturae Sinica,2012, 39(4): 669-676. (in Chinese)
[18] 付雪清, 王俊玲, 高志奎. NaHSO3和Na2SO4配施对小白菜叶片硝酸盐含量及营养品质的影响. 河北农业大学学报, 2013, 36(6):43-47. FU X Q, WANG J L, GAO Z K. Effects of NaHSO3and Na2SO4combination on the nitrate and nutritional quality non-heading Chinese cabbage. Journal of Agricultural University of Hebei, 2013,36(6): 43-47. (in Chinese)
[19] 李合生. 植物生理生化实验原理和技术. 北京: 高等教育出版社,2000, 123-137. LI H S. Theory and Technology of Plant Physiology and Biochemistry Experiments. Beijing: Higher Education Press, 2000: 123-137. (in Chinese)
[20] REN J, CHEN Z W, DUAN W K, SONG X M, ZHOU J, LIU T K,WANG J J, HOU X L, LI Y. Comparison of ascorbic acid biosynthesis in different tissues of three non-heading Chinese cabbage cultivars. Plant Physiology and Biochemistry, 2013, 73: 229-236.
[21] TAKAHASHI H, KOPRIVA S, GIORDANO M, SAITO K, HELL R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annual Review of Plant Biology, 2011, 62: 157-184.
[22] DUBOUSSET L, ABDALLAH M, DESFEUX A S, ETIENNE P,MEURIOT F, HAWKESFORD M J, GOMBERT J, SEGURA R,BATAILLE M P, REZE S, BONNEFOY J, AMELINE1 A F, OURRY A, DILY F L, AVICE J C. Remobilization of leaf S compounds and senescence in response to restricted sulphate supply during the vegetative stage of oilseed rape are affected by mineral N availability. Journal of Experimental Botany, 2009, 60(11): 3239-3253.
[23] RUSLAN Y, SARAH G M, COLETTE M, TAMARA G, HENNING F, SEAN D, ANNA K, ULF-INGO F STANISLAV K. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. The Plant Journal, 2010, 62: 1-11.
[24] RIEMENSCHNEIDER A, NIKIFOROVA V, HOEFGEN R, DE K,KOK L J D, PAPENBROCK J. Impact of elevated H2S onmetabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiology and Biochemistry, 2005, 43:473-483.
[25] DAVIDIAN J C, KOPRIVA S. Regulation of sulfate uptake and assimilation—the same or not the same? Molecular Plant, 2010, 3(2):314-325.
[26] GIORDANO M, NORICI A, HELL R. Sulfur and phytoplankton:acquisition, metabolism and impact on the environment. New Phytologist, 2005, 166(2): 371-382.
[27] ABDALLAH M, DUBOUSSET L, MEURIOT F, ETIENNE P,AVICE J C, OURRY A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L.. Journal of Experimental Botany, 2010,61(10): 2635-2646.
[28] ZHANG B, PASINI R, HANBIN D, NAVEEN J, ZHAO Y H,THOMA, ZHENG Z. Aberrant gene expression in the Arabidopsis SULTR1;2 mutants suggests a possible regulatory role for this sulfate transporter in response to sulfur nutrient status. The Plant Journal,2014, 77: 185-197.
[29] SIMONA C, VINCENZA V, VITTORIA D M, SERGIO E,CARMELO R. Nitrogen assimilation and cysteine biosynthesis in barley: Evidence for root sulphur assimilation upon recovery from N deprivation. Environmental and Experimental Botany, 2011, 71:18-24.
[30] LEE B R, MUNEER S, KIM K Y, AVICE J C, OURRY A, KIM T H. S-deciency responsive accumulation of amino acids is mainly due to hydrolysis of the previously synthesized proteins not to de novo synthesis in Brassica napus. Physiologia Plantarum, 2013, 147:369-380.
[31] XU Y, ZHU X, CHEN Y, GONG Y Q, LIU L. Expression profiling of genes involved in ascorbate biosynthesis and recycling during fleshy root development in radish. Plant Physiology and Biochemistry, 2013,70: 269-277.
[32] PROSSER I M, PURVES J V, SAKER L R, CLARKSON D T. Rapid disruption of nitrogen metabolism and nitrate transport in spinach plants deprived of sulphate. Journal of Experimental Botany, 2001, 52:113-121.
[33] NIKIFOROVA V J, BIELECKA M, GAKIERE B, KRUEGER S,RINDER J, KEMPA S R, MORCUENDE R, SCHEIBLE W R,HESSE H, HOEFGEN R. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids, 2006, 30: 173-183.
[34] LEA P J, MIFLIN B J. Alternative route for nitrogen assimilation in higher plants. Nature, 1974, 251: 614-616.
[35] SUÁREZ M F, AVILA C, GALLARDO F, CANTÓN F R, GARCÍAGUTIÉRREZ A, CLAROS M G, CÁNOVAS F M. Molecular and enzymatic analysis of ammonium assimilation in woody plants.Journal of Experimental Botany, 2002, 53: 891-904.
[36] TEIXEIRA J, FIDALGO F. Salt stress affects glutamine synthetase activity and mRNA accumulation on potato plants in an organdependent manner. Plant Physiology and Biochemistry, 2009, 47:807-813.
[37] CASTRO-RODRÍGUEZ V, GARCÍA-GUTIÉRREZ A, CANALES J,AVILA C, KIRBY E G, CÁNOVAS F M. The glutamine synthetase gene family in Populus. BMC Plant Biology, 2011, 11: 119-134.
[38] BERNARD S M, HABASH D Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytologist, 2009, 182: 608-620.
[39] LAM H M, COSCHIGANO K T, OLIVEIRA I C, MELO-OLIVEIRA R, CORUZZI G. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annual Review of Plant Biology, 1996, 47: 569-593.
[40] LU Y E, LUO F, YANG M, LI X H, LIAN X M. Suppression of glutamate synthase genes significantly affects carbon and nitrogen metabolism in rice (Oryza sativa L.). Sciences China Life Sciences,2011, 54(7): 651-663.
[41] LANCIEN M, MARTIN M, HSIEH M H, LEUSTEK T, GOODMAN H, CORUZZI G M. Arabidopsis glt1-T mutant defines a role for NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. The Plant Journal, 2002, 29: 347-358.
[42] LIANG G, YANG F, YU D. MicroRNA395 mediates regulation of sulphate accumulation and allocation in Arabidopsis thaliana. The Plant Journal, 2010, 62, 1046-1057.
(责任编辑 赵伶俐)
Effect of Sulphur Availability on Nitrate Accumulation and Expression of Nitrogen and Sulphur Assimilation Related Genes in Non-Heading Chinese Cabbage
XU Yao, MU Jian-mei, ZHANG Guo-qin, MA Jia-jia, XU Jun, LI Jun, LIU Feng-jun, SHE Xu-dong
(Institute of Agricultural Sciences in Taihu Lake District, Suzhou 215155, Jiangsu)
Abstract:【Objective】The objective of this paper is to identify the best sulfate to reduce nitrate accumulation in non-heading Chinese cabbage, and analyze the key genes involved in nitrogen and sulphur assimilation. The results of this study will provide new insights into sulphur fertilization and investigation of the molecular mechanisms of nitrate accumulation with the goal of molecular breeding.【Method】Nitrate content of non-heading Chinese cabbage leaves and petioles were measured under treatments of 4 forms of sulphur with 3 concentrations. The expression of 30 nitrogen and sulphur assimilation related genes were analyzed by semi-quantitative RT-PCR. 【Result】Sulphur treatments were significantly increased the aboveground biomass of non-heading Chinese cabbage, and the treatments of Na2SO4had the best effect, and 30 mg·kg-1Na2SO4was the most effective treatment as the aboveground biomass increased by 49.76% compared with the control. Na2SO4and Na2S2O3reduced nitrate content of non-headingChinese cabbage more significantly than others. Na2SO4decreased nitrate content by 12.23%-23.55% in leaves and by 33.08%-41.98% in petioles compared with the control, and it was also found a positive correlation between the reduction and concentration of Na2SO4, and 30 mg·kg-1Na2SO4also had the best effect. Na2S2O3decreased nitrate content by 15.34%-33.08% compared with the control in leaves and by 11.95%-19.68% in petioles. Sulphur promoted nitrogen assimilation, and the expression of NR-1, NADH-GOGAT-1, NADH-GOGAT-2, Cytoplasm-GS-4, Cytoplasm-GS-5, and GDH-3 were higher than the control in leaves,and the expression of NR-1, NADH-GOGAT-2, Cytoplasm-GS-1, and GDH-2 were higher than the control in petioles. The expression of NADH-GOGAT-2 was correlated with nitrate levels. Sulphur treatments also had an effect on sulphur assimilation genes, and the expressions of ATPS-2, ATPS-3, ATPS-4, APSR-3, SIR, SAT1.1, and SAT2.1 were higher than the control in leaves, and only the expressions of SIR and OASTL-A were higher than the control in petioles. 【Conclusion】 Na2SO4had the best effect in reducing nitrate content and increasing yield of non-heading Chinese cabbage, and 30 mg·kg-1Na2SO4was the most effective treatment. The expression of NADH-GOGAT-2 was correlated with nitrate levels. The data suggested that NADH-GOGAT-2 may be the key gene in nitrogen assimilation.
Key words:non-heading Chinese cabbage; sulphur; nitrate; nitrogen and sulphur assimilation; gene expression
收稿日期:2015-11-16;接受日期:2016-03-29
基金项目:苏州市科技计划项目(SYN201421)

