肿瘤错配修复基因PMS2及活化Akt1在不同卵巢癌细胞株中的表达及相关性
贾静辉,李春东,陈 冰,李景轩,童 英
(中国人民解放军空军总医院妇产科,北京 100142)
肿瘤错配修复基因PMS2及活化Akt1在不同卵巢癌细胞株中的表达及相关性
贾静辉,李春东,陈冰,李景轩,童英△
(中国人民解放军空军总医院妇产科,北京 100142)
[摘要]目的检测Akt1、P-Akt S473和PMS2蛋白在人类不同卵巢癌细胞株(A2780、Caov3、C13*和ES2)中的表达水平及相关性。方法应用Western blot检测Akt1、P-Akt S473和PMS2蛋白分别于A2780、Caov3、C13*和ES2细胞中表达水平;应用Akt1激动剂胰岛素样生长因子-1(IGF-1)及Akt1特异性抑制剂API-2调节Akt1活化水平,观察PMS2蛋白表达水平变化。结果Akt1,P-Akt S473及PMS2 3种蛋白在A2780,Caov3,C13*和ES2卵巢癌细胞内均表达,但表达水平高低不一,即Akt1的活化形式P-Akt S473与PMS2蛋白表达呈负相关;应用IGF-1上调Akt1的活性后,ES2和Caov3中PMS2表达水平明显下降,且与IGF-1的作用存在时间依赖性;应用特异性抑制剂API-2抑制Akt1的活性后,A2780中PMS2表达水平明显升高,且与API-2的作用存在时间依赖性。结论人类卵巢癌细胞中PMS2蛋白的表达水平可能受活化Akt1的直接调控。
[关键词]卵巢肿瘤;蛋白激酶类;胰岛素样生长因子1
Akt1又称蛋白激酶B(protein kinase B,PKB),是一种丝/苏氨酸蛋白激酶,它是细胞生存信号通路PI3K/Akt的枢纽分子,Akt1活化后可通过磷酸化作用,活化其下游生长因子及其旁路分子,从而抑制细胞凋亡、促进细胞周期、促使细胞侵袭和转移,促进血管生成等生物学效应。Akt1存在两个磷酸化位点Thr308和Ser473,只有当Ser473活化后Akt1才能完全活化,Akt1通过磷酸化下游靶分子中保守序列RXRXXS/TB(X:任意氨基酸;R:精氨酸;B:疏水氨基酸)发挥其生物学效应[1]。
PMS2是MMRs蛋白家族中的重要一员,它在保持基因组稳定性中起至关重要的作用。PMS2基因突变或表达缺失与HNPCC及15%的实体肿瘤发生、发展密切相关[2]。然而有关PMS2与肿瘤相关性报道不尽相同,有研究证实:前列腺癌中PMS2表达异常增高[3]。因此,研究PMS2蛋白的表达调控及稳定性,可能成为新的肿瘤防治靶点。应用DNAmanda软件分析发现:PMS2蛋白中存在Akt1识别及磷酸化的共有基因序列YPRPRGT156TVSV。因此推测Akt1可能通过活化共有序列中T156位点调控PMS2蛋白的表达及其稳定性。
本研究通过Western blot技术检测不同人类卵巢癌细胞株中Akt1、P-Akt S473和PMS2蛋白的表达水平;应用Akt1激动剂和Akt1特异的抑制剂调节其活化水平,检测PMS2的蛋白表达变化情况。初步探讨PMS2蛋白与活化Akt1的关系,为进一步研究活化的Akt1与PMS2的作用机制奠定基础。
1材料与方法
1.1细胞培养将A2780、Caov3,C13*和ES2卵巢癌细胞株于10 %胎牛血清RPMI-1640中培养,每2天传代1次,选择生长状态好的细胞进行试验。
1.2Western blot检测PMS2、Akt1和P-Akt S473的表达各组细胞中加RIPA,超声裂解,BCA法检测蛋白浓度,SDS-PAGE电泳分离蛋白,转膜于PVDF膜,室温封闭1 h,加入Akt1抗体(CST)、P-Akt S473抗体(CST)、PMS2抗体(Epitomics)、GAPDH抗体(CST),稀释度均为1∶1 000,4 ℃过夜,加二抗(稀释度1∶5 000)室温孵育1 h,曝光,利用Quantity-one软件进行分析,本试验重复3次。
1.3Akt1活性上调选取已验证低Akt1活化状态的ES2及Caov3卵巢癌细胞,于无血清培养基中饥饿过夜;应用Akt1活化剂胰岛素样生长因子-1(IGF-1,100 ng/mL)刺激细胞[4-6],观察Akt1的活化是否与IGF-1作用存在时间依赖性。
1.4Akt1活性下调选取已验证高Akt1活化的A2780卵巢癌细胞,于无血清培养基中饥饿过夜;应用Akt1特异抑制剂API-2 4 μM抑制其活性,观察Akt1活性抑制是否与API-2存在时间依赖性。
2结果
2.1Western blot检测不同卵巢癌细胞株中Akt1、P-Akt S473和PMS2的表达情况P-Akt S473在卵巢癌细胞株ES2中表达缺失(图1)。PMS2、Akt1和P-Akt S473蛋白在A2780、Caov3及C13*细胞株中均表达,且表达水平不一。如图1示,C13*、Caov3、ES2细胞株中P-Akt S473表达处于低水平,与此同时PMS2蛋白高水平表达;A2780细胞株中,P-Akt S473蛋白表达明显增高,但伴随PMS2蛋白的低表达。因相关报道证实,Hela宫颈癌细胞株中既无MMR蛋白表达缺失[7],又无Akt1蛋白异常活化[8],故本试验中将其作为阳性对照。综上所述,在不同的卵巢癌细胞株中,P-Akt S473蛋白高表达对应PMS2蛋白低表达,Akt1低活化水平对应PMS2蛋白高表达。卵巢癌细胞株中Akt1的活化形式P-Akt S473蛋白与PMS2蛋白表达水平呈负相关。
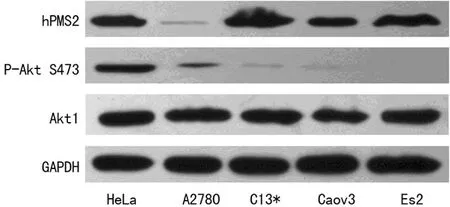
GAPDH:内参照,Hela:阳性对照。
图1Western Blot检测卵巢癌细胞中PMS2,Akt1和P-Akt S473蛋白的表达水平
2.2Western Blot 检测不同卵巢癌细胞中Akt1活化与PMS2蛋白表达的关系
2.2.1激活Akt1后卵巢癌细胞中PMS2蛋白表达水平为进一步检验Akt1的活性是否与其下游PMS2蛋白表达相关,应用Akt1激动剂IGF-1上调Akt1蛋白激酶活性,检测卵巢癌细胞株中PMS2蛋白表达情况。试验结果表明:应用100 ng/mL IGF-1作用于卵巢癌细胞ES2和Caov3后活化Akt1蛋白的表达水平明显增高,且与IGF-1作用呈时间依赖性;活化的Akt1表达上调卵巢癌细胞株中PMS2的蛋白表达水平明显下降,且亦存在时间依赖性,结果见图2。
2.2.2抑制Akt1活性后卵巢癌细胞中PMS2蛋白表达水平为进一步检测抑制卵巢癌细胞中Akt1活性后PMS2蛋白表达变化情况,应用Akt1特异抑制剂API-2作用于卵巢癌细胞A2780不同时间,如图3示:API-2可使卵巢癌细胞A2780中P-Akt S473的表达明显下调,且存在时间依赖性;在卵巢癌细胞A2780中API-2作用48 h对Akt1活性抑制最明显,PMS2蛋白表达达峰值。
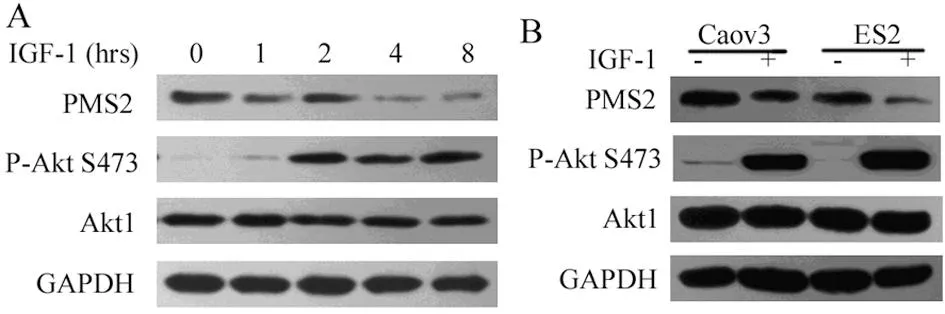
A:在PMS2高表达的ES2细胞株中,IGF-1上调P-AktS473表达水平,且呈时间依赖性;Akt1活化后PMS2蛋白的表达明显下降,亦存在时间依赖性。B:Caov3与ES2 细胞中IGF-1上调Akt1活性后PMS2蛋白表达水平明显下调。
图2不同卵巢癌细胞株中活化Akt1后PMS2的蛋白表达水平
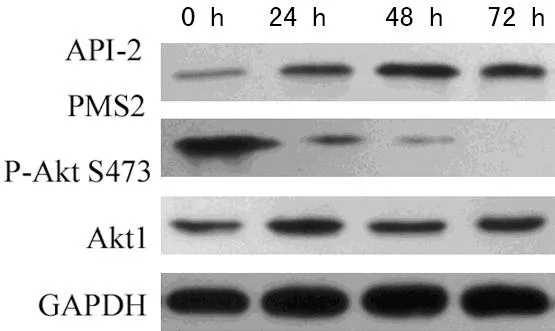
图3 Western Blot 检测卵巢癌细胞中PMS2的表达
3讨论
肿瘤的发生、发展有明显的遗传倾向及相关性。DNA错配修复即MMR系统,它是生物进化过程中演化出一种高度保守的错配修复系统。MMR不仅能够识别和修复DNA重组和复制过程中的单个碱基错配,还可矫正小片段DNA聚合酶滑链的插入和缺失(IDLs),以保持整个基因组的稳定性及高度保真性;MMR亦可通过诱导细胞凋亡抑制细胞癌变[9]。人类对MMR的认识源于对Ecoli的研究[10],且MMR的缺失与人类多种肿瘤的发生密切相关,如:宫颈癌、乳腺癌、儿童肿瘤综合征、Turcot综合征[11-14]等。
在对散发性结直肠癌的研究中首次发现人类肿瘤错配修复基因,目前MMR家族公认有MutS同源物hMSH2~hMSH6及MutL的同源物hMLH1、hMLH3、hPMS1和hPMS2。只有当错配识别复合物MutSα(hMSH2-hMSH6)和(或)MutSβ(hMSH2-hMSH3)与DNA序列上错配位点结合后,使得ATP依赖水解酶构象发生改变,复合物MutLα(MLH1-PMS2)与MutSα或MutSβ结合,才能激活MMR系统,从而发挥其基因错配修复功能[15-16]。以往经典研究发现,MMRs绝大部分突变发生在MLH1、MSH2和MSH6,发生于PMS1和PMS2少见。随着研究深入,PMS2在人类肿瘤中的作用日益受关注,与其他错配修复蛋白不同,PMS2具有高度不稳定性,在人类多种肿瘤中表达缺失或功能缺失[17]。本试验发现,在卵巢癌细胞株A2780、Caov3、ES2和C13*中PMS2均有表达,但在A2780中PMS2表达水平极低,伴随高活化水平的Akt1,即PMS2的表达水平与活化的Akt1呈负相关。
Akt1是细胞生存通路PI3K/Akt的枢纽分子,广泛存在于哺乳动物各组织中,参与细胞的生长、增殖及凋亡[18-19]。通常情况下,Akt1处于非激活状态,只有它被上游激酶磷酸化完全活化后,磷酸化下游靶分子中保守序列,从而发挥其生物学效应。Akt1异常活化与多种恶性肿瘤的发生、发展和转归关系密切。在人类卵巢癌、前列腺癌、胰腺癌、骨髓瘤、直肠癌及肾细胞癌中Akt1异常活化;应用抑制剂抑制Akt1磷酸化水平后,明显抑制以上肿瘤的生长[20-23]。
大量研究证实,已知Akt1下游靶分子,如GSK3、FKHRL1、BAD及BRCA1等,均含有其磷酸化的保守序列RXRXXS/TB[4,24]。本研究前期实验发现,PMS2蛋白中存在YPRPRGT156TVSV共有基序,这一结果提示蛋白PMS2可能被活化的Akt1磷酸化,影响稳定性,进而影响其生物学功能。本研究发现,不同卵巢癌细胞株中蛋白PMS2及活化Akt1表达水平呈负相关,提示PMS2蛋白的表达水平可能受活化Akt1的直接调控,PMS2可能为PI3K/Akt信号通路下游新的靶分子,为人类卵巢癌的治疗寻找新靶点奠定基础。
参考文献
[1]Alessi DR,Caudwell FB,Andjelkovic M,et al.Molecular basis for the substrate specificity of protein kinase B;comparison with MAPKAP kinase-1 and p70 S6 kinase[J].Febs Lett,1996,399(3):333-338.
[2]Fink D,Aebi S,Howell SB.The role of DNA mismatch repair in drug resistance[J].Clin Cancer Res,1998,4(1):1-6.
[3]Norris RD,Woodruff RB,D′Agostino RB,et al.Elevated levels of the mismatch repair protein PMS2 are associated with prostate cancer[J].Prostate,2007,67(2):214-225.
[4]Datta SR,Brunet A,Greenberg ME.Cellular survival:a play in three Akts[J].Genes Dev,1999,13(22):2905-2927.
[5]Yang L,Dan HC,Sun M,et al.Akt/protein kinase B signaling inhibitor-2,a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt[J].Cancer Res,2004,64(13):4394-4399.
[6]Hu Y,Qiao L,Wang S,et al.3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K,Akt,and cancer cell growth[J].J Med Chem,2000,43(16):3045-3051.
[7]Boyer JC,Umar A,Risinger JI,et al.Microsatellite instability,mismatch repair deficiency,and genetic defects in human cancer cell lines[J].Cancer Res,1995,55(24):6063-6070.
[8]Su CH,Wang CY,Lan KH,et al.Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase[J].Cell Signal,2011,23(11):1824-1830.
[9]Fishel R.The selection for mismatch repair defects in hereditary nonpolyposis colorectal cancer:revising the mutator hypothesis[J].Cancer Res,2001,61(20):7369-7374.
[10]Modrich P.Mismatch repair,genetic stability,and cancer[J].Science,1994,266(5193):1959-1960.
[11]Liu B,Parsons R,Papadopoulos N,et al.Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients[J].Nat Med,1996,2(2):169-174.
[12]Tan TY,Orme LM,Lynch E,et al.Biallelic PMS2 mutations and a distinctive childhood cancer syndrome[J].J Pediatr Hematol Oncol,2008,30(3):254-257.
[13]Miyaki M,Nishio J,Konishi M,et al.Drastic genetic instability of tumors and normal tissues in Turcot syndrome[J].Oncogene,1997,15(23):2877-2881.
[14]De Rosa M,Fasano C,Panariello L,et al.Evidence for a recessive inheritance of Turcot′s syndrome caused by compound heterozygous mutations within the PMS2 gene[J].Oncogene,2000,19(13):1719-1723.
[15]Modrich P,Lahue R.Mismatch repair in replication fidelity,genetic recombination,and cancer biology[J].Annu Rev Biochem,1996(65):101-133.
[16]Stojic L,Brun R,Jiricny J.Mismatch repair and DNA damage signalling[J].DNA Repair (Amst),2004,3(8/9):1091-1101.
[17]Chang DK,Ricciardiello L,Goel A,et al.Steady-state regulation of the human DNA mismatch repair system[J].J Biol Chem,2000,275(24):18424-18431.
[18]Chen WS,Xu PZ,Gottlob K,et al.Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene[J].Genes Dev,2001,15(17):2203-2208.
[19]Cho H,Thorvaldsen JL,Chu Q,et al.Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice[J].J Biol Chem,2001,276(42):38349-38352.
[20]Numahata K,Satoh M,Handa K,et al.Sialosyl-Le(x) expression defines invasive and metastatic properties of bladder carcinoma[J].Cancer,2002,94(3):673-685.
[21]Marsh Rde W,Rocha Lima CM,Levy DE,et al.A phase Ⅱ trial of perifosine in locally advanced,unresectable,or metastatic pancreatic adenocarcinoma[J].Am J Clin Oncol,2007,30(1):26-31.
[22]Cirstea D,Hideshima T,Rodig S,et al.Dual inhibition of akt/mammalian target of rapamycin pathway by nanoparticle albumin-bound-rapamycin and perifosine induces antitumor activity in multiple myeloma[J].Mol Cancer Ther,2010,9(4):963-975.
[23]Floryk D,Thompson TC.Perifosine induces differentiation and cell death in prostate cancer cells[J].Cancer Lett,2008,266(2):216-226.
[24]Pearce LR,Komander D,Alessi DR.The nuts and bolts of AGC protein kinases[J].Nat Rev Mol Cell Biol,2010,11(1):9-22.
The expression and the relativity of PMS2 and P-Akt S473 in different human ovarian cancer cell lines
Jia Jinghui,Li Chundong,Chen Bing,Li Jingxuan,Tong Ying△
(1.Department of Obstetrics and Gynecology,Air Force General Hospital of PLA,Beijing 100142,China)
[Abstract]ObjectiveTo evaluate the expression and the relativity of PMS2,Akt1 and P-Akt S473 protein in A2780,Caov3,C13* and ES2 ovarian cancer cell lines.MethodsThe expression of PMS2,Akt1 and P-Akt S473 protein in A2780,Caov3,C13* and ES2 ovarian cancer cells was detected by Western Blot.After treated with IGF-1 (Akt1 activator) and API-2 (specific Akt1 inhibitor),Caov3,ES2 and A2780 cells were collected and the level of PMS2 was detected by Western Blot.ResultsPMS2,Akt1 and P-Akt S473 proteins were detected in all of the four ovarian cancer cell lines with varied expression levels,and the activity of Akt1 was inversely related to PMS2 expression in ovarian cancer cells.Exposed to Akt kinase stimulator IGF-1,ES2 and Caov3 cells were detected with a dramatically PMS2 decreasing.Meanwhile,the decreasing of PMS2 protein was time-dependent on IGF-1.Treated with API-2,Akt kinase specific inhibitor,A2780 was detected with PMS2 dramatically increasing,and the increasing of PMS2 protein was time-dependent on API-2.ConclusionIn ovarian cancer cells,PMS2 expression could be directly regulated by activated Akt1.
[Key words]ovarian neoplasms;protein kinases;insulin-like growth factor 1
doi:论著·基础研究10.3969/j.issn.1671-8348.2016.09.010
作者简介:贾静辉(1982-),博士,主治医师,主要从事妇科肿瘤研究。△通讯作者,E-mail:tongying7326@sina.com。
[中图分类号]R737.31
[文献标识码]A
[文章编号]1671-8348(2016)09-1183-03
(收稿日期:2015-10-08修回日期:2015-12-26)

