Vanadium oxide nanotubes for selective catalytic reduction of NO x with NH3
Seyed Mahdi Mousavi*
Faculty of Chemistry,University of Kashan,Kashan 87317,Iran
1.Introduction
Over the pastdecade,nanostructure materials have been considered as a new and fast developing research area of technology.These novel materials have gained intense interest due to their extraordinary physical and chemical properties that may be tailored for various applications.New one-dimensional nanostructured materials attract great attention due to their future possible applications[1,2].Among them,the tubular morphology nanomaterials are particularly attractive since it provides access to the three different contact regions:inner and outer surfaces as well as the tube ends[3–5].
Recently,great interest was assigned to synthesis of metal oxide nanotubes such as titanium dioxide nanotubes(TiO2-NTs)and vanadium oxide nanotubes(VOx-NTs),because of their potential applications as catalysts and electrochemical devices[6–10].However,study of their applications such as catalytic performance is still under the way.VOx-NTs can be prepared with a unique structure of multilayer scrolls with a hydrothermal process[11,12].The interlayer distance and inner and outer diameters of the tube can be easily controlled by a proper choice of structure directing agents,hydrolysis and hydrothermal conditions[9].Due to the special morphology of VOx-NTs,it may have high performance in a wide range of catalytic processes,especially in NOxremoval technologies[8].
Nitrogen oxides(NOx),generally referring to nitric oxide(NO)and nitrogen dioxide(NO2)are mainly generated fromcombustion processes.The emission of NOxis directly responsible for various environment problems including the formations of photochemical smog and acid rain,depletion of the stratospheric ozone layer as well as respiratory diseases[13].A well-known NOxcontrol technology is the selective catalytic reduction(SCR)of NOx.In SCR processes,NH3and hydrocarbons are usually used as reducing agents,which convert NOxinto N2and H2O via catalytic reactions[14,15].Many catalytic systems including transition,noble and rare earth metal containing catalysts(supported,unsupported and/or their mixed oxides)have been reported to be active for NH3-SCR of NOx[16–20].Vanadia based catalysts(V2O5/TiO2or V2O5–WO3/TiO2and V2O5supported on other supports)are the most commonly employed for NH3-SCR,due to their high resistance towards SO2poisoning[20–22].However,most of the developed Vanadia catalysts are active at high temperature(>350 °C)[13,21,23,24].Therefore,the trend is to develop new low-temperature catalysts that are more selective to the production of inert product(N2).
The aim of the present work is to develop some Vanadia oxide nanostructure catalysts and to investigate their efficiency in NH3-SCR of NOxat low temperatures.Vanadia oxide nanostructures were prepared by hydrothermalprocess by varying some preparation parameters such as volume ratio of the EtOH/(EtOH+H2O)and time of hydrolysis.
2.Materials and Methods
2.1.Synthesis and characterization of VOx nanostructures
The synthesis of the VOxnanostructure was adapted from the technique developed by Spahr et al.(1998).VOxnanostructure was synthesized from a suspension of V2O5and dodecylamine as a structure directing agent,with a molar ratio of 1:1,prepared in a mixture of distilled water and ethanol with a variety of volume ratios(EtOH/(EtOH+H2O)=0,25%,50%,75%and 100%).After preparation of the suspension,hydrolysis was performed under stirring for a desired time(24 and 48 h).The resulting suspension was hydrothermally treated at 180°C for 7 days in a Te flon-lined autoclave with a stainless steel shell,then it was allowed to naturally cool down to room temperature.The obtained solid was filtered,repeatedly washed with ethanol and hexane and finally dried at 110°C for 10 h.
The phase composition of the samples was characterized by X-ray powder diffraction(XRD)using a Siemens D5000 dual goniometer diffractometer by a graphite monochromator CuKαradiation(λ =1.54 nm).The diffractograms were recorded within the 2θ range of 20°–80°with a scanning rate of 0.04 s−1.
The Brunauer,Emmett,Teller(BET)specific surface areas of the VOxsamples were measured with a Micromeritics ASAP 2010 using nitrogen adsorption at 77 K.Prior to the measurements,the samples were outgassed to eliminate volatile adsorbents on the surface at 453 K for 4 h under high vacuum.
The morphology and nanostructure of samples were characterized by a LEO 1430 VP scanning electron microscope.Transmission Electron Microscopy(TEM)studies of the nanotube samples were performed using a JEOL(JEM-2010)microscope.For TEM analysis,few droplets of an ultrasonically dispersed suspension of each sample in ethanol were placed on a copper grid with lacey carbon film and dried at ambient conditions.
2.2.Catalytic activity tests
The NH3-SCR activities of the prepared VOxnanostructure were measured in a tubular(i.d.=10 mm) fixed bed glass reactor at atmospheric pressure.At steady state,a gas mixture containing 1000 ml·L−1NO,1000 ml·L−1NH3,5%O2and Ar as balance,was introduced into the reactor.In all the tests,0.2 g of the prepared catalyst was dispread between glass wool plugs;the total flow rate was fixed at 200 cm3·min−1,which corresponded to a gas hourly space velocity(GHSV)of 12,000 h−1.The SCR experiments were carried out at a temperature range of 100–400 °C.
The concentrations of NOxat the inlet and outlet of the reactor were monitored by a Flue Gas Analyzer(Testo 350M/XL).A gas chromatograph(SHIMADZU model 2010 plus)equipped with a molecular sieve(HP-Molesieve,30 m length,0.53 mm diameter)column and a thermal conductivity detector(TCD)was used to determine the concentration of N2and N2O.According to the concentration of NOx(NO+NO2),N2and N2O in the inlet and outlet flow,NOxconversion and N2selectivity were calculated using the following equations:

The subscripts in and outindicate the inletand outletconcentrations at steady state,respectively.
3.Results and Discussion
3.1.Characterization of VOx nanostructures
Recent studies have shown that the physico-chemical properties of vanadium oxide nanostructures are sensitive to the initial materials and the synthesis procedures,especially the vanadium source,alkylamine used as a structure directing agent and the applied hydrolysis and hydrothermal conditions[9,11].Many studies in the literature are available on the investigation of the effects of vanadium precursors such as VO(OPr)3[25],NH4VO3[11]and V2O5sols[26],structure directing agent[27]and hydrothermal time and temperature[28].To the best of our knowledge there is no report on the investigation of the solventtype in reaction mixture as wellas time ofhydrolysis.Therefore,it has a great significance to study the effect of the solvent type in reaction mixture and time ofhydrolysis on structure and morphology of VOxnanostructure.For this purpose,various experiments were performed which are shown in Table 1.According to Table 1,six VOxnanostructures(VOx0–VOx5)were prepared with different EtOH/(EtOH+H2O)volume ratios and times of hydrolysis.All prepared VOxmaterials were in black color.As regards mixed-valent vanadium(IV,V)oxides being generally black[29],it can be concluded that some vanadium is in 4+oxidation states in prepared VOxmaterial.
The XRDpatterns ofVOxsamples prepared atdifferentvolume ratios

Table 1 The hydrolysis and hydrothermal condition of different VO x samples
of EtOH/(EtOH+H2O)were shown in Fig.1.The XRD patterns of the VOx1 and VOx2 feature the low-angle re flection peaks(2θ< 10°),corresponding to well-ordered layered structures.The weak re flection peaks of VOx0 confirm the weakening degree of the crystallinity of layered structures.No characteristic peak is recognized in the XRD pattern of VOx4 and VOx5,which indicates thatthe layered structure is notformed.From Fig.1,it can be seen that with the rise of the EtOH/(EtOH+H2O)volume ratio(VOx1 to VOx2)the intensity of the re flection peaks increased,which represents an increase of crystallinity in VOx2.
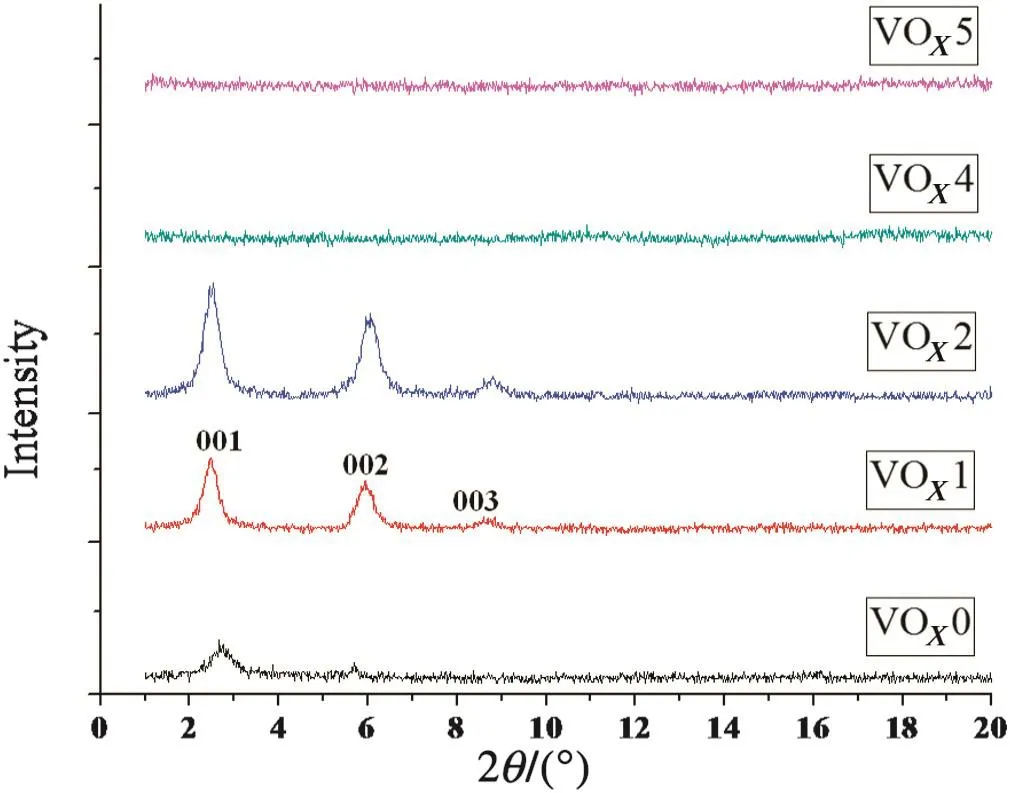
Fig.1.XRD patterns of prepared VO x s at different volume ratios of EtOH/(EtOH+H2O).
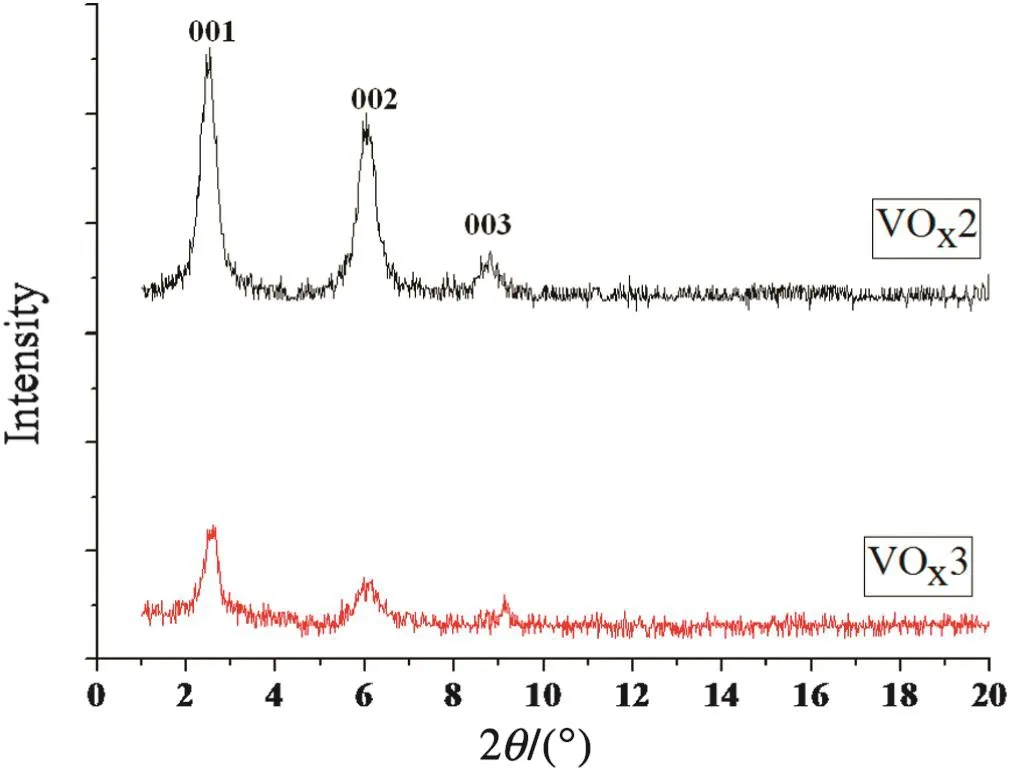
Fig.2.XRD patterns of VO x s prepared at 50 vol%EtOH/(EtOH+H2O)and different times of hydrolysis.
The XRD patterns of the VOx2 and VOx3 samples prepared at differenttimes ofhydrolysis were compared in Fig.2.According to this figure,an obvious decrease in intensity of the re flection peaks with a decrease of the time of hydrolysis process was observed,indicating a decrease in crystallinity of layered structures of VOx.In the formation of the layered structure of vanadium oxide,two main processes take place during hydrolysis:surfactant adsorption and vanadium reduction[12].These processes start at the first minutes of stirring and continue during the hydrolysis process.Therefore,the time of hydrolysis is an important parameterin the formation and completion ofthe layered structure ofVOx.With an increase in EtOH/(EtOH+H2O)volume ratio,the adsorption of surfactant has probably reduced and led to abatement of order in layered structures of VOx.
The average interlayer distance(d001values)calculated from the sharpest peak(001 re flection peak)is listed in Table 2.The average interlayer distances of VOx1,VOx2 and VOx3 are close to the values reported in the literature for a similar synthesis procedure[11,12].These d001values are also consistent with the length of the dodecylamine chain used as a structure directing agent.The d001value calculated for VOx0 was longer than others.Probably,the water molecules intercalated together with the protonated amines in dodecylamine chain are increased the interlayer distance.
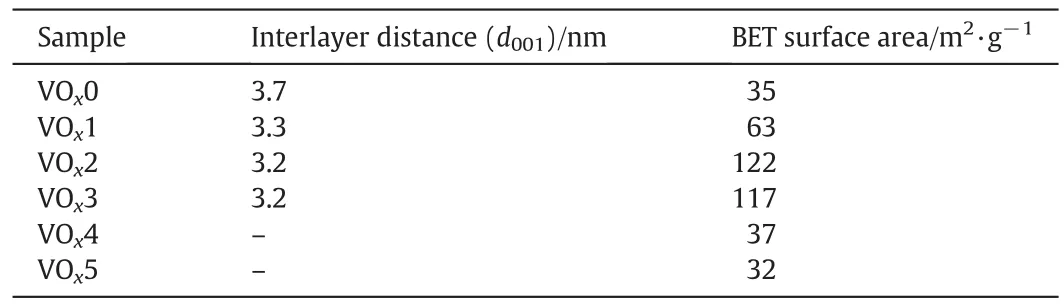
Table 2 Structural properties of the prepared VO x nanostructures
A general impression of the prepared samples is provided by the SEMimages,which evidence the successfulformation ofVOxnanostructure.From the SEM images of VOx1(Fig.3),it can be seen that the VOx1 clearly reveals the presence of well-developed nanorods with 80–120 nm diameter and 1–4 μm length.The SEM images of VOx2 and VOx3 are shown in Fig.4.The nanotube structures can be observed for both the VOx2 and VOx3 samples.However,it can be clearly seen that the VOx2 has a more regular nanostructure than VOx3.In VOx2,most of the nanotubes have open ends,but a small part of the nanotubes with closed ends was noticed.The side of the other tube wrapped around the end to close it off.The dimensions of VOx2 nanotubes were 0.5–2 μm long,110–180 nm for their outer diameter and 50–100 nm for theirinner diameter.As shown in Fig.5,the VOx0 had notany regular structure.The VOx4 and VOx5 had no special morphology(SEM images not shown).
The TEM image of the VOx2 sample is presented in Fig.6.From this figure,it can be clearly seen that the nanostructures of VOx2 are multilayered wall nanotubes,formed by several parallel layers.In the TEM image,the dark lines represent the VOxlayers,while the bright ones are the interlayer.The interlayer distance was approximately 3.2 nm,corresponding to the estimated values obtained by XRD(d001).
The nanotubes and nanorods of VOxare formed by scrolling up VOxlayers in hydrothermal process[25].The VOx1,VOx2 and VOx3 samples which had a well-ordered layered structure(confirmed by XRD and TEM results)provide ideal condition for scrolling up VOxlayers,resulting in the formation of nanotubes and nanorods(confirmed by SEM images).But,in the VOx0,VOx4 and VOx5 samples which had no layered structure(confirmed by XRD results),the formation of a tube or rod structure would not be possible.
The BET surface areas of the prepared VOxare listed in Table 2.It is apparent that the volume ratio of the EtOH/(EtOH+H2O)and time of hydrolysis have a notable impact on the surface area of VOxnanostructures.A BET specific surface area of 122 and 117 m2·g−1was measured for the VOx2 and VOx3 samples,respectively,which are significantly highervalues than thatfound forthe other samples.Although,itexpected to be have a higher specific surface area(due to nanotube morphology),however,this because ofresidualsolventmolecules covering parts of the surface of nanotubes.
3.2.NH3-SCR performance of VOx nanostructures
The NH3-SCR activity of all prepared VOxcatalyst and pure V2O5in terms of NOxconversion(%)as function of reaction temperature is presented in Fig.7.The N2O was not detected in any of the tests,suggesting that an effective selectivity to N2can be achieved over all the studied catalysts.According to Fig.7,the V2O5,VOx0,VOx4 and VOx5 samples exhibited quite low activity in the whole temperature range.As reaction temperature increases,the NOxconversion of these samples slightly increases,however,the maximum NOxconversion over these catalysts was less than 45%.
From Fig.7,itcan be clearly seen thatas the reaction temperature increases,the NOxconversion ofthe VOx2 and VOx3 samples extremely increases,however,the NOxconversion decreases above 250°C.The VOx2 exhibited relatively higher NOxconversion than VOx3 in the low temperature range(100–250 °C).Under identical operating conditions,VOx2 catalyst which had a well-ordered nanotube morphology(confirmed by XRD,SEM and TEM results),showed more than 85%NOxconversion at 250°C.Newly designed VOxnanotubes show a very good NH3-SCRactivity in the low temperature range,while mostofthe developed Vanadia base catalysts are active at high temperature(>350 °C)[21,24,30].According to Fig.7,it can be observed that the NH3-SCR activity of VOx1 catalyst,which had a nanorod morphology,was lower than the activity of the VOx2 and VOx3 nanotubes.
From Fig.7,it can be seen that with the rise of reaction temperature above 250°C,the NOxconversion of VOx2 and VOx3 nanotube catalysts extremely decreases,which is probably due to the thermal instability of VOxnanostructures.Thermogravimetric analysis(TGA)results of VOxnanostructuresin the presence ofoxygen have shown one majorweight loss between 250 and 400°C[31].Itsuggested thatloss ofweightcorresponds to the decomposition of template and the loss of water between vanadiumoxide layers.In fact,the decline ofNOxconversion over 250°C might be caused by the destruction of the well-ordered crystalline structure of VOxnanotubes through combustion and elimination of template.Itis interesting to note thatallthe VOxnanostructure catalysts show the orange color like V2O5after the NO reduction tests.

Fig.3.SEM images of VO x1 nanostructure(25 vol%EtOH/(EtOH+H2O),hydrolysis time of 48 h−1).

Fig.4.SEMimages of(a,b)VO x2(50 vol%EtOH/(EtOH+H2O)and hydrolysis time of48 h−1)and(c,d)VO x3 nanostructures(50 vol%EtOH/(EtOH+H2O)and hydrolysis time of24 h−1).
The O2is an important operation parameter in the NH3-SCR of NOxreaction.The NOoxidation to nitrites and/or nitrates and NH3activation to NH2/OH are the key steps in the SCR reaction[17,32].Increasing O2content can improve these two steps.However,the thermal instability of VOxnanotube catalysts strongly depends on the presence of oxygen.Fig.8 shows the effectofO2concentration on the NH3-SCRperformance ofVOx2 nanotubes as function ofreaction temperature.The NOxconversion was only 65%at 250°C when the O2content was 1%,however,it achieved 89%with 3%O2,indicating the obvious promoter action of O2in SCR of NOx.In addition,the tests showed that the NOxconversion declined with increasing O2contentabove 3%.Itmightbe caused by the increase in the probability ofdestruction ofthe crystalline structure ofVOxnanotubes through combustion of template.
3.3.Concluding remarks
The mechanism of the NH3-SCR process involves the adsorption of reactants(NO,NH3and O2)and redox reaction on the catalyst surface[23].Three obvious differences of the VOxnanostructure catalysts prepared in this study are their morphology,crystallinity and specific surface area,which may be the possible reasons why the nanotubular VOxcatalystexhibits such superiorcatalytic activity.The high crystallinity nanotubes provide access to the three different contact regions:inner and outer surface as wellas the tube ends.Therefore the nanotube morphology leads to an increase in adsorption of reactant and consequently an increase in NOxconversion.Furthermore VOx2 nanotubes are more crystalline in the layered structure than VOx3 nanotubes(see XRD patterns).It can be concluded that the excellent activity of nanotube VOx2 catalyst is mainly due to the specific surface structure and the well-ordered crystalline phase.
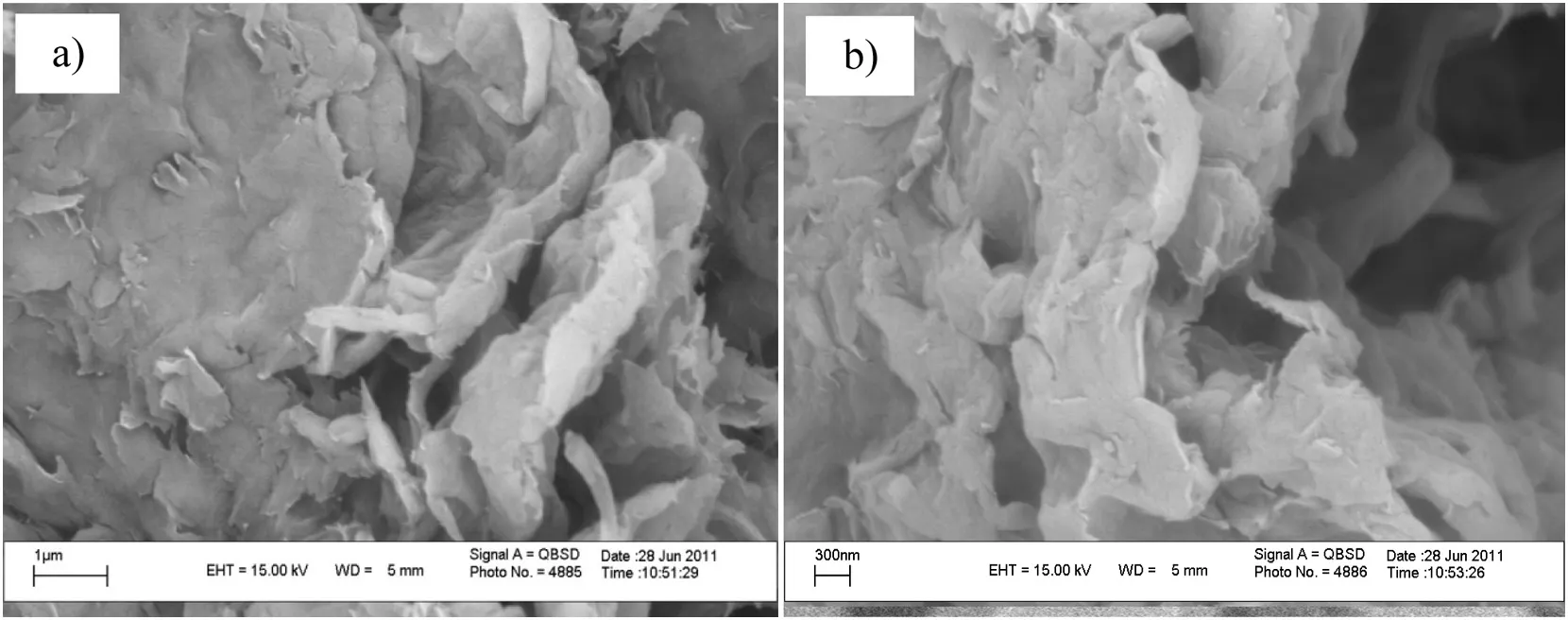
Fig.5.SEM images of VO x0(0 vol%EtOH/(EtOH+H2O)and hydrolysis time of 48 h−1).

Fig.6.TEM image of VO x2 nanotubes.

Fig.7.NO x conversion(%)ofdifferent VO x catalysts(reaction conditions:1000 ml·L−1 NO,NH3/NO is 1,5%O2,GHSV=12000 h−1).
4.Conclusions
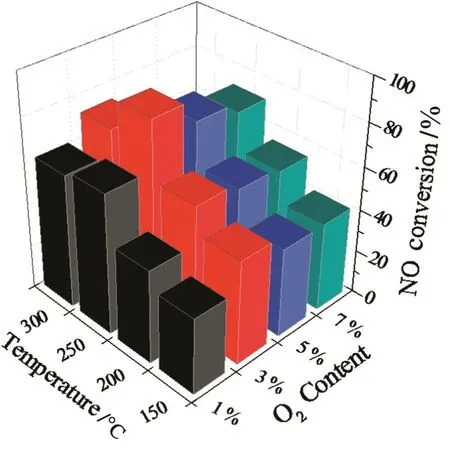
Fig.8.Effect of O2 concentration on NH3-SCR performance of VO x2 nanotubes.
VOxnanotubes and nanorods were successfully synthesized by the hydrothermal method and their performance was evaluated in NH3-SCR of NOx.The results of the current investigation revealed that the solvent type in the reaction mixture(EtOH/(EtOH+H2O))and time of hydrolysis were two important parameters in the synthesis of VOxnanostructures.VOxnanorods with 80–120 nm diameter and 1–4 μm length were prepared in 25 vol%EtOH/(EtOH+H2O),whereas VOxnanotubes with 0.5–2 μm length,110–180 nm for their outer diameter and 50–100 nm for their inner diameter were synthesized in 50 vol%EtOH/(EtOH+H2O).The VOxnanotubes prepared athigher time of hydrolysis showed more regular nanostructure tubes.The prepared VOxnanotubes exhibited superior NH3-SCR activity(89%NOxconversion and 100%N2selectivity)at low temperature of 250°C,while most of the developed VOxbase catalysts are active at high temperature(>350 °C).The excellent NH3-SCR activity of VOxnanotubes may have been caused by their well-ordered crystalline phases,specific nanotube morphology as well as high surface area,confirmed by XRD,SEM,TEM and BET analysis.The decrease of NH3-SCR activity of VOxnanotubes above 250°C could be related to thermal instability of VOxnanostructures.
Acknowledgments
The authors would like to gratefully acknowledge the Iran Nanotechnology Initiative Council for the financial and other supports.
[1]B.Azambre,M.J.Hudson,Growth of copper nanoparticles within VOxnanotubes[J],Mater.Lett.57(20)(2003)3005–3009.
[2]W.Shan,C.Liu,H.Guo,L.Yang,X.Wang,Z.Feng,Synthesis of zero,one,and three dimensional CeO2particles and CO oxidation over CuO/CeO2[J],Chin.J.Catal.32(6–8)(2011)1336–1341.
[3]N.M.Mubarak,E.C.Abdullah,N.S.Jayakumar,J.N.Sahu,An overview on methods for the production of carbon nanotubes[J],J.Ind.Eng.Chem.20(12)(2014)1186–1197.
[4]Z.Tang,X.Yin,Y.Zhang,N.Zhang,Y.Xu,Inhibiting Pd nanoparticle aggregation and improving catalytic performance using one-dimensional CeO2nanotubes as support[J],Chin.J.Catal.34(6)(2013)1123–1127.
[5]F.Ji,C.Cao,H.Xu,Z.Yang,Mechanosynthesis of boron nitride nanotubes[J],Chin.J.Chem.Eng.14(3)(2006)389–393.
[6]R.Rouhani,H.R.Aghabozorg,Asadabad M.Asadi,Synthesis and characterization of Re-,Mo-,and W-doped vanadium oxide nanotubes[J],Synth.React.Inorg.41(8)(2011)1018–1021.
[7]J.Li,L.F.Zheng,K.F.Zhang,X.Q.Feng,Z.X.Su,J.T.Ma,Synthesis of Ag modified vanadium oxide nanotubes and their antibacterial properties[J],Mater.Res.Bull.43(10)(2008)2810–2817.
[8]K.F.Zhang,D.J.Guo,X.Liu,J.Li,H.L.Li,Z.X.Su,Vanadium oxide nanotubes as the support of Pd catalysts for methanol oxidation in alkaline solution[J],J.Power Sources 162(2)(2006)1077–1081.
[9]G.T.Chandrappa,N.Steunou,S.Cassaignon,C.Bauvais,J.Livage,Hydrothermal synthesis of vanadium oxide nanotubes from V2O5gels[J],Catal.Today 78(1)(2003)85–89.
[10]A.Liu,M.Ichihara,I.Honma,H.Zhou,Vanadium-oxide nanotubes:Synthesis and template-related electrochemical properties[J],Electrochem.Commun.9(7)(2007)1766–1771.
[11]X.Chen,X.Sun,Y.Li,Self-assembling vanadium oxide nanotubes by organic molecular templates[J],Inorg.Chem.41(17)(2002)4524–4530.
[12]A.V.Grigorieva,E.A.Goodilin,A.V.Anikina,I.V.Kolesnik,Y.D.Tretyakov,Surfactants in the formation of vanadium oxide nanotubes[J],Mendeleev Commun.18(2)(2008)71–72.
[13]S.Roy,M.S.Hegde,G.Madras,Catalysis for NOx abatement[J],Appl.Energy 86(11)(2009)2283–2297.
[14]S.M.Mousavi,D.Salari,A.Niaei,P.N.Panahi,S.Sha fiei,A modelling study and optimization of catalytic reduction of NO over CeO2–MnOx(0.25)–Ba mixed oxide catalyst using design of experiments[J],Environ.Technol.35(5)(2014)581–589.
[15]S.Saravanan,G.Nagarajan,R.Ramanujam,S.Sampath,Controlling NOxemission of crude rice bran oil blend for sustainable environment[J],Clean Soil Air Water 39(6)(2001)515–521.
[16]Panahi P.Nakhostin,D.Salari,A.Niaei,S.M.Mousavi,NO reduction over nanostructure M–Cu/ZSM-5(M:Cr,Mn,Co and Fe)bimetallic catalysts and optimization of catalyst preparation by RSM[J],J.Ind.Eng.Chem.19(6)(2013)1793–1799.
[17]J.Amanpour,D.Salari,A.Niaei,S.M.Mousavi,P.N.Panahi,Optimization of Cu/activated carbon catalyst in low temperature selective catalytic reduction of NO process using response surface methodology[J],J.Environ.Sci.Health A 48(8)(2013)879–886.
[18]S.M.Mousavi,A.Niaei,M.J.Illán Gómez,D.Salari,Panahi P.Nakhostin,V.Abaladejo-Fuentes,Characterization and activity of alkaline earth metals loaded CeO2–MOx(M=Mn,Fe)mixed oxides in catalytic reduction of NO[J],Mater.Chem.Phys.143(3)(2014)921–928.
[19]Panahi P.Nakhostin,A.Niaei,H.H.Tseng,D.Salari,S.M.Mousavi,Modeling of catalyst composition–activity relationship of supported catalysts in NH3–NO-SCR process using artificial neural network[J],Neural Comput.&Applic.26(7)(2015)1515–1523.
[20]S.Bai,S.Jiang,H.Li,Y.Guan,Carbon nanotubes loaded with vanadium oxide for reduction NO with NH3at low temperature[J],Chin.J.Chem.Eng.23(3)(2015)516–519.
[21]A.Boyano,M.J.Lazaro,C.Cristiani,F.J.Maldonado-Hodar,P.Forzatti,R.Moliner,A comparative study of V2O5/AC and V2O5/Al2O3catalysts for the selective catalytic reduction of NO by NH3[J],Chem.Eng.J.149(1–3)(2009)173–182.
[22]G.J.Dong,Y.F.Zhang,Y.Zhao,Y.Bai,Effect of the pH value of precursor solution on the catalytic performance of V2O5–WO3/TiO2in the low temperature NH3-SCR of NOx[J],J.Fuel Chem.Technol.42(12)(2014)1455–1463.
[23]P.Forzatti,Present status and perspectives in de-NOx SCR catalysis[J],Appl.Catal.A Gen.222(2001)221–236.
[24]Y.Gao,T.Luan,T.Lü,K.Cheng,H.Xu,Performance of V2O5–WO3–MoO3/TiO2catalyst for selective catalytic reduction of NOx by NH3[J],Chin.J.Chem.Eng.21(1)(2013)1–7.
[25]F.Krumeich,H.J.Muhr,M.Niederberger,F.Bieri,B.Schnyder,R.Nesper,Morphology and topochemical reactions of novel vanadium oxide nanotubes[J],J.Am.Chem.Soc.121(36)(1999)8324–8331.
[26]W.Chen,Synthesis of vanadium oxide nanotubes from V2O5sols[J],Mater.Lett.58(17–18)(2004)2275–2278.
[27]M.Niederberger,H.J.Muhr,F.Krumeich,F.Bieri,D.Gunther,R.Nesper,Low-cost synthesis of vanadium oxide nanotubes via two novel non-alkoxide routes[J],Chem.Mater.12(7)(2000)1995–2000.
[28]Krishnan S.Pillai,F.Krumeich,H.J.Muhr,M.Niederberger,R.Nesper,The first oxide nanotubes with alternating inter-layer distances[J],Solid State Ionics 141-141(185-190)(2001).
[29]L.Mai,W.Chen,Q.Xu,Q.Zhu,C.Han,J.Peng,Cost-saving synthesis of vanadium oxide nanotubes[J],Solid State Commun.126(10)(2003)541–543.
[30]H.J.Chae,I.S.Nam,S.W.Ham,S.B.Hong,Characteristics of vanadia on the surface of V2O5/Ti-PILC catalyst for the reduction of NOx by NH3[J],Appl.Catal.B Environ.53(2004)117–126.
[31]W.Chen,L.Q.Mai,J.F.Peng,Q.Xu,Q.Y.Zhu,FTIR study of vanadium oxide nanotubes from lamellar structure[J],J.Mater.Sci.39(2004)2625–2627.
[32]S.M.Mousavi,A.Niaei,D.Salari,P.N.Panahi,M.Samandari,Modelling and optimization of Mn/activate carbon nanocatalysts for NO reduction:comparison of RSM and ANN techniques[J],Environ.Technol.34(11)(2013)1377–1384.
 Chinese Journal of Chemical Engineering2016年7期
Chinese Journal of Chemical Engineering2016年7期
- Chinese Journal of Chemical Engineering的其它文章
- Permeabilization of Escherichia coli with ampicillin for a whole cell biocatalyst with enhanced glutamate decarboxylase activity☆
- A unified graphical method for integration of hydrogen networks with purification reuse☆
- Optimal design for split-and-recombine-type flow distributors of microreactors based on blockage detection☆
- Theoreticalpredictions ofviscosity ofmethane under confined conditions☆
- Formation of crystalline particles from phase change emulsion:In fluence of different parameters
- The effect of SiO2 particle size on iron based F–T synthesis catalysts
