The effect of SiO2 particle size on iron based F–T synthesis catalysts
Xiuying Guo*,Yijun Lu,Peng Wu,Kui Zhang,Qinghua Liu,Mingsheng Luo
National Institute of Clean and Low Carbon Energy,Beijing 102209,China
1.Introduction
The current interest in Fischer–Tropsch synthesis(FTS)has been growing up as a consequence of environmental demands,technological developments,and changes in fossil energy reserves.The use of ironbased catalysts is attractive due to their high FTS activity,low cost,as well as their water–gas shift activity,which is good for making up the deficit of H2in the syngas from modern energy-efficient coal gasifiers[1].In recent years,many researchers began to pay attention to ironbased FT catalyst[2,3].
A number of oxides(SiO2,Al2O3,TiO2,ZrO2,AC/CNT and zeolite)have been used to support iron-based catalysts for FTS[4–9].The role of the support is to disperse the active phase,reduce the amount of active component,stabilize the active species and effectively remove the heat generated in such an exothermal reaction.As generally known,the texturalproperties ofthe supports have a greatin fluence on the catalytic performances for it affects active particle size and dispersion.A high dispersion of iron oxide particles enhances heat and mass transfer as well as high resistance against coke formation and deposition[10].Kang Cheng[11]proved that the catalytic performance ofiron nanoparticles in the catalysts with differentpore sizes principally correlates with the extent of iron carbidization.A.N.Pour[12]had studied the effect of catalyst particle size on product distribution of nanostructured iron catalystin Fischer–Tropsch synthesis,and found thatthe carbon numberof produced hydrocarbon decreased with decreasing catalyst particle size.
A comparison of silica with other supports for iron-based F–T catalysts shows that silica materials have high activity and wax selectivity[13].Typically,silica is added to iron-based Fischer–Tropsch catalysts as a binder to reduce attrition during Fischer–Tropsch synthesis under turbulent environments.Now the slurry bubble column reactor(SBCR)is the most popular FT reactor due to its nearly isothermal operation and large catalyst loadings,as well as high productivity[14].Development of attrition-resistant iron catalysts for commercial SBCRs is a critical challenge.Y.W.Li's group[15,16],J.G.Goodwin's group[17–20]and H.N.Pham[21]did a lot of work on the amount and addition procedures of silica during iron based FT catalyst synthesis,but the particle size effect of silica sol on the activity and attrition of iron based FT catalyst was not reported in the open publication.
The addition of silica sol to iron based FT catalyst may result in the formation of Fe–O–Si bonds.The Fe–O–Si bonds help in reducing attrition,especially attrition induced by the chemical transformation of the catalytically active phase.The interaction of silica with iron not only depends on the procedure of silica sol addition during catalyst synthesis[22],but also the particle size of silica sol used.In this paper,the silica sol particle size effect on iron based FT catalysts will be studied.
2.Experimental
2.1.Catalyst preparation
These catalysts were prepared by the co-precipitation method using an aqueous solution containing Fe(NO3)3·9H2O(0.5 mol·L−1)(99.7%,Chinese medicine group Reagent,China)and Cu(NO3)2·2.5H2O(0.5 mol·L−1)(99.7%,Chinese medicine group Reagent,China)in the Fe/Cu atomic ratio of 23,which were precipitated at 80ºC,maintaining the pHat~7 using 0.5 mol·L−1Na2CO3(99.7%,Chinese medicine group Reagent,China)solution as the precipitating agent.After precipitation and several times filtration,the final cakes were reslurried in DI water.K2CO3(99.7%,Chinese medicine group Reagent,China)aqueous solution in a K/Fe atomic ratio of0.06 was subsequently added.Then the welldispersed slurry was divided into five parts,and a series ofdifferent sized silica sols of 20 wt%based on the total catalyst mass were separately added into five parts.A series of silica sols from Nalco(5 nm,8 nm,15 nm,20 nm,sodiumstabilized),and one from Qingdao Haiyang Ltd.(average size of 13 nm,sodium stabilized)were used.The five slurries were further separately stirred to make silica sol uniformly distributed,and then spray-dried at 250ºC.Finally,the spray dried samples were calcined at 500ºC for 5 h in a muf fle furnace,and labeled by their corresponding silica sol size.For example,Si-5,Si-8,Si-13,Si-15 and Si-20 are prepared by 5 nm,8 nm,13 nm,15 nm and 20 nm silica sol,respectively.
In additional,sodium silicate was used as a source of the smallest size silica sol,and it was added after precipitation but before filtration to get its sodium ions removed together with sodium ions of Na2CO3precipitant by filtration.The catalyst sample was labeled as Si-0.
2.2.Catalyst characterization
The BET surface area and pore size distribution of catalysts were determined by N2physisorption using a Micromeritics TriStar II 3020 automative system.These parameters were determined both for catalyst samples as-prepared(in the calcined state).Each sample was degassed in the Micromeritics system at 90 ºC for 1 h then at 350 ºC for 3 h prior to each measurement.
The hydrogen reduction behavior was measured in a Micromeritics AutoChem II 2920 system.X-ray powder diffraction patterns were obtained using a Rigaku D/MAX2600 X-ray unit with a CuKαradiation.The SEM micrographs were taken using an FEI Nova NanoSEM 450.The TEM micrographs were taken using a JEOL JEM-ARM200F.The FTIR spectra were obtained with a VERTEX 70(Bruker)FT-IR spectrophotometer.Powdered samples were diluted with KBr and pressed into translucent disks at room temperature.All spectra were taken in the range of 4000–400 cm−1at a resolution of 4 cm−1.
2.3.Attrition resistance measurement
The calcined catalysts were sieved with standard sieves of 53 and 120 μm before attrition testing.The sieving process was applied until particles no longer passed through.
The attrition of the iron-based catalysts was tested using an ASTM method in a 3-hole attrition tester.In the jet cup test,50 g of each sample was used with an air jethaving a flowrate of10 L·min−1(with a relative humidity of(60±5)%)at room temperature for 5 h.The fines were collected with a thimble filter at the outlet of the jet cup chamber.The mass of the fines collected was divided by the mass of the total sample recovered to calculate the mass percentage of fines lost,then divided by 5 to get mass loss hourly.
2.4.Activity testing
The FT activity of these iron based FT catalysts was measured in a fixed-bed microreactor(8.0 mm inner diameter)system.1.5 g catalyst of 53–120 μm was diluted with similar size quartz beads and held in the middle of the reactor.The catalyst was reduced in-situ at an H2/CO ratio of 20 at 0.5 MPa,260°C,1800 h−1for 24 h.After reduction,the reactor temperature was lowered to 200°C.The system was then pressurized to 1.6 MPa,and synthesis gas(H2/CO=0.67)was introduced at a gas space velocity of 3000 h−1.The reactor temperature was then increased gradually to 235 °C.The catalysts were tested at 235 °C for more than 100 h.
Reactant and stream products were analyzed using an on-line Agilent gas chromatograph(6890N)equipped with a thermal conductivity detector(TCD)and a Chromosorb column.The heavy hydrocarbon products were analyzed off-line using an Agilent 1260 with a fused silica capillary column and a flame ionization detector(FID).
3.Results and Discussion
3.1.Textural characteristics and attrition resistance
The BET surface area and the average pore size of iron-based FT catalysts with different SiO2particles were measured using N2physisorption for the calcined catalysts and their attrition resistances were test by the ASTM air jet cup test method,as shown in Table 1.

Table 1 Textural characteristics and attrition resistance
The BET surface area decreases with the increase of silica sol particle size,but the area for Si-13(130.3 m2·g−1)was less than that of the Si-15 sample.It is because 13 nm is an average size.As shown in Fig.1,the particle size of Nalco 8 nm silica sol is very uniform,but Qingdao Haiyang 13 nm silica sol presents above 40 nm large particles and less than 5 nm smallparticles coexisting.The Si-0 catalystprepared using sodiumsilicate as a silica source had a high surface area of 200 m2·g−1,average pore radius of 10.06 nm and pore volume of 0.505 cm3·g−1.
The main problemassociated with the operation ofslurry bubble column reactors(SBCRs)is separation of catalyst particles from hydrocarbon wax produced during F–T synthesis.The problem is attributed to the break-up of the original catalyst particles to fine particles.Catalysts used in the SBCR are required to be resistant to both fracture(breakup into smaller fragments)and abrasion/erosion(the process during which particle surface layers or corners are removed).Separation of catalyst particles from wax is particularly difficult as it generates fine particles(micron size range)[23].Therefore,attrition resistance is an important character for FT catalyst.The ASTM air jet cup test method has been demonstrated to be more severe than what would be experienced in an SBCR,so the mechanical strength of all samples were tested by the ASTM method in our experiment,and their attrition loss percent was listed in Table 1.High attrition loss indicates a poor attrition resistance.All samples presented attrition loss less than 5%,indicating that the spray dried iron based catalyst was adequate for use in SBCR[19].Si-0 prepared by sodium silicate and Si-20 prepared by a colloidal silica solof20 nm showed a relative high attrition loss ofabove 4%.In contrast,catalysts prepared by silica sols of medium particle size from 5 nm to 15 nm showed better attrition resistance,especially Si-13 prepared by average 13 nm silica sol presented the lowest attrition loss of 1.89%.
When SiO32−was used as a source of silica,a porous and weak silica network formed,and it was not strong enough to resist a strong air impact force in the jet cup tester.The silica network formed by silica sols is strong,but if the silica sol particle size is too large,a continuous silica network may not be formed due to insufficient silica sol particles.Under the condition of same silica weight percent in all samples,large silica sol particle size means a less amount of particles.When silica sol particles are insufficient,its bonding effectis greatly weakened.We suppose thatthe relative high attrition loss ofSi-20 isoriginated frominsufficient silica particles.The good attrition resistance of Si-13 is attributed to a firm silica network formed by small size silica sol particles linking large size silica sol particles.Thus,we propose the use of a mixed particle size silica sol to improve the mechanical strength of iron based FT catalyst.In R.K.Eller's book[24],he also indicated that a mixed particle size silica sol provides a stronger mechanical strength.

Fig.1.TEM of silica sols.
Compared to Si-0,the average pore radius and pore volumes of all samples prepared by silica sol were small.It may be explained by the pore size distribution,as shown in Fig.2.All samples prepared by silica sols present a very narrow pore size distribution between 2 and 40 nm,but Si-0 which was prepared by sodium silicate presents a broad pore size distribution.Notably,the incremental pore volume of 2–8 nm decreases with the increase of silica sol particle size,especially the Si-0 sample shows an obvious bump between 2 and 3 nm.We know that the BET surface area mainly comes from small pores,so Si-0 had the highest surface area.
2–8 nm pores in iron-based FT catalyst are supposed to be mainly formed by small preliminary ferrihydrite/hematite particles.SiO32−anions or silica sol particles interact with iron hydroxide particles,and form Fe–Si–O bonding in iron based FT catalysts during the calcining process.Iron hydroxide particles'agglomeration is restrained due to the formation of a Fe–Si–O structure,thus a high-dispersion state of iron oxides is kept.Because the mass amount of silica is the same in all catalysts,the quantity of SiO32−anions is obviously more than silica sol particles,and the negative ions are easily bonding with iron hydroxides.Therefore,lots of preliminary iron hydroxides are kept by Fe–Si–O bonds in the calcined Si-0 sample,and the following FT-IR also proved that the Si-0 sample had the strongest Fe–Si–O structure.
The preliminary particles of the Si-0 sample are the smallest among all catalysts,but there are large pores above 8 nm,which are formed by their differentsize aggregates.Usually,silica easily protects the particles close to its size,thus small preliminary ferrihydrite particles aggregate to form large ones in samples prepared by silica sols.And lots of aggregated particles further build different size aggregates from 4 to 100 nm in the sintering process.
3.2.XRD patterns
The X-ray diffraction spectra are plotted over 2θ values from 10°to 80°.The iron phases in the catalyst samples are assigned by matching the lattice spacing values to the data reported in JCPDS(XRD analysis).The XRD patterns of all calcined samples are presented in Fig.3.Two broad diffraction regions around 35°and 63.5°are presented in the patterns of the catalysts incorporated with different silica sizes.It shows the characteristic diffraction peaks of two-line ferrihydrite in samples prepared by silica sol less than 15 nm[25].When the particle size of silica sol is more than 20 nm,the characteristic diffraction peaks of hematite(α-Fe2O3)at 2θ values of 33.1°appeared.The result indicates that the crystalline of precipitated iron FT catalyst enhanced with increasing silica sol size.

Fig.2.Pore size distributions of samples with different silica sizes.

Fig.3.XRD patterns of samples prepared by different silica particles.
Ferrihydrites(FHs)formed by co-precipitation interacts with silica sol or silicate after silica addition.When the precipitate is exposed to thermal treatment,the interaction is strengthened to form a Fe–O–Si structure[26].Due to the formation of this interaction,FH particles'agglomeration is restrained,which results in a high-dispersion state of ferrihydrites or iron oxides.The interaction of ferrihydrite with silica sol decreases with silica sol size increasing.Thus,hematite appeared in samples Si-15 and Si-20 for ferrihydrite aggregations'dehydration in calcination.
3.3.TEM micrographs
From Fig.4,it is found that the Si-0 sample prepared by sodium silicate forms uniform particles about 4 nm.The Si-5 sample prepared by 5 nm silica sol formed particles about 6 nm.The Si-8 sample presented 8 nm and about 4 nm particles.Some large silica oxide particles with several small ferrihydrite/hematite particles were seen in the Si-13 and Si-20 samples,as indicated by arrow lines.And several obvious hematite crystal fringes(as indicated by cycle)appeared in the 15 nm and 20 nm samples.It agrees with the XRD results that there are very weak hematite peaks appearing in the Si-15 and Si-20 samples'X-ray diffraction patterns.
The small ferrihydrite or hematite particles are bonded to silica oxide particles by a Fe–O–Si structure.In the Si-0 sample,almost all ferrihydrites are coated by SiO32−ions,and form a Fe–O–Sistructure network to preventferrihydrite aggregation in calcination.Ferrihydrites'aggregation occurred at different degrees from Si-5 to Si-20 during their sintering process.When silica sol particle with size less than 8 nm was used,large ferrihydrite particles cannot be formed due to the existence of Fe–O–Si and Si–O–Si networks.For silica sol above 8 nm,a small amount of ferrihydrites is attached to large silica oxide particles,and others are aggregated into large ferrihydrite or hematite particles.Thus,small and large ferrihydrite/hematite particles coexisted,as shown in Si-20.
3.4.Fourier Transform Infrared Spectro(FT-IR)analysis
Fig.5 shows the FT-IR spectra of the calcined samples with different SiO2particle sizes.The four main adsorption bands locate at 3000–3700 cm−1,1600–1700 cm−1,800–1250 cm−1and 400–750 cm−1.The very broad absorption band is centered at 3449 cm−1and the small adsorption peak at 1632 cm−1is assigned to the stretching and bending vibrations of the hydroxyl groups and/or water molecules,respectively[27].The strong and broad band with peaks at 456 and 573 cm−1is typical for low crystalline ferrihydrite or the Fe–O bond vibrations in hematite[28].The adsorption peak at 986 cm−1is ascribed to Fe–O–Si stretching frequency or a bidentate((≡FeO)2–Si(OH)2)[29,30],and the peak at 1115 cm−1is attributed to the asymmetric Si–O–Si vibration in solid state.
The typical adsorption band for the asymmetric Si–O–Si vibration at 1115 cm−1[30]is absentin the Si-0 sample prepared by sodium silicate,and a very strong Fe–O–Si peak appeared.It is indicated that almost allanions are bonded to ferrihydrites,and formed Fe–O–Si after calcination.It is like charges repel each other while opposite charges attract.So negativeis notaptto attracteach other to form a Si–O–Si structure.
With increasing silica sol size,the number of silica sol particles decreases at the same silica content,and their chance of contacting with ferrihydrites is reduced.So the Si–O–Si vibration adsorption band at 1115 cm−1gradually enhances from samples Si-5 to Si-20.It is proven that more silica oxides exist in samples prepared with large silica sol particles.Compared to Si-0,the Fe–O–Si absorption peak at 986 cm−1is very weak in catalysts prepared by silica sol,indicating that the chance or ability of ferrihydrites to bond with silica sol is not as easy as.
3.5.H2 and CO reduction
H2-TPR is shown in Fig.6.All pro files of six catalysts present two reduction peaks indicating thatthe reduction process occurred in two distinct stages.The firstpeak near300°C is corresponding to the reduction of α-Fe2O3to Fe3O4,and the second wide peak range from 600 °C to 900 °C is corresponding to the reduction of Fe3O4to FeO or α-Fe[31,32].The reduction of Fe3O4to FeO or α-Fe is difficult.In view of Fischer Tropsch reaction temperature below 300°C,we mainly considered the reduction peak of α-Fe2O3to Fe3O4.The Si-0,Si-5,Si-8 and Si-13 samples presented the reduction temperature of α-Fe2O3to Fe3O4at 298.8 °C,320.8 °C,272 °C and 310.6 °C,respectively.The Si-15 to Si-20 samples present a reduction temperature at about 303.4°C.The reduction peak temperature of the Si-8 sample is lower than those prepared from silicate or silica sols.Itis proven that the 8 nmsample has a proper Si–O–Fe interaction and iron oxide particle size,which make it more easily reduced from α-Fe2O3to Fe3O4.Though Si-0 had a high surface area and small ferrihydrite particles,its reduction was not easier than Si-8 for a strong Fe–O–Si interaction.The samples prepared by above 8 nm silica sol present large ferrihydrite/hematite particles or aggregates,as shown in Fig.4,which is difficult to be reduced.Specially,the Si-5 sample shows the highest reduction temperature,which is probably due to strong Si–O–Fe interactions and ferrihydrite aggregation coexisting effect.
4.F–T Reaction Performance

Fig.4.TEM micrographs of catalysts with different silica sizes.
The performance of iron based FT catalysts prepared by different sized silica sol is summarized in Table 2.The CO conversion of Si-0 catalystprepared by sodiumsilicate shows a very differentcatalytic performance with time on stream.The Si-0 sample shows a high CO conversion above 60%in FT starting period,but its CO conversion gradually declined with time on stream.The CO conversion ofSi-0 decreases from 60.5%at 20 h to 50.2%at 100 h.It is because small iron particles in the Si-0 sample are easier to aggregate during complex iron phase change.On the other hand,carbon deposition may occur for its high initial activity.The Si-5–Si-20 samples present a similar FT activity.Their COconversion shows a very smallincrease from20 h to 100 h,which indicates that the samples prepared by silica sols is not activated completely in the reduction process,and more active sites appear in the FT reaction.The above XRD and TEM results show that the ferrihydrite/hematite particle or aggregation of samples prepared by large silica sol size is big.So its internal iron oxides'activation needs a more severe condition.Compared to Si-0,the iron based FT catalysts prepared by silica sols show a more stable FT activity for a proper Fe3O4and iron carbide dynamic balance.
Table 2 shows the effect of silica size on the distribution of hydrocarbons under reaction conditions.It is indicated that the average molecular weightofFTS products increases with the increase ofsilica size.The selectivity is mainly affected by the pore structure and potassium effect.On the one hand,smallpores contribute to smallweightmolecularformation for diffusion limitation and large pores bene fit heavy hydrocarbons formation.Fig.2 shows that 2–8 nm pores are suppressed with the increase of silica sol size.On the other hand,the interaction between K and Si is weakened with increasing silica size in the condition ofthe same potassium and silica amount.Weak K–Si interaction makes potassium more effectively promote carbon chain growth,and restrains the formation of methane and gaseous products.The effect of potassium on the hydrocarbon distribution observed in the presentstudy is in good agreementwith the results obtained in earlierstudies with a variety ofiron-based FTS catalysts[33,34].
5.Conclusions
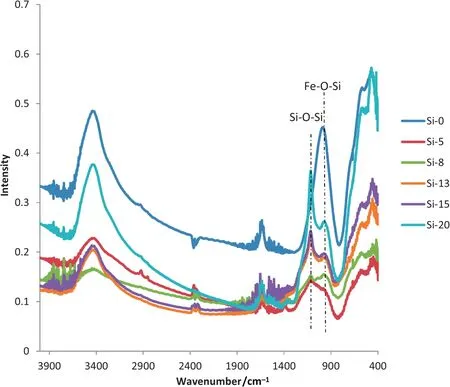
Fig.5.FT-IR spectra of catalysts with different silica sizes.
The BET surface area and small pores of 2–8 nm decrease with increasing silica sol particle size.Sodium silicate used as a silica source make mores smaller pores of 2–4 nm than silica sol.XRD patterns show that samples from Si-0 to Si-15 are mainly two line ferrihydrite,and hematite appeared when silica sol with size exceeding 15 nm is used.Silica in the Si-0 sample presents a Fe–O–Si structure,but silica in catalysts prepared by silica sols is mainly in a Si–O–Si structure.With increasing silica sol size,Si–O–Si bonds in calcined catalysts strengthened.Silica network derived by silica sol of 5–15 nm,especially with mixing sized silica sols,is stronger than that derived by silicate.
The Si-0 sample prepared by sodium silicate shows poor attrition resistance and unstable F–T activity with time on stream forsilica network formed by silicate is not strong enough to prevent active iron aggregation in complex iron phase change.The Si-5–Si-15 samples show good mechanical strength(attrition loss<4%)and stability of FT activity.All catalysts prepared by silica sols show similar CO conversion of about 50%.The average molecular weight of FTS products increases with the increase of SiO2particle.
Acknowledgments
The authors are very grateful for the financial support from Shenhua Group and Zhejiang University of Technology is highly acknowledged for the catalyst test.

Fig.6.H2-TPR results from Si-0,Si-5,Si-8,Si-13,Si-15 and Si-20.
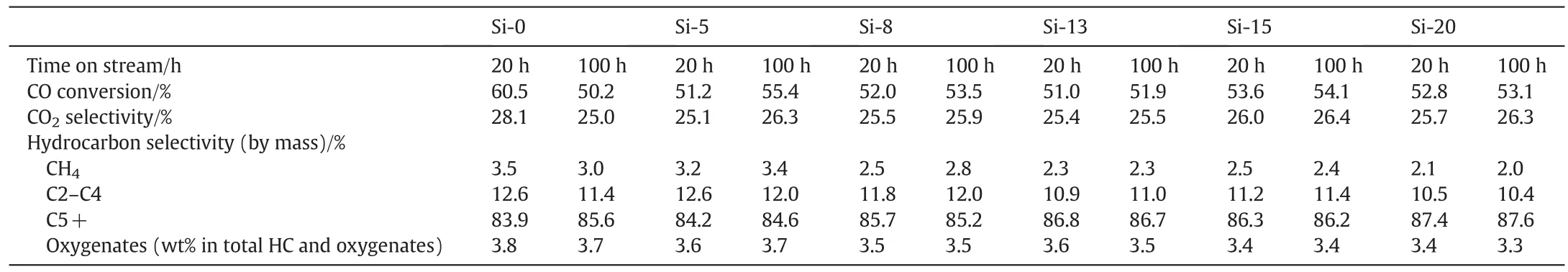
Table 2 Effects of SiO2 particle size on iron based FT catalyst activity and selectivity
[1]Y.M.Jin,A.K.Datye,Phase transformations in iron Fischer–Tropsch catalysts during temperature-programmed reduction,J.Catal.196(2000)8–17.
[2]N.Lohitharn,J.G.Goodwin,E.Lotero,Fe-based Fischer–Tropsch synthesis catalysts containing carbide-forming transition metal promoters,J.Catal.255(2008)104–113.
[3]S.H.Kang,J.W.Bae,K.J.Woo,P.S.S.Prasad,K.W.Jun,ZSM-5 supported iron catalysts for Fischer–Tropsch production of light ole fin,Fuel Process.Technol.91(2010)399–403.
[4]S.H.Kang,J.W.Bae,P.S.S.Prasad,K.W.Jun,Fischer–Tropsch synthesis using zeolitesupported iron catalysts for the production of light hydrocarbons,Catal.Lett.125(2008)264–270.
[5]H.Y.Suo,C.H.Zhang,B.S.Wu,J.Xu,Y.Yang,H.W.Xiang,Y.W.Li,A comparative study of Fe/SiO2Fischer–Tropsch synthesis catalysts using tetraethoxysilane and acidic silica sol as silica sources,Catal.Today 183(2012)88–95.
[6]J.Y.Park,Y.J.Lee,P.K.Khanna,K.W.Jun,J.W.Bae,Y.H.Kim,Alumina-supported iron oxide nanoparticles as Fischer–Tropsch catalysts:Effect of particle size of iron oxide,J.Mol.Catal.A Chem.323(2010)84–90.
[7]W.B.Wang,M.Y.Ding,L.L.Ma,X.Yang,J.Li,N.Tsubaki,G.Yang,T.Wang,X.J.Li,Fe2O3nanoparticles encapsulated in TiO2nanotubes for Fischer–Tropsch synthesis:The confinement effect of nanotubes on the catalytic performance,Fuel 164(2016)347–351.
[8]Z.H.Li,R.J.Liu,Y.Xu,X.B.Ma,Enhanced Fischer–Tropsch synthesis performance of iron-based catalysts supported on nitric acid treated N-doped CNTs,Appl.Surf.Sci.347(2015)643–650.
[9]H.B.Zhao,Q.J.Zhu,Y.J.Gao,P.Zhai,D.Ma,Iron oxide nanoparticles supported on pyrolytic graphene oxide as model catalysts for Fischer Tropsch synthesis,Appl.Catal.A 456(2013)233–239.
[10]R.J.Liu,Y.Xu,Y.Qiao,Z.H.Li,X.B.Ma,Factors in fluencing the Fischer–Tropsch synthesis performance ofiron-based catalyst:iron oxide dispersion,distribution and reducibility,Fuel Process.Technol.139(2015)25–32.
[11]K.Cheng,M.Virginie,V.V.Ordomsky,C.Cordier,P.A.Chernavskii,M.I.Ivantsov,S.Paul,Y.Wang,A.Y.Khodakov,Pore size effects in high-temperature Fischer–Tropsch synthesis over supported iron catalysts,J.Catal.328(2015)139–150.
[12]A.N.Pour,M.R.Housaindokht,Studies on product distribution of nanostructured iron catalyst in Fischer–Tropsch synthesis:effect of catalyst particle size,J.Ind.Eng.Chem.20(2014)591–596.
[13]M.V.Cagnoli,S.G.Marchetti,N.G.Gallegos,A.M.Alvarez,R.C.Mercader,A.A.Yeramian,In fluence of the support on the activity and selectivity of high dispersion Fe catalysts in the Fischer–Tropsch reaction,J.Catal.123(1990)21–30.
[14]Iliuta,F.Larachi,J.Anfray,N.Dromard,D.Schweich,Comparative simulations of cobalt-and iron-based Fischer–Tropsch synthesis slurry bubble column reactors,Ind.Eng.Chem.Res.47(2008)3861–3869.
[15]H.Y.Suo,S.G.Wang,C.H.Zhang,J.X.,B.S.Wu,Y.Yang,H.W.Xiang,Y.W.Li,Chemical and structural effects of silica in iron-based Fischer–Tropsch synthesis catalysts,J.Catal.286(2012)111–123.
[16]Y.Yang,H.W.Xiang,L.Tian,H.Wang,C.H.Zhang,Z.C.Tao,Y.Y.Xu,B.Zhong,Y.W.Li,Structure and Fischer–Tropsch performance of iron–manganese catalyst incorporated with SiO2,Appl.Catal.A 284(2005)105–122.
[17]K.Jothimurugesan,J.G.Goodwin,S.K.Gangwal,J.J.Spivey,Development of Fe Fischer–Tropsch catalysts for slurry bubble column reactors,Catal.Today 58(2000)335–344.
[18]K.Jothimurugesan,J.J.Spivey,S.K.Gangwal,J.G.Goodwin,Effect of silica on ironbased Fischer–Tropsch catalysts,natural gas conversion V,Stud.Surf.Sci.Catal.119(1998)215–220.
[19]R.Zhao,J.G.Goodwin,K.Jothimurugesan,S.K.Gangwal,J.J.Spivey,Spray-dried iron Fischer–Tropsch catalysts.1.Effect of structure on the attrition resistance of the catalysts in the calcined state,Ind.Eng.Chem.Res.40(2001)1065–1075.
[20]K.Sudsakorn,J.G.Goodwin,K.Jothimurugesan,A.A.Adeyiga,Preparation of attrition-resistant spray-dried Fe Fischer–Tropsch catalysts using precipitated SiO2,Ind.Eng.Chem.Res.40(2001)4778–4784.
[21]H.N.Pham,A.Viergutz,R.J.Gormley,A.K.Datye,Improving the attrition resistance of slurry phase heterogeneous catalysts,Powder Technol.110(2000)196–203.
[22]W.J.Hou,B.H.Wu,X.An,T.Z.Li,Effect of the ratio of precipitated SiO2to binder SiO2on iron-based catalysts for Fischer–Tropsch synthesis,Catal.Lett.119(2007)353–360.
[23]D.B.Bukur,V.C.Vazquez,H.N.Phamb,A.K.Datye,Attrition properties of precipitated iron Fischer–Tropsch catalysts,Appl.Catal.A 266(2004)41–48.
[24]R.K.Iller,The Chemistry of Silica—Solubility,Polymerization,Colloid and Surface Properties,and Biochemistry,John Wiley&Sons,New York,1979 230–235.
[25]U.Schwertmann,J.Friedl,H.Stanjek,From Fe(III)ions to ferrihydrite and then to hematite,J.Colloid Interface Sci.209(1999)215–223.
[26]M.Qing,Y.Yang,B.Wu,J.Xu,C.H.Zhang,P.Gao,Y.W.Li,Modification of Fe–SiO2interaction with zirconia for iron-based Fischer–Tropsch catalysts,J.Catal.279(2011)111–122.
[27]K.Supattarasakda,K.Petcharoen,T.Permpool,A.Sirivat,W.Lerdwijitjarud,Control of hematite nanoparticle size and shape by the chemical precipitation method,Powder Technol.249(2013)353–359.
[28]S.M.Rodulfo-Baechler,S.L.Gonalez-Cortes,J.Orozco,V.Sagredo,B.Fontal,A.J.Mora,G.Delgado,Characterization of modified iron catalysts by X-ray diffraction,infrared spectroscopy,magnetic susceptibility and thermogravimetric analysis,Mater.Lett.58(2004)2447–2450.
[29]P.J.Swedlund,R.D.Hamid,G.M.Miskelly,Insights into H4SiO4surface chemistry on ferrihydrite suspensions from ATR-IR,Diffuse Layer Modeling and the adsorption enhancing effects of carbonate,J.Colloid Interface Sci.352(2010)149–157.
[30]L.Dyer,P.D.Fawell,O.M.G.Newman,W.R.Richmond,Synthesis and characterisation of ferrihydrite/silica co-precipitates,J.Colloid Interface Sci.(2010)34865–34870.
[31]D.B.Bukur,X.Lang,D.Mukesh,W.H.Zimmerman,M.P.Rosynek,C.Li,Binder/support effects on the activity and selectivity of iron catalysts in the Fischer–Tropsch synthesis,Ind.Eng.Chem.Res.29(1990)1588–1599.
[32]C.H.Zhang,Y.Yang,B.T.Teng,T.Z.Li,H.Y.Zheng,H.W.Xiang,Y.W.Li,Study of an iron-manganese Fischer–Tropsch synthesis catalyst promoted with copper,J.Catal.237(2006)405–415.
[33]H.Pichler,Twenty- five years of synthesis of gasoline by catalytic conversion of carbon monoxide and hydrocarbon,in:W.G.Frankenberg,V.I.Komarewsky,E.K.Rideal(Eds.),Advances in Catalysis,vol.4,Academic Press,New York 1952,p.271.
[34]C.Li,Effect of Potassium and Copper Promoters on Reduction Behavior of Precipitated Iron Catalysts(Ph.D.Dissertation)Texas A&M University,College Station,1988.
 Chinese Journal of Chemical Engineering2016年7期
Chinese Journal of Chemical Engineering2016年7期
- Chinese Journal of Chemical Engineering的其它文章
- Permeabilization of Escherichia coli with ampicillin for a whole cell biocatalyst with enhanced glutamate decarboxylase activity☆
- A unified graphical method for integration of hydrogen networks with purification reuse☆
- Optimal design for split-and-recombine-type flow distributors of microreactors based on blockage detection☆
- Theoreticalpredictions ofviscosity ofmethane under confined conditions☆
- The biomethane producing potential in China:A theoretical and practical estimation☆
- Formation of crystalline particles from phase change emulsion:In fluence of different parameters
