Formation of crystalline particles from phase change emulsion:In fluence of different parameters
Javed Iqbal*,Zul fiqar AliMurid Hussain Rizwan Sheikh Khaliq Majeed Asad Ullah Khan Joachim Ulrich
1 COMSATS Institute of Information Technology,Department of Chemical Engineering,Defence Road,Off Raiwind Road,Lahore,Pakistan
2 Martin-Luther-Universität Halle-Wittenberg,Zentrum für Ingenieurwissenschaften,Verfahrenstechnik/TVT,D-06099 Halle(Saale),Germany
1.Introduction
The materials used for the storage of thermalenergy for a shortspan of temperature are called phase change materials(PCMs).These materials absorb and release the thermal energy in the form of heat during the loading and unloading of PCM storage,respectively.The phase of these materials shifts from liquid to solid with the slight gradient of temperature.The phase change materials(PCMs)have great importance in industry keeping in view their storage and transportation.The smaller temperature interval in fluences the loading and unloading capacity of PCMs significantly;hence,the properties of the PCM play an importantrole for the proper design of the system.The storage energy is termed as phase change enthalpy[1,2].Although,phase change materials have been extensively studied for a number of applications during the pastfew decades,theirapplication in the form ofsolid spherical particles below the freezing pointhas received great attention in recent years[1–3].Despite the fact that PCMs have a wide range of potential applications in different fields,their use in the form of emulsion solid particles is limited,especially,in the area of pharmaceutical industries.Therefore,the use of emulsion of phase change materials in the formof solid particles is of greatimportance.Emulsion solidification is one of the techniques among others for PCM systems.Generally,a variety of PCMs may be employed for the preparation of solid particles,however,oils,fats and theircommonly derivates available in the market are observed to meltatambienttemperatures.Hence,theiruse as PCMs is of great interest.They possess the properties that make them suitable for the preparation of phase change materials(PCMs)in the temperature range of 0–110 °C.Below 0 °C,water,water–salts and water–organics eutectics exhibit high latent heats and above 110°C,fatty acids are not suitable for thermal application due to the thermal degradation.Hence,palm oil with melting temperature range 35–40 °C was selected as PCM despite the fact that it is widely used in the food industry.The solidification behavior and drop formation pattern were obtained from palm oil emulsion which was used as model substance in our previous studies[4,5].
Emulsions comprise the systems in which droplets of one liquid dispersed in another immiscible liquid.They are generally classified into two types,namely,w/o and o/w emulsions.In the first type of emulsions,water droplets dispersed in the oil phase,and in the second type oil droplets distributed in the water medium.Food emulsions lie in the first type while water in the crude oil in the petroleum industry belongs to the second category[6].The emulsions can also be further classified into more complex forms like:w/o/w or o/w/o emulsions.These complex emulsions are referred as double emulsions.Emulsions are not stable systems,so it is accepted that emulsifiers or surfactants are used to stabilize the emulsion matrix[7].Moreover,emulsifiers have more affinity to the continuous phase than to the dispersed one;thus Span,a nonionic emulsifiers with higher hydrophobic than hydrophilic character is adequate to form w/o emulsion of palm oil,a phase flowing medium[17,18],uniform wax particles in coolant below the freezing point[19]have been studied extensively.The solidification behavior of drops produced from phase change materials(PCMs)has got great importance.Therefore,the different parameters which in fluence the process were studied.The purpose of this study was to monitor the different factors like:the emulsion characteristic i.e.,preparation methods and energy inputs,viscosity ofthe emulsions,effectofcapillary size,interfacial tension and the temperature of the emulsion were observed.In addition,the crystallization mechanismthathappened within the solid particles during solidification was also examined.
2.Experimental
2.1.Chemicals
Refined palm oil obtained from Fluka(Sigma-Aldrich Chemie GmbH,Germany)having m.p.30–40 °C and composition linoleic acid 6%–13%,myristic acid 0.5%–6%,oleic acid 35%–50%,palmitic acid 35%–48%and stearic acid 3%–7%was used as model substance for the preparation of emulsions.Sorbitan laurate i.e.,Span 20 received from Merck(Merck Schuchardt OHG,Germany)was used as an emulsifierforthe stabilization of the emulsion.Berliner Blau löslich(Riedel-de Haën AG,Germany)and Alizarin Red S(Fluka Chemie AG,Germany)were used as dyes for the microscope analysis of the emulsions and the particles produced from the emulsions.Polysorbate Tween 20(Carl-Roth GmbH,Germany)was used in the coolant to reduce the interfacial tension.All the chemicals were used as obtained without further purification.Distilled water was used for all the scheme of experiments.
2.2.Experimental method
The emulsions were prepared by using palm oil as a continuous phase at different concentrations,distilled water as dispersed phase and Span 20 as an emulsifier.Both palm oil and Span 20 were heated at different temperatures separately before mixing.The surfactant was then dissolved in the oil at the same temperature.After that distilled water was added to the mixture ofoiland surfactantatthe same temperature.The water compositions in the emulsion matrices were varied from 5%–30%by volume.The type of emulsion remains the same until 30%of water concentration in the emulsion.The mixture was then stirred continuously for 30 min with a magnetic stirrer at 700 r·min−1and the other matrices of emulsions were prepared with an ultra turrax T25(IKA®Labortechnik)atfour differenttip speeds(8000,9500,13,500 and 20,500 r·min−1).The temperature was kept constant throughout the experiment.The emulsion was stable up to 30 min as reported previously[4,5].The properties of the emulsions produced by a magnetic change material.Generally emulsions can be produced by different systems like:high pressure systems,membrane systems,ultrasonic systems,rotor systems and disc like systems as described brie fly[8].Different parameters like:thermal and physical properties,preparation method,surfactant type,mass ratio,and microstructure that in fluence the emulsion properties of PCMs have been extensively studied[1,4,5,9–12].
The mechanism of drop formation from a capillary tube,nozzle or orifice into another immiscible liquid has received great attention for over a century.Different variables that governed the drop formation mechanism are density of dispersed and continuous phases,interfacial tension,nozzle design including shape and size,and viscosities of both phases[13].When a disperse phase is injected into another immiscible liquid at very low flow rate through an orifice or the capillary,two different drop formation mechanisms are observed.Qualitatively,these mechanisms may be classified as: firstly,almost static growth at the capillary tips,and secondly,the necking and the detaching.It is obvious that the static approach as described here is insufficient to get through the insightofthe dripping dynamics[14],therefore,the effectofthe viscosity, flow rate and capillary diameters[15,16],drop generation in the stirrer and a rotor stator system were obtained with the help of a light microscope(×100,VHX-500F)supplied by Keyence,Neu-Isenburg,Germany attached to the microscope cell to maintain the temperature of the emulsions alike with that of emulsion preparation.Whereas the size and the shape of the solidified emulsion particles were measured by a light microscope(×16.5,BH2)provided by Olympus,Tokyo,Japan,also connected to the microscope cell to keep the temperature similarto thatoftemperature ofcoolantduring solidification.The viscosity of the emulsions was measured by standard operating procedures with the help of a Rotational Viscometer(VT550),Thermo Haake GmbH,Germany.The schematic diagram of the experimental setup is described by Fig.1.In this figure,photographs ofthe actual experimental setup and magnified image of the solid particles obtained have also been included.
The prepared emulsions were pumped using a syringe pump(Cole-Parmer,VERNON HILLS,USA)through a capillary of various sizes situated at the bottom of the coolant vessel.The purpose of the emulsion feeding by capillary of two different sizes was to generate uniform drops of various sizes.The inner diameters of the capillaries used were of 0.5 mm and 1.0 mm,respectively.The total volume of the emulsion used for the preparation of emulsion drops from the capillary was 2–3 ml and the experiments were terminated between 18 and 20 min,so there was not much concern regarding the instability of the emulsion.The produced emulsion drops in the coolant(water)turned into solid form in the coolant at low temperature.The height of the coolant used in the jacketed vessel was 60 cm and it starts from the injection point of the drops till the collection point of the particles.The total volume of the coolant was 2000 ml.The temperature of the coolant was kept at 10°C.Spherical drops of the emulsions were formed at the tip of the capillary and started ascending in the continuous coolant(water)system.The drops were almostsphericalatthe startoftheirupward acceleration.As the drops ascended in the coolant water,solidi fication started.These semi-solid spherical drops hereafter called particles were turned somewhat into an ellipsoid shape i.e.,circular from top view and disc like shape from side view[4].The particles were collected at the top of the coolant for further measurements.Tween 20 was used in the coolant to reduce the interfacial tension between the coolant and the emulsion drops.
3.Results and Discussion
The experiments were carried out in order to observe the in fluence of different parameters on the rheology of emulsions and their effects on the size and the shape of the particles produced from these emulsions.These parameters include:emulsifier concentration,energy input,viscosity,size of the capillary used for the drop formation,temperature of the emulsions,and the effect of the interfacial tension between the working materials and the coolant.The drops of the emulsion are then called particles after solidification in the coolant.The size of the particles,obtained,was measured by a light microscope.When the particles are not exactly spherical,but somewhat ellipsoid in shape,then the particle diameter is described by an equivalent diameter.The equivalentdiameter was calculated by Eq.(1)as described elsewhere[3].

where,dpis the equivalent diameter of single particle and “a”and “b”are the major and minor diameters of the individual particle measured by Eq.(1),the equivalent diameter of single particle was then calculated.In these experiments,20–30 particles were measured and then the average value was used similarto the method described in the literature[3]where they used 40–60 particles forthe measurements oftheir sizes and then mean value was calculated in case of solid particles.
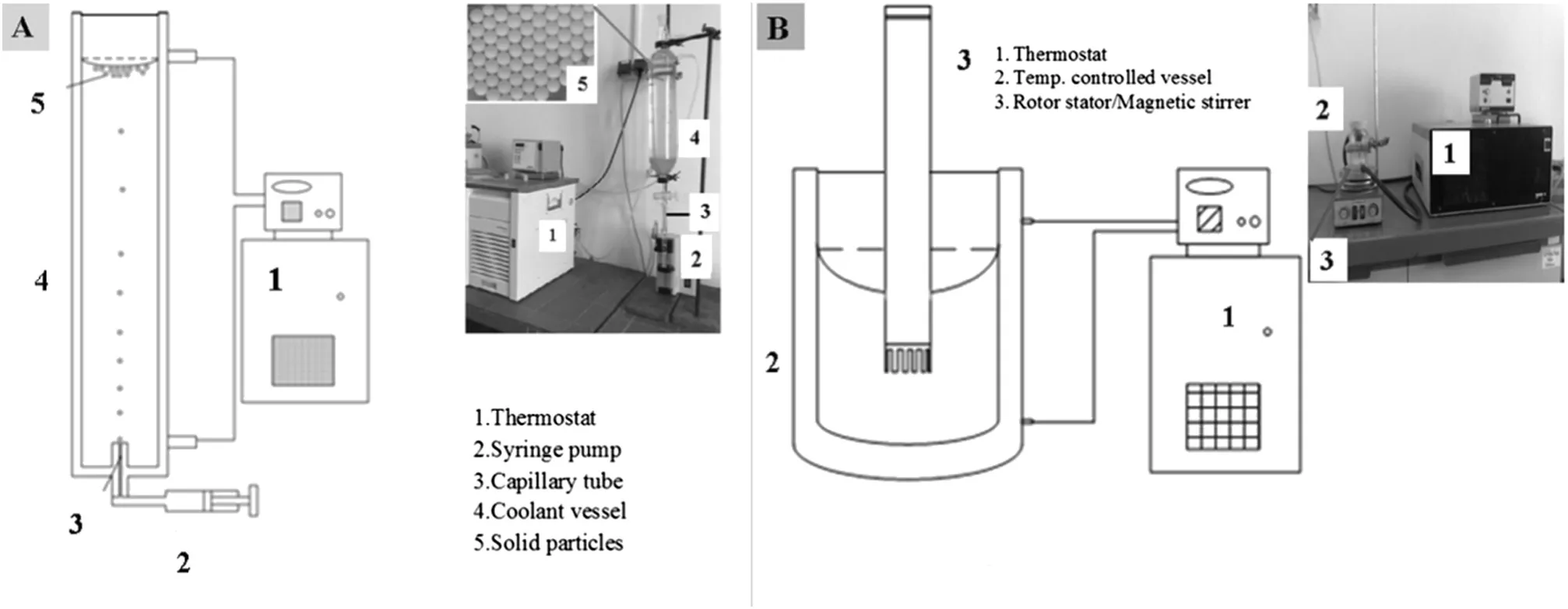
Fig.1.Schematic diagram along with experimental setup of emulsion preparation(A)and drop launching unit(B).
3.1.Effect of surfactant concentration on the size of the particle
The equivalent diameter of particles at different surfactant concentrations was measured at various flow rates.The effect of surfactant concentrations on the size of the particles is shown in Fig.2.The flow rate of the injected emulsion stream was varied from 8 to 18 ml·h−1.The surfactant concentration was varied from 0.2%to 5%by volume.Fig.2 shows that the particles are up to 5 mm in size at 0.2%surfactant,even atlow flow rates.In Fig.2,the column bar graph was also included which showed the effect of surfactant concentration on the particle size for clarity and better understanding.The size was reduced to 3.5 mm when a surfactant concentration of 2%by volume was used,and there was no further decrease in size even when the surfactant concentration was increased to 5%.It was observed that the surfactant concentration of 2%by volume is sufficientfor the stability of the emulsion.The observations are well in agreement with the results as reported in the literature[20]in the case of preparation of w/o emulsion with a magnetic stirrer by the use of a mixture of Span and Tween surfactants with an overall concentration of 5%by mass.Similarly,in the case of a rotor stator system,the use of a surfactant concentration for the production of stabilized w/o and w/o/w emulsions with Span series is monitored[7].The concentration was varied from 0.5 to 5%also by weight for the stability of emulsions.Moreover,the concentration was increased to decrease the interfacial tension between the drop at the capillary exit and the coolant water.The interfacial tension was further decreased by the use of detergent in the coolant.Furthermore,the size of particles was observed in three different size patterns[3].The classification was made for the observation ofthe flowrate effecton the size ofthe particle leaving the capillary exit.
It is noted that the particle equivalent diameter at a flow rate of 8 ml·h−1and a surfactant concentration of 0.2%by volume was 5.0 mm.The equivalent diameter of particles decreased to 3.5 mm at 2.0%at the same flow rate.The formation of pure hexadecane particles by 1.0 mm capillary in a coolant system was reported in the literature[3].They observed the formation pattern of the particles at three different flow rates and categorize them into three different regions.Hence,the particles obtained in our system with the similar size ofthe capillary are in the range of 5.0 to 3.5 mm at different conditions.Likewise,the formation of wax particles in direct coolant water system was studied by Fang et al.[19],They achieved the spherical wax particles of good shape and desirable flowability in a water-cooling tower.The semire fined and crystalline wax was used to produce wax particles at different temperatures of the coolant.They obtained wax particles of size 4–5 mm using the capillary or nozzle with corresponding inner and outer diameters of the nozzle of 0.96 and 1.28 mm,respectively.Therefore,the results obtained in this study are wellin agreementas reported[19].

Fig.2.Effectofsurfactantconcentration on the equivalentdiameter ofthe particles atdifferent flow rates with a capillary size of1.0 mm with a)line symboland b)column bar graphs.The emulsions were produced by a magnetic stirrer at 60 °C and at 700 r·min−1 and 30%water by volume.

Fig.3.(a)Lightmicroscopic image ofthe emulsion prepared at700(A),8000(B),9500(C),13,500(D),and 20,500(E)rpm at60 °Cand 30%water by volume.Scale bar:50 μm.(b)Droplet size distribution corresponding to statistical values of the droplet diameter of the emulsion prepared at 700(A)8000(B)9500(C),13500(D),and 20500(E)r·min−1 at 60 °C and 30%water by volume.
3.2.Effect of energy inputs on the emulsion characteristics
The emulsions prepared at different energy inputs by a magnetic stirrer and an ultra turrax system and their characteristics were measured with the help of a light microscope.The microscopic images of the emulsions and the distribution of the water droplets in the emulsionsare shown in Fig.3(a)and(b),respectively.The dropletsize distribution of the emulsion matrices prepared by different methods and at various energy inputsis plotted againstthe statisticalvaluesofdiameter of the droplets distributed in the w/o emulsions for better understanding and more clarity.Itis noteworthy that as the energy input increases,it is possible to produce smaller droplets and emulsions with a narrow droplet size distribution.It was observed that the droplets distributed in the emulsion produced at9500 r·min−1are finely distributed around the average value.However,the reverse mechanism was observed as the energy input was increased by increasing the tip speed as shown in Fig.3(b)(D,E)where the size distribution is not a narrow distribution.Moreover,some images showed creamy or gel-like character of the matrix,which may be due to the coalescence of fine water droplets present in the emulsions as observed in Fig.3(a)(D,E).It might be that the coalescence increased more than the droplet break-up as the speed was increased.
3.3.Size of the particles
The size of particles plays a vital role in carrying and transportation of different materials for their fruitful use.Therefore,attempts were made to reduce the size of particles obtained by the emulsion drops.Different parameters like:interfacial tension,effect of temperature of the emulsions and the viscosity of the working materials were monitored.
3.3.1.Effect of interfacial tension on the size and shape of the particles
The interfacialtension is one of the principalfactors determining the size of the drops from the capillary or the orifice.It is the responsible force for keeping the drop attached to the capillary pore until the interfacial force is higher than the shear force applied.Moreover,the drop grows at the capillary pore until the shear force is higher than the interfacial tension force.Therefore,hydrophilic detergent or an emulsifier(Tween 20)was used in the coolantto reduce the interfacialtension between the coolant and the drops so that the two forces will attain equilibrium much earlier.The concentration of emulsifier(Tween 20)was 0.5%by volume.

Fig.4.Comparison between the sizes of the particles in the presence and absence of detergent in the coolant obtained by 0.5 and 1.0 mm pore size of capillaries with 15%water by volume and at 9500 r·min−1.
It is to be noted that the hydrophobic emulsifier(Span 20)was used for the emulsion stability.Figs.4 and 5 illustrated the size and the shape of the particles obtained in the presence and absence of the detergent in the coolant.The emulsions were prepared at different temperature rangesfrom50–9°C.In Fig.4,the equivalentdiameterofthe particlesobtained from emulsions of various temperatures is plotted against the temperature of the emulsion.After drop detachment,an adequate amount of surfactant(detergent)present in the coolant adsorbs at the hemispherical interface before the critical pressure is reached to form the next drop.The adsorption of the surfactant is a kinetic process and is exhibited by the dynamic interfacial tension of the given surfactant[21].Hence,Tween 20,a non-ionic surfactant was used in the coolant to provide a sufficient amount that adsorbs on the hemispherical interface to reduce the interfacial tension between the forming drops at the pore of the capillaries used.From Fig.4,the equivalent diameters of particles observed at50 and 90°C withoutan emulsifier(0.5%by volume)in the coolantare 3.8 and 3.2 mm,respectively.However,the equivalentdiameter ofparticles falls to 2.7 and 2.2 mm in the presence ofTween 20 at the corresponding conditions.These measurements were obtained when the capillary size of1.0 mm was used.Similarly,the corresponding observations with a capillary size of0.5 mm were 3.6 to 3.1 and 2.3 to 1.8 in the absence and presence of the detergent in the coolant at the specified temperature conditions of the emulsion.
On the other hand,the shape of the particles obtained at the conditions described in the above paragraph is shown in Fig.5.The shape of the particles is described by the roundness ofparticles which is given by the ratio of the minor to major diameters of the particle as illustrated in Eq.(1).The shape or the roundness of the particles was not affected by the temperature of the emulsions,however,the presence of emulsifier in fluenced the particle roundness.It is recognized that the particle roundness reached 89%–90%in the presence of an emulsifier when a capillary size of 0.5 mm was used.This value falls in the range of 77%–78%when there was no emulsifier in the coolant and a capillary size of 1.0 mm was used.So,from these examinations,it can be revealed that the smallest particles give the highest roundness.It is monitored that the drop size decreases up to 29%–31%and 36%–41%by the capillary of 1.0 and 0.5 mm inner diameter in the presence of detergent(0.5%by volume)in the coolant.Also,the roundness of the particles obtained was increased to 12%–13%in the presence of an emulsifier.The percent decreases and increases indicate the reduction in size of the drop and the increment in the shape of particles when there was no emulsifier in the coolant with the same mentioned capillary sizes.The deviation or the decrease in the drop(particle)size in the presence of an emulsifier(detergent)is higher compared to the results reported[22],which is an advantage.The deviation observed was 12.9%and the difference in the results reported in this work could be due to the different liquid–liquid systems for drop generations.
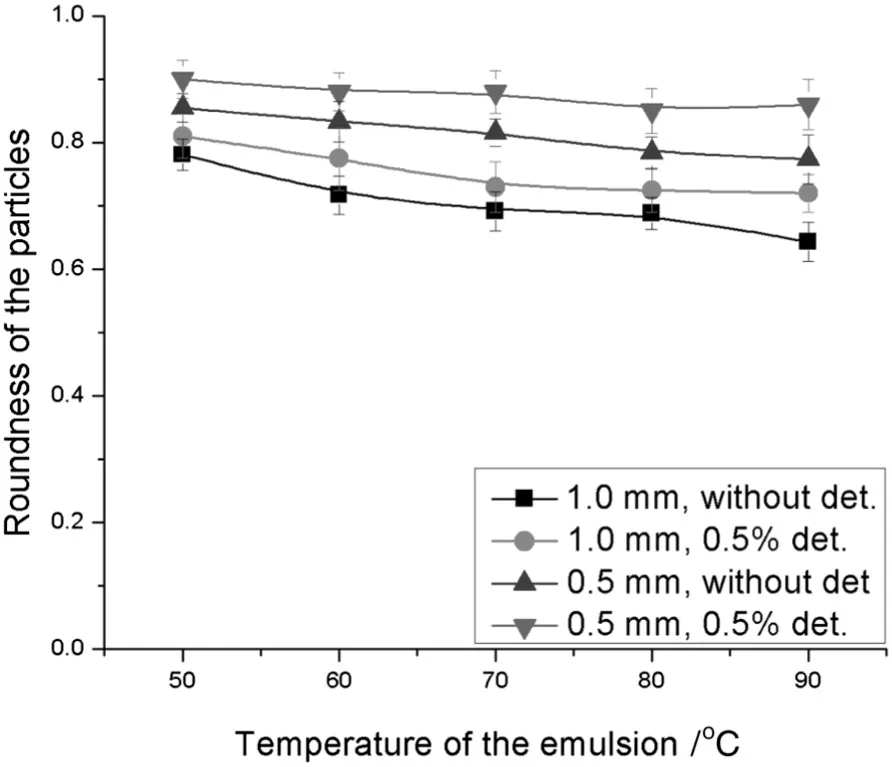
Fig.5.Comparison between the roundness ofthe particles in the presence and absence ofa detergent in the coolant obtained by 0.5 and 1.0 mm pore size of capillaries with 15%water by volume and at 9500 r·min−1.
3.3.2.Effect of the emulsion temperature on the size of the particle
Temperature plays an important and significant role in the preparation and stabilization ofemulsionsespecially water-in-oilemulsions.Itinfluences the differentvariables such as the viscosity ofeach phase and the solubility of the non-ionic surfactants in either phase.Fig.6 shows the effect of temperature of the prepared emulsion on the equivalent diameter of the particles.The equivalent diameter of the particles was plotted against temperature on a column bar scale for clarity which is the replica ofFig.5.It is mandatory to observe the in fluence oftemperature on the w/o emulsion stability.Itisnoted thatata highertemperature,the stability of the emulsions is slightly reduced in the first hour after preparation and then with time,the effect becomes significant[23].The stability of the emulsions plays a key role for the formation of particles.Moreover,the size,shape and structure ofthe materials(particles)also play a significant role in their storage and transportation.The materials having small size and ofregulargeometry possess excellentproperties like flowability,handling,and their energy storage capacity in the case of phase change materials(PCMs).In the current study,the material used for the storage of energy is the emulsion of palm oil,therefore,the properties of the emulsion have importance in the formation ofparticles being used forenergy storage for short interval of time.Hence,the less aggregation of the droplets in the emulsion and its stability for a long time have greater significance.Therefore,the particles obtained from fine and stable emulsion are considered as potential candidate for the better storage and transportation of the particles On the other hand,the viscosity of either phase reduces significantly with temperature.Therefore,the water droplets distributed in the emulsion gained much kinetic energy which ultimately raised the water droplet collision with each other.The collision between the water droplets then finally leads to the instability of the emulsions with time.Also,at lower temperature,the viscosity is high for the dense emulsions,but when the temperature increases,there will be no further change in the viscosity as reported[24].

Fig.6.In fluence of temperature of the emulsions prepared at 9500 r·min−1 on the size of the particles produced by the capillary of pore sizes 0.5 and 1.0 mm.
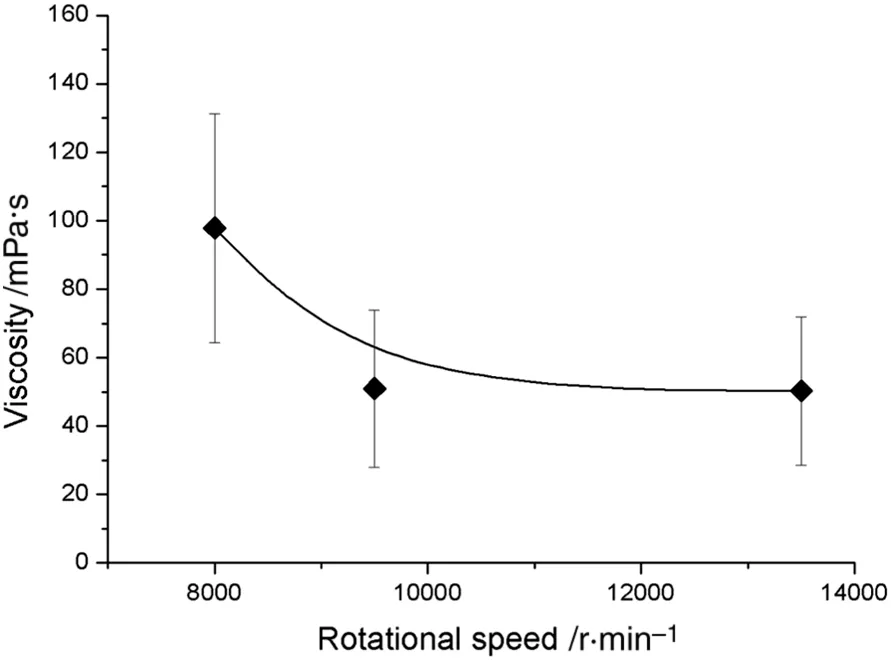
Fig.7.The viscosity versus rotational speed with 20%water by volume and at 60°C.
3.3.3.Effect of energy input on the viscosity of the emulsion
The effect of different rotational speeds of a rotor stator on the viscosity of the emulsions prepared was measured.The viscosity initially decreases with the increase ofspeed and then becomes almost constant with a further increase in the rotational speed.Fig.7 shows the effect of rotationalspeed on the viscosity ofthe emulsions produced.From Fig.7,it can be revealed that the viscosity of the emulsions stays almost the same if the rates per minute are above 9500 and at 60°C and 20%water by volume.Therefore,the flow behavior of the emulsions and drop formation from the capillary of two different pore sizes was done at various temperatures of the emulsions.
In our previous observations[4],it was noted that water concentration in the emulsion from 5 to 25%by volume has no effect on the size and shape of the particles obtained from the emulsion.When the water concentration in the emulsion is low(5%), fine droplets are distributed in the emulsions which results in a viscosity higher compared to higher concentrations of water.The smaller the droplet size,the higher is the viscosity of the emulsion[25].As the water concentrations in the emulsions increase,the fine droplets come closertogether and coalescence of the droplets occurs and this eventually reduces the viscosity of the emulsion.The reduction in viscosity of the emulsion at higher water concentration is due to droplet coalescence or flocculation.At a higher water concentration the rheological properties like the viscosity of the emulsion are according to the ratio of the surface tension and radius of the droplet[25].Therefore,it is expected that,with the increase in the drop radius,the surface tension,viscosity,stress and rheological properties decreases.It is also reported that the effect of the viscosity on the droplet diameter in water-in-oil emulsion increases when the concentration of water increases in the dispersed phase,the rheology of the dispersed phase changes considerably due the interaction of the droplets[24].Therefore,coalescence at higher water contents would be faster and hence the decrease in the viscosity might broaden the droplet size distribution.Therefore,the water concentration in the emulsions discussed in terms ofviscosity ofthe emulsion is nota unique factor which governs the drop formation by a capillary.
3.4.Crystallization mechanism within the solid particles

Fig.8.Droplet distribution at 9500 r·min−1 and at 60 °C and 30%water by volume:light microscope image of emulsion(A),size distribution(B),light microscope images of thin layer of solidified particles generated from emulsion(C,D).Scale bar:50 μm.
Prior to the crystallization occurring in the solidified particles,the particles solidify in a direct contact coolant below the freezing point of the emulsion.In the solidification,the emulsion drops exchange heat with the coolant and this heat transfer results in two steps.At first,the emulsion drops releases heat to the liquid coolant surrounding the drop.When the surface temperature of a liquid drop reaches its solidification temperature,a thin solidified layer of the emulsion begins to envelop the emulsion drop.In this first cooling step,which is terminated by the start of solidification,the sensible heat of the drop is dissipated into the coolant by convective heat transfer inside as well as outside the liquid drop which now becomes a particle,a solidified emulsion particle.In the second cooling step,which may initiated by the start of solidification ofthe particle surface,the latentheatofsolidification released by freezing is transferred by conductive heat transfer through the solid enveloping layer forming on the particle and is transferred by convective heat transfer from the particle surface to the coolant.At this time,the sensible heat transferrate fromliquid PCMs contained in the core ofdrops is smallcompared to the latentheatofsolidification transfer rate.Thus,the heattransfer in the liquid core is of little significance[3,4].
Fig.8 shows the microscopic images of emulsion prepared with 30%water,2%Span 20 and the balance is the palm oil by volume at 9500 r·min−1and at 60 °C,size distribution of water droplets in the emulsions and the images of thin solid layer of emulsion particles.The crystallization mechanism and the structure inside the solid are shown in Fig.8(C,D).The droplets froze in the particles of size 50 μm and above are shown in “D”while the fine droplets less than 30 μm are exhibited in “C”of the said figure.It is also observed that the fine frozen droplets show a hairy like structure around the periphery which indicates the crystallization behavior of the fat which was emulsified and then solidified in the coolant below the freezing point.It means that the water droplets distributed carry out the crystallization of the emulsified fat surrounding it and grow in the form of fine needle like crystals.Generally,fats are naturally occurring triglycerides(TAGs)and fatty acids that contribute an appropriate oil phase for the production of most of the food.Pure and mixture of TAGs grow as spherulites which are made of several crystalline ribbons that grow radially from a similar central nucleus.The ribbons that result the spherulite are needle like shape in most of the cases.Irregularstructuresofspherulite are frequently observed due to deformation and the interface with the liquid may diffuse.Different morphologies of TAG spherulites are due to difference of the driving force which causes changes to the mechanism of secondary nucleation of crystal layer.
Crystallization mechanism of different palm oil emulsions shows the heterogeneous nucleation mechanism according to the kinetic measurements of solid fat contents,but the mechanism alters when the emulsion matrix was diluted with sun flower oil as described elsewhere[26].They proposed a self-nucleating mechanism for complex fat emulsion matrixes and noted that a supercooling is needed to induce the crystallization mechanism.It is assumed that the crystallization within the solid particles may follow the mechanism as described.
4.Conclusions
The process of emulsion drops to particle formation has been developed by optimizing and modifying the parameters which have effects on the process.The smallerparticles have theiradvantage in betterstorage and transportation properties.The fine particles are also considered as good carrier of the hydrophilic and hydrophobic materials.The formation of solid particles from the phase change materials was done under different working conditions.The emulsions prepared by different methods were characterized and noted that 2%surfactant concentration is enough for sufficient stability.The results for the stability of emulsions are well in agreement with the reported results[7].It was also observed that the droplets in the emulsions produced at 9500 rpm are fine and showed a narrow size distribution around the mean value.The particles of diverse sizes atvarious conditions were obtained.The particles of the emulsions from the capillary oftwo different sizes and the effect of temperature were monitored.Particles are smaller when the interfacial tension between the coolant and the injected drops was reduced by mixing of the detergent(0.5%by volume)in the coolant.The particle size was reduced to 41%by the use ofthe detergent in the coolant.Waterconcentration in the emulsion showed no effecton the viscosity of the emulsion and its value remains the same when an energy input in the form of rotational speed was 9500 r·min−1and above.The crystallization mechanism within the solid particles was observed and noted that needle like crystal grows at the periphery of the droplets which leads to the spherulitic growth of the emulsified fats.Nevertheless,the crystallization mechanism within the emulsion particles is discussed brie fly,but it is also noteworthy that the mechanism is still and certainly worthy of further study and observations.
Acknowledgments
The authors would like to acknowledge the Departmentof Chemical Engineering,COMSATS Institute of Information Technology,Lahore,Pakistan,for relieving them from their duties,and Higher Education Commission,Pakistan(A/07/96851)for providing the financial assistance to carry out PhD study in cooperation with the German Academic Exchange Service(DAAD).
[1]H.Inaba,K.Sato,Fundamental study on latent cold heat storage by means of oil droplets with low freezing point,Trans.Japan Soc.Mech.Eng.62(1996)325–332.
[2]H.Inaba,A.Horibe,K.Ozaki,K.Emoto,H.Kakiuchi,Heat release characteristics of middle temperature latent heat storage vessel by means of direct contact heat exchange method,Trans.Japan Soc.Mech.Eng.65(1999)2454–2461.
[3]Y.Nakao,M.Hishida,G.Tanaka,Y.Shiina,Solidification characteristics of rising immiscible oil droplets in coolant,Int.J.Heat Mass Transf.47(2004)5339–5349.
[4]J.Iqbal,J.Ulrich,Spherical-particle generation by phase change materials:Nearmonosize particles from emulsions,Chem.Eng.Technol.33(2010)1011–1014.
[5]J.Iqbal,S.Petersen,J.Ulrich,Emulsion solidification:in fluence of the droplet size of the water-in-oil emulsion on the generated particle size,Chem.Eng.Technol.34(2011)530–534.
[6]D.Clausse,F.Gomez,C.Dalmazzone,C.Noik,A method for the characterization of emulsions,thermogranulometry:application to water-in-crude oil emulsion,J.Colloid Interface Sci.287(2005)694–703.
[7]A.L.Márquez,G.G.Palazolo,J.R.Wagner,Water in oil(w/o)and double(w/o/w)emulsions prepared with spans:microstructure,stability,and rheology,J.Colloid Interface Sci.285(2007)1119–1128.
[8]K.Urban,G.Wagner,D.Schaffner,D.Roglin,J.Ulrich,Rotor–stator and disc systems for emulsification processes,Chem.Eng.Technol.29(2006)24–31.
[9]X.Hui,Y.Rui,Z.Yip,H.Zue,L.Jia,W.Xin,Thermal physical properties and key in fluence factors of phase change emulsion,Chin.Sci.Bull.50(2005)88–93.
[10]H.Inaba,S.Morita,Flow and cold heat-storage characteristics ofphase-change emulsion in a coiled double-tube heat exchanger,J.Heat Transf.117(1995)440–446.
[11]K.Kousksou,A.Jamil,S.Gibout,Thermalanalysis ofphase change emulsion,J.Therm.Anal.Calorim.96(2009)841–852.
[12]Z.Zhennan,W.Ting,L.Lixin,Fundamental properties and application prospect of the phase change emulsion as a cold storage material,Energy Eng.4(2000)28–29(In Chinese).
[13]C.B.Hayworth,R.E.Treybal,Drop formation in two-liquid-phase systems,Ind.Eng.Chem.42(1950)1174–1181.
[14]C.Cramer,P.Fischer,E.J.Windhab,Drop formation in a co- flowing ambient fluid,Chem.Eng.Sci.59(2004)3045–3058.
[15]X.Zhang,O.A.Basaran,An experimental study of dynamics of drop formation,Phys.Fluids 7(1995)1184–1203.
[16]D.Zhang,H.Stone,Drop formation in viscous flows at a vertical capillary tube,Phys.Fluids 9(1997)2234–2242.
[17]P.Umbanhowar,V.Prasad,D.Weitz,Monodisperse emulsion generation via drop break off in a co flowing stream,Langmuir 16(2000)347–351.
[18]S.L.Anna,N.Bontoux,H.A.Stone,Formation of dispersions using “ flow focusing”in microchannels,Appl.Phys.Lett.82(2003)364–366.
[19]J.Y.Fang,Y.Z.Xi,T.J.Wang,Y.Jin,A novel granulation technology for producing spherical wax particles,Chem.Eng.Technol.27(2004)1039–1047.
[20]M.Porras,C.Solan,C.Gonzalez,A.Martinez,A.Guinart,J.M.Gutierrez,Studies of formation of W/O nano-emulsions,Colloids Surf.A 249(2004)115–118.
[21]M.J.Geerken,R.G.Lammertink,M.Wessling,Interfacial aspects ofwater drop formation at micro-engineered orifices,J.Colloid Interface Sci.312(2007)460–469.
[22]A.H.P.Skelland,E.A.Slaymaker,Effects of surface-active agents on drop size in liquid–liquid systems,Ind.Eng.Chem.Res.29(1990)494–499.
[23]M.T.Ghannam,Water-in-crude oil emulsion stability investigation,Pet.Sci.Technol.23(2005)649–667.
[24]A.I.Anisa,A.H.Nour,Affect of viscosity and droplet diameter on water-in-oil(w/o)emulsions:an experimentalstudy,J.World Acad.Sci.Eng.Technol.38(2010)692–694.
[25]R.Pal,Effect of droplet size on the rheology of emulsions,AIChE J.42(1996)3181–3190.
[26]W.Kloek,P.Walstra,T.V.Vliet,Nucleation kinetics of emulsified triglyceride mixtures,JAOCS 77(2000)643–652.
 Chinese Journal of Chemical Engineering2016年7期
Chinese Journal of Chemical Engineering2016年7期
- Chinese Journal of Chemical Engineering的其它文章
- Permeabilization of Escherichia coli with ampicillin for a whole cell biocatalyst with enhanced glutamate decarboxylase activity☆
- A unified graphical method for integration of hydrogen networks with purification reuse☆
- Optimal design for split-and-recombine-type flow distributors of microreactors based on blockage detection☆
- Theoreticalpredictions ofviscosity ofmethane under confined conditions☆
- The biomethane producing potential in China:A theoretical and practical estimation☆
- The effect of SiO2 particle size on iron based F–T synthesis catalysts
