Exploring aramid as emerging contender for CO2 capture
Sonia Zul fiqar *,Muhammad Ilyas Sarwar 2,*
1 Department of Chemistry,School of Natural Sciences(SNS),National University of Sciences and Technology(NUST),Islamabad 44000,Pakistan
2 Department of Chemistry,Quaid-i-Azam University,Islamabad 45320,Pakistan
1.Introduction
The level of greenhouse gases is continuously rising due to their anthropogenic emissions,especially that of carbon dioxide,which has increased by 30%over the past 200 years[1].This alarming situation is a serious public concern leading to environmental and economic threats caused by climate change.CO2is mainly discharged from the combustion offossilfuels,and also fromthe naturalgas sweetening,the production of synthesis gas,and certain chemical plants.Accumulation of CO2in the atmosphere causes globalwarming and according to the latest findings,the CO2levelraised up to 397 parts permillion in 2015[2].The anthropogenic CO2emissions(ca.44%)come from coal,oil or natural gas based power plants[3].In near future,energy consumption pattern of the society is notgoing to be shifted drastically and fossilfuels remain the predominant source of energy than other sources such as biomass-based fuels,solar and nuclear energy,which are not CO2emitter.Besides,the energy demand is expected to increase 53%by 2030[3].Subsequently,immediate mitigation of CO2-emission is incredible.Therefore,a lot of efforts have been dedicated globally to develop novel techniques for CO2capture,sequestration and utilization[4].Thus,it is essential to develop preventive measures against this jeopardy generated by global warming.CO2can be captured by various techniques and many research efforts have been focused in recent years including physical/chemical absorption,adsorption;membrane processing and cryogenic distillation[5].
Sorption-based technologies and processes usually involve solid CO2-adsorbents,e.g.,for large scale reduction in CO2-emission from flue gas power plants;three kinds of technologies have been exploited including post-combustion,pre-combustion and oxyfuel[6].Various solid CO2-adsorbents have been employed in the post-combustion and precombustion capture processes[7,8],still almost all commercial processes for capturing CO2utilize liquid amine solutions.Unlike liquid adsorbents,solid adsorbents can be used overa widertemperature range fromambient temperature to 700°C,yield less waste during cycling,and the consumed solid adsorbents can be disposed ofwithoutundue environmentalprecautions[9].The solid absorbents like supported amine and carbonates,carbon-based sorbents,zeolites,polymeric ionic liquids(PILs),microporous crystalline organic networks(COFs),covalent triazine framework(CTF),conjugated microporous polymers(CMPs)[10–33],porous poly(aryleneethylene)(PAE)[34–36],porous aromatic frameworks(PAFs)[37],polymers of intrinsic microporosity(PIMs)[38]and hypercross-linked polymers(HCPs)[39,40]have been designed and synthesized.
Polyamides are the macromolecules containing amide groups in their backbones.The aromatic polyamides differ from the aliphatic polyamides(nylons)on basis of their highly aromatic nature of the polymer backbone and are known as aramids.Both nylons and aromatic polyamides are considered engineering materials,but aromatic chain structure of the aramids endow them special features making them less sensitive to oxidation,higher solvent resistant,and conferring the materials with superior thermal and mechanical resistance,thus being classified as high-performance materials.The commercial aramids can be transformed into flame,cut-resistant and high-tensile strength synthetic fibers,with technological applications in the field of coatings and fillers in the aerospace and armament industry,in asbestos substitutes,electrical insulation,bullet-proof body armor,industrial filters,and sport fabrics[41,42].Thus,owing to their chemical structure,enormous research efforts have been directed toward exploiting the special high-performance features of the polyamides to obtain electro-or photoluminescent materials,reverse osmosis,gas or ion-exchange membranes,optically active materials,nanocomposites,etc.with superior thermomechanicalperformances.Aramid offered more structuralrigidity to the materials and therefore,the synthesized polyamides are anticipated to be promising materials for CO2adsorption in hostile environment.
In the present work,aramid was fabricated as a solid adsorbent for CO2capture and mitigation of its emission to the atmosphere.Aramid chains were synthesized by condensing 1,3,5-benzenetricarbonyl trichloride and 1,3-phenylenediamine in 1,4-dioxane as a solvent medium.The resulting aramid chains were analyzed for fourier transform infrared spectroscopy(FTIR),X-ray diffraction(XRD),thermogravimetric analysis(TGA),BET surface area and pore size analysis, field emission scanning electron microscopy(FESEM)and CO2adsorption measurements.The results obtained from various analyses were described in the imminent sections.
2.Experimental
2.1.Materials
1,3-Phenylenediamine flakes(≥99%),1,3,5-benzenetricarbonyl trichloride(98%),anhydrous 1,4-dioxane(99.8%)and acetone(≥99.5%)were procured from Sigma-Aldrich and used as such.
2.2.Synthesis of aramid
1,3,5-Benzenetricarbonyl trichloride(5 mmol)was placed into a 500 ml flask equipped with a dropping funnel and a magnetic stirrer.The solvent 1,4-dioxane was added to the flask containing 1,3,5-benzenetricarbonyl trichloride with continuous stirring for complete dissolution under inert atmosphere.Flakes of 1,3-phenylenediamine(7.5 mmol)were also separately dissolved in the same solvent.To the above acid chloride solution,1,3-phenylenediamine was added dropwise with help of dropping funnel with continuous stirring.The exothermicity of the reaction was controlled by cooling the contents of the flask to 0°C for 1 h in order to avoid any side reaction.The reaction mixture was allowed for constantstirring for 72 h at room temperature.The condensation reaction leading to the formation of the aramid is given in Fig.1.The white precipitates formed were isolated and washed with fresh solvent.These precipitates were immersed in acetone for three days with decantation and freshly replacing of acetone thrice.The white powder was dried under vacuum at room temperature and was further used for the adsorption measurements of CO2.
2.3.Characterization
FTIR spectrum of aramid was taken over the range 550–4000 cm−1at a resolution of 4 cm−1using Jasco FTIR 4100 Spectrometer.The diffraction pattern of powdered aramid sample was recorded by a Rigaku(D/Max-2500)HR-X-ray diffractometer using Ni- filtered Cu Kαradiation at 40 kV and 300 mA.Measurements were performed for 2θ in the range of 2°to 80°with a step size of 0.01°and scan speed of 2(°)min−1.Thermal stability of aramid was determined using NETZSCH TG 209 F3 thermogravimetric analyzer using 1–5 mg of the sample in Al2O3crucible heated from 25 to 900°C at a heating rate of 10 °C·min−1under both nitrogen and air atmosphere with a gas flow rate of 20 ml·min−1.A Tristar 3020,Micromeritics(USA)porosimetry analyzerwas exploited to measure Nitrogen adsorption–desorption isotherms of aramid at 77 K.For surface area determination,Brunauer–Emmett–Teller(BET)method was employed using a nitrogen molecule surface area of 0.162 nm2.Field-emission scanning electron microscopy was used to study the surface morphology of the synthesized aramid using Nova 230 FE-SEM.The internal morphology of aramid was investigated using Transmission Electron Microscope,TECNAI G2 20 TWIN(FEI),operating at an accelerating voltage of 200 kV in a bright- field image mode.A Tristar 3000,Micromeritics(USA)CO2analyzer was used to determine CO2adsorption capacity at pressure of 0.1 MPa.The aramid sample was degassed at 120°C for 6 h under vacuum prior to surface area and CO2adsorption analysis.
3.Results and Discussion
Aramids are lightweight possessing low flammability,no melting point,and good fabric integrity at elevated temperatures,outstanding strength-to-weight properties,high tenacity,high modulus and extraordinarily strength,with five times the strength of steel on an equal-weight basis.These heat-resistant synthetic materials can be transformed into fibers, filaments,or sheets and used especially in textiles and as plastics.Best known for its use in ballistic and stab-resistant body armor,aramid fiber has saved thousands of lives around the globe.Since its inception,high performance aramid served a wide range of end-uses including filtration,insulation,automotive,accessories,flame-resistantclothing,protective vestsand helmets,asbestos replacement,hot air filtration fabrics,ropes and cables,sail cloth,sporting goods,aerospace and composites.In this study,aramid was synthesized to verify its novel relevance for CO2capture and storage.The results obtained from FTIR,XRD,TGA,FESEM,BET analysis and CO2adsorption measurements are described below.
3.1.FTIR spectroscopy
To elucidate the structure of synthesized aramid,IR spectrum of powdered sample was taken at 25°C and various bands appeared in the spectrum are presented in Fig.2.The bands at 3263 and 1608 cm−1assigned to the N–H stretching and bending vibrations of the polyamide respectively.The band at the 3070 cm−1was due to the aromatic C–H stretching.C=O group of the aramid gave band at 1665 cm−1and broadening of the band showed the C=O in different environments,i.e.,amide C=O both free and combined.The stretching vibrations of amide linkage indicated the condensation of monomers to yield aramid.Band at1540 cm−1attributed to aromatic C=C stretching vibration.The FTIR data confirmed the formation of the aramid.
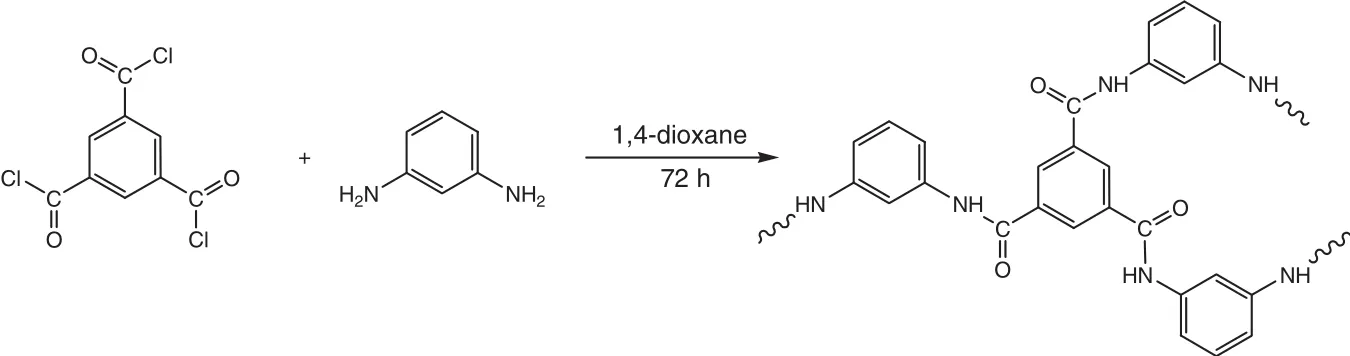
Fig.1.Reaction for the synthesis of aramid.

Fig.2.FTIR spectrum of aramid.
3.2.X-ray diffraction
The crystallinity of aramid was examined by X-ray diffraction scan with 2θ ranging from 2°to 80°and the diffractogram is illustrated in Fig.3.The diffraction pattern showed diffuse halo exhibiting an amorphous structure and the absence ofcrystallinity in the synthesized aramid depicting less ordered chain symmetry with weak crystalline diffraction pattern.The formation of hydrogen bonds between the amide groups of aramid promoted non-planar,three-dimensional architectures suggesting an extended three-dimensional porous network.Aramid network was predominately amorphous with only a low degree of molecular ordering.
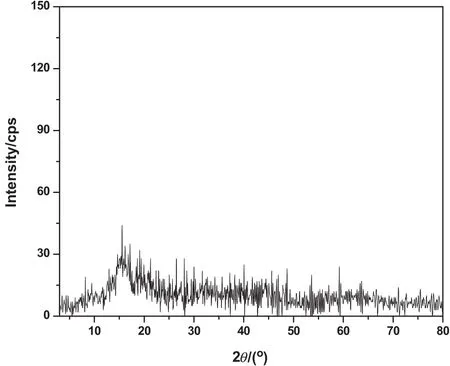
Fig.3.PXRD pattern of aramid.
3.3.Thermogravimetric analysis
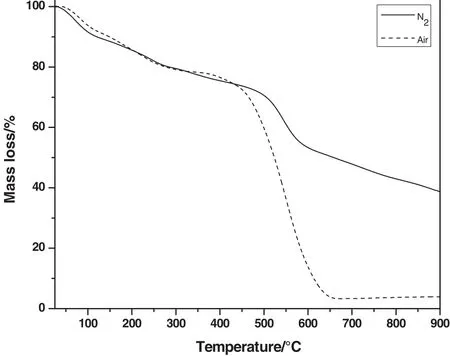
Fig.4.TGA thermograms of aramid.
Thermal stability of aramid was monitored by thermogravimetric analysis in both N2and air atmospheres and the thermograms are presented in Fig.4.It is a very important parameter to evaluate the potential of a given material to be exploited for CO2scrubbing and cleaning the atmosphere.The thermograms gave initial slight mass loss around 100°C due to loss of water molecules that may be produced by the condensation of terminal amine and acid groups of the polyamide.Further mass loss between 200 and 300°C may be due to removal of solvent molecules entrapped in the pores of sorbent.In air atmosphere,the polymer started decomposing athigher temperature and maximum decomposition occurred at 530°C.This observation indicated that aramid is thermally very stable even in the air atmosphere.Similarly,in N2atmosphere,sorbent showed initial mass loss due to removal of water and solvent molecules from the pores and aramid decomposed beyond 500°C.The thermal decomposition temperatures of aramid were in the range of 450–530 °C and 500–560 °C in air and N2atmospheres respectively.However,mass loss in both the atmospheres is comparable except beyond 550°C where mass loss in N2is less relative to air atmosphere as a result of enhanced degradation.This thermally stable aramid can potentially be used as best contender for CO2adsorption in severe environmental conditions.
3.4.Porosity measurement
Nitrogen sorption isotherms provide information regarding the porosity of the materials and the gas sorption data stipulate important parameters,such as surface area,pore volume and pore size.In order to determine the porous nature of the aramid,sample was activated at 120°C for6 h to remove the moisture prior to adsorption measurement.N2adsorption isotherms measured at 77 K are given in Fig.5.BET surface area of the synthesized aramid was found to be 114.08 m2·g−1while Langmuir surface area was 155.74 m2·g−1.Aramid has average pore size of 20.85 nm and pore volume using Barrett–Joyner–Halenda(BJH)method was found to be 0.66 cm3·g−1and also exhibited a“Type-III”N2sorption isotherms depicting a porous nature of the polyamide.The nitrogen sorption isotherms showed a rise above P/P0=0.9 implying the existence ofmesopores in the structure[35].The direct synthetic methodology was employed to prepare porous polymer network structures using solution polycondensation route.By selecting suitable monomers,porous polymeric networks have been fabricated through solution polymerization technique in different media[38].After evaporating the solvent,the extended polymer networks exhibit permanent porosity with wider size distribution ranging from micropores(2 nm)through mesopores(2–50 nm)to macropores(>50 nm).The pores within polymer networks are believed to be formed from the entrapped solvent molecules as they form,whereas the larger pores begin from phase separation of the polymer from the solvent.This methodology can lead to the formation of pores during solution polymerization upon removal of the solvent.Microporous polymers with exceptionally high surface area and permanent porosity can be prepared by direct synthetic approach using rigid and contorted monomer building blocks inhibiting efficient packing.In this study,the same standard condensation technique was exploited to yield the porous polymeric network structures from different monomers.The resulting polyamide showed a better BET surface area,which may be due to orientation of the pores in the network.

Fig.5.N2 gas isotherms for aramid measured at 77 K(inset:differential pore size distribution curve from BJH method).
3.5.Microscopic analysis
The surface morphology of aramid was investigated using field emission scanning electron microscopy.The microscopic analysis was performed on the powdered sample and the micrograph is presented in Fig.6(a).Aramid showed aggregation ofsmallparticles resulting in irregular shapes depicting the presence of porous particles clumped together forming a continuous porous network structure of the compound.The surface morphology exhibited porosity in the aramid due to more free volume with less compact structure,thus indicating a porous nature of the sample.This observation was further verified by Transmission electron microscopy and the internal morphology of the aramid also confirmed the formation of porous structure(Fig.6(b)).The microscopic analysis matched with the porosity data measured through N2uptake rendering porous aramid network structure.Similarnetwork structures have been reported in severalmicroporous organic polymers[43].
3.6.CO2 adsorption measurements

Fig.6.(a)FESEM and(b)TEM image of aramid.

Fig.7.CO2 gas sorption isotherms of aramid(a)273 K(b)298 K.
Aramids are high performance polymers having basic amide groups in their back bones.These amide groups have ability to develop interactionswith otherorganic moieties,especially with carbon dioxide.In this study,aramid was synthesized to produce porous network structure capable of capturing CO2.CO2uptake of aramid was measured at two different temperatures(273 K and 298 K)at 0.1 MPa as depicted in Fig.7.The CO2adsorption at 273 K is well established route for the analysis of porous materials[44]aswell as porous polymers[32].The final amount of carbon dioxide adsorbed was found to be 23.14 mg·g−1at 273 K(Fig.7(a))which further decreased to 14.74 mg·g−1with rise in temperature to 298 K(Fig.7(b)),thus exhibiting the temperature dependence of adsorption capacity.CO2adsorption capacities of aramid decreased with temperature due to the exothermic nature of the binding[45].The higher uptake of CO2by aramid may be ascribed on the basis of the porous network structure of the aramid.Although surface area,pore size and pore volume play a vital role in the CO2uptake but also other factors do in fluence the CO2adsorption that include groups having strong affinity toward CO2like –COO,C=O,–OH,–NH2[46].The polar sites of these groups develop strong interactions with CO2through hydrogen bonding.Aromatic polyamides possess binary interaction sites–NH and C=O.Also,a strong Lewis acid–base interaction may exist between CO2and oxygen of the amide carbonyl as well as π–π interactions with the aromatic phenyl group along with the development of weak H-bonding with phenyl C–H.Aramid CO2uptake profile followed Type-I behavior at 273 K and 298 K,rendering monolayer adsorption.Similar results have been reported with other organic microporous polymers[47].In a typical Type I isotherm,gas molecules may only find sufficient space to form a simple monolayer.This behavior is characteristic of mainly porous solids such as activated carbons and zeolites,with negligible inter-particle voids.Isosteric heat of adsorption(Qst)has also been calculated for aramid using Clausius Clapeyron equation and found to be 22.72 kJ·mol−1which is typical for physisorption process and considerably below the values expected for a chemisorption process(>40 kJ·mol−1)(Fig.8).The comparison of CO2capture capacity ofaramid with othersorbents is also listed in Table 1[48–50].The aramid possess higher CO2sorption capacity as compared to other similar sorbents reported in literature depicting its superior potential for CO2capture.
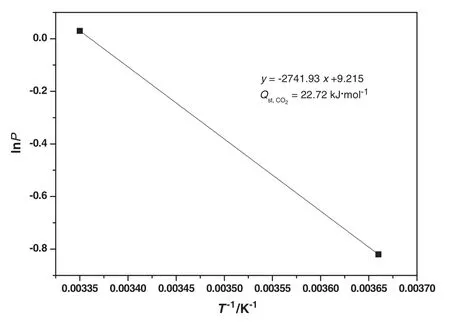
Fig.8.Gas adsorption data of aramid fitted with the Clausius–Clapeyron equation for calculation of isosteric heat of adsorption(Q st).
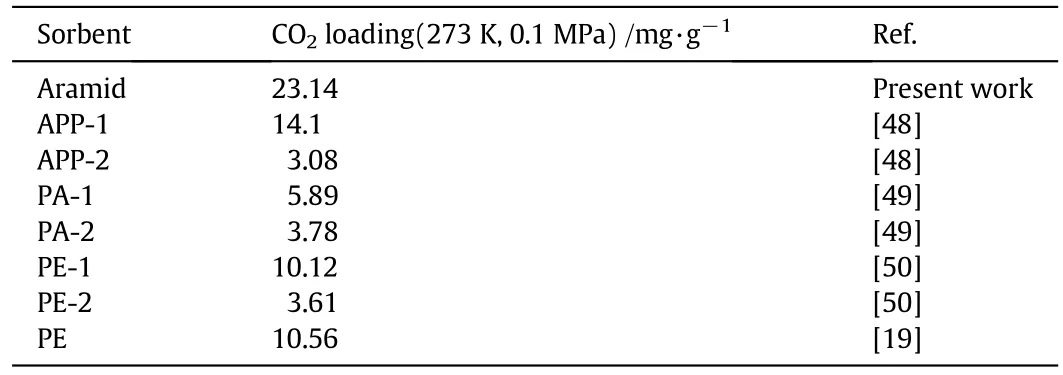
Table 1 Comparison of CO2 sorption of selected polymers reported in present work and literature
4.Conclusions
Porous aramid was successfully synthesized and the amide groups of the polymer were exploited as CO2scavengers,which made these macromolecules as promising contenders for CO2capture and sequestration.The maximum adsorption of carbon dioxide was found to be 23.14 mg·g−1at273 K at0.1MPa thatreduced with increase in temperature.The high adsorption of CO2may be attributed to the porous network structure of the aramid.Additionally,the carbonyl oxygen of amide groups may develop interaction with CO2and also undergoes weak H-bonding with the phenyl C–Hand π–π interactions with the aromatic phenyl group,thus rendering improved CO2sorption capacity of the aramid.The thermally stable porous aramid can be exploited as good CO2sorbent to clean the atmosphere.
[1]S.Zul fiqar,F.Karadas,J.Park,E.Deniz,G.D.Stucky,Y.Jung,M.Atilhan,C.T.Yavuz,Amidoximes:Promising candidates for CO2capture,Energy Environ.Sci.4(2011)4528–4531.
[2]U.S.Department of Commerce,National Oceanic and Atmospheric Administration(NOAA)/Earth System Research Laboratory(ESRL),Global monitoring division,http://www.esrl.noaa.gov/gmd/ccgg/trends/,accessed October 2015
[3]K.M.K.Yu,I.Curcic,J.Gabriel,S.C.E.Tsang,Recent advances in CO2capture and utilization,ChemSusChem.1(2008)893–899.
[4]M.G.Plaza,C.Pevida,B.Arias,M.D.Casal,C.F.Martın,J.Fermoso,F.Rubiera,J.J.Pis,Different approaches for the development of low-cost CO2 adsorbents,J.Environ.Eng.135(2009)426–432.
[5]D.Aaron,C.Tsouris,Separation of CO2from flue gas:A review,Sep.Sci.Technol.40(2005)321–348.
[6]F.M.Orr Jr.,Carbon capture and storage:Are we ready,Energy Environ.Sci.2(2009)449–458.
[7]R.Steeneveldt,B.Berger,T.A.Torp,CO2capture and storage:Closing the knowing–doing gap,Chem.Eng.Res.Des.84(2006)739–763.
[8]J.Blamey,J.Anthony,J.Wang,P.S.Fennel,An overview of CO2capture technologies,Prog.Energy Combust.Sci.36(2010)260–279.
[9]D.P.Harrison,The role of solids in CO2capture:A mini review,Greenhouse Gas Control Technol.7(2)(2005)1101–1106.
[10]G.P.Knowles,J.V.Graham,S.W.Delaney,A.L.Chaffee,Aminopropyl-functionalized mesoporous silicas as CO2adsorbents,Fuel Process.Technol.86(2005)1435–1448.
[11]M.L.Gray,Y.Soong,K.J.Champagne,J.Baltrus,R.W.Stevens Jr.,P.Toochinda,S.S.C.Chuang,CO2capture by amine-enriched fly ash carbon sorbents,Sep.Purif.Technol.35(2004)31–36.
[12]Y.He,X.Zhu,Y.Li,C.Peng,J.Hu,H.Liu,Ef ficient CO2capture by triptycene-based microporous organic polymer with functionalized modification,Microporous Mesoporous Mater.214(2015)181–187.
[13]T.Filburn,J.J.Helble,R.A.Weiss,Development of supported ethanolamines and modified ethanolamines for CO2capture,Ind.Eng.Chem.Res.44(2005)1542–1546.
[14]M.Radosz,X.Hu,K.Krutkramelis,Y.Shen,Flue-gas carbon capture on carbonaceous sorbents:Toward a low-cost multifunctional carbon filter for“green”energy producers,Ind.Eng.Chem.Res.47(2008)3783–3794.
[15]M.M.Maroto-Valer,Z.Tang,Y.Zhang,CO2capture by activated and impregnated anthracites,Fuel Process.Technol.86(2005)1487–1502.
[16]M.G.Plaza,C.Pevida,A.Arenillas,F.Rubiera,J.J.Pis,CO2capture by adsorption with nitrogen enriched carbons,Fuel 86(2007)2204–2212.
[17]C.Lu,H.Bai,B.Wu,F.Su,J.F.Hwang,Comparative study of CO2capture by carbon nanotubes,activated carbons,and zeolites,Energy Fuels 22(2008)3050–3056.
[18]C.Pevida,M.G.Plaza,B.Arias,J.Fermoso,F.Rubiera,J.J.Pis,Surface modification of activated carbons for CO2capture,Appl.Surf.Sci.254(2008)7165–7172.
[19]S.Zul fiqar,M.I.Sarwar,Probing the potential of polyester for CO2capture,J.Environ.Sci.26(2014)1423–1427.
[20]C.Pevida,T.C.Drage,C.E.Snape,Silica-templated melamine-formaldehyde resin derived adsorbents for CO2capture,Carbon 46(2008)1464–1474.
[21]T.C.Drage,J.M.Blackman,C.Pevida,C.E.Snape,Evaluation of activated carbon adsorbents for CO2capture in gasification,Energy Fuel 23(2009)2790–2796.
[22]H.Hayashi,J.Taniuchi,N.Furuyashiki,S.Sugiyama,Ef ficient recovery of carbon dioxide from flue gases of coal- fired power plants by cyclic fixed-bed operations over K2CO3-on-carbon,Ind.Eng.Chem.Res.37(1998)185–191.
[23]N.Shigemoto,T.Yanagihara,S.Sugiyama,H.Hayashi,Material balance and energy consumption for CO2recovery from moist flue gas employing K2CO3-on-activated carbon and its evaluation for practical adaptation,Energy Fuel 20(2006)721–726.
[24]S.Zul fiqar,M.I.Sarwar,D.Mecerreyes,Polymeric ionic liquids for CO2capture and separation:Potential,progress and challenges,Polym.Chem.6(2015)6435–6451.
[25]J.Merel,M.Clausse,F.Meunier,Experimental investigation on CO2post-combustion capture by indirect thermal swing adsorption using 13X and 5 A zeolites,Ind.Eng.Chem.Res.47(2008)209–215.
[26]D.Ko,R.Siriwardane,L.T.Biegler,Optimization of a pressure-swing adsorption process using zeolite 13X for CO2sequestration,Ind.Eng.Chem.Res.42(2003)339–348.
[27]S.Cavenati,C.A.Grande,A.E.Rodrigues,Adsorption equilibrium of methane,carbon dioxide,and nitrogen on zeolite 13X at high pressures,J.Chem.Eng.Data 49(2004)1095–1101.
[28]A.P.Cote,A.I.Benin,N.W.Ockwig,M.O'Keeffe,A.J.Matzger,O.M.Yaghi,Porous,crystalline,covalent organic frameworks,Science 310(2005)1166–1170.
[29]H.M.El-Kaderi,J.R.Hunt,J.L.Mendoza-Cortes,A.P.Cote,R.E.Taylor,M.O’Keeff,O.M.Yaghi,Designed synthesis of 3D covalent organic frameworks,Science 316(2007)268–272.
[30]F.J.Uribe-Romo,J.R.Hunt,H.Furukawa,C.Kloeck,M.O'Keeffe,O.M.Yaghi,A crystalline imine-linked 3-D porous covalent organic framework,J.Am.Chem.Soc.131(2009)4570–4571.
[31]P.Kuhn,M.Antonietti,A.Thomas,Porous,covalent triazine-based frameworks prepared by ionothermal synthesis,Angew.Chem.Int.Ed.47(2008)3450–3453.
[32]P.Kuhn,A.Forget,D.Su,A.Thomas,M.Antonietti,From microporous regular frameworks to mesoporous materials with ultrahigh surface area:Dynamic reorganization of porous polymer networks,J.Am.Chem.Soc.130(2008)13333–13337.
[33]R.Chinchilla,C.Najera,The Sonogashira reaction:A booming methodology in synthetic organic chemistry,Chem.Rev.107(2007)874–922.
[34]J.-X.Jiang,F.Su,A.Trewin,C.D.Wood,N.L.Campbell,H.Niu,C.Dickinson,A.Y.Ganin,M.J.Rosseinsky,Y.Z.Khimyak,A.I.Cooper,Conjugated microporous poly(aryleneethynylene)networks,Angew.Chem.Int.Ed.46(2007)8574–8578.
[35]J.-X.Jiang,F.Su,A.Trewin,C.D.Wood,H.Niu,J.T.A.Jones,Y.Z.Khimyak,A.I.Cooper,Synthetic control of the pore dimension and surface area in conjugated microporous polymer and copolymer networks,J.Am.Chem.Soc.130(2008)7710–7720.
[36]J.-X.Jiang,A.Trewin,F.Su,C.D.Wood,H.Niu,J.T.A.Jones,Y.Z.Khimyak,A.I.Cooper,Microporous poly(tri(4-ethynylphenyl)amine)networks:Synthesis,properties,and atomistic simulation,Macromolecules 42(2009)2658–2666.
[37]T.Ben,H.Ren,S.Ma,D.Cao,J.Lan,X.Jing,W.Wang,J.Xu,F.Deng,J.M.Simmons,S.Qiu,G.Zhu,Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area,Angew.Chem.Int.Ed.48(2009)9457–9460.
[38]N.B.McKeown,P.M.Budd,Exploitation of intrinsic microporosity in polymer-based materials,Macromolecules 43(2010)5163–5176.
[39]Y.Luo,B.Li,W.Wang,K.Wu,B.Tan,Hypercrosslinked aromatic heterocyclic microporous polymers:A new class of highly selective CO2capturing materials,Adv.Mater.24(2012)5703–5707.
[40]J.Y.Lee,C.D.Wood,D.Bradshaw,M.J.Rosseinsky,A.I.Cooper,Hydrogen adsorption in microporous hypercrosslinked polymers,Chem.Commun.2670-2672(2006).
[41]M.Karahan,A.Kus,R.Eren,An investigation into ballistic performance and energy absorption capabilities of woven aramid fabrics,Int.J.Impact Eng.35(2008)499–510.
[42]M.Karahan,Comparison of ballistic performance and energy absorption capabilities of woven and unidirectional aramid fabrics,Text.Res.J.78(2008)718–730.
[43]Q.Chen,J.-X.Wang,F.Yang,D.Zhou,N.Bian,X.-J.Zhang,C.-G.Yan,B.-H.Han,Tetraphenylethylene-based fluorescent porous organic polymers:Preparation,gas sorption properties and photoluminescence properties,J.Mater.Chem.21(2011)13554–13560.
[44]R.V.Siriwardane,M.S.Shen,E.P.Fisher,J.A.Poston,Adsorption of CO2on molecular sieves and activated carbon,Energy Fuel 15(2001)279–284.
[45]D.M.Ruthven,Principles of adsorption and adsorption processes,Wiley,NY,1984.[46]R.Dawson,D.J.Adams,A.I.Cooper,Chemical tuning of CO2sorption in robust nanoporous organic polymers,Chem.Sci.2(2011)1173–1177.
[47]Y.-C.Zhao,T.Wang,L.-M.Zhang,Y.Cui,B.-H.Han,Facile approach to preparing microporous organic polymers through benzoin condensation,ACS Appl.Mater.Interfaces 4(2012)6975–6981.
[48]S.Zul fiqar,S.Awan,F.Karadas,M.Atilhan,C.T.Yavuz,M.I.Sarwar,Amidoxime porous polymers for CO2capture,RSC Adv.3(2013)17203–17213.
[49]S.Zul fiqar,M.I.Sarwar,C.T.Yavuz,Melamine based porous organic amide polymers for CO2capture,RSC Adv.4(2014)52263–52269.
[50]S.Zul fiqar,M.I.Sarwar,Effect of solvent on the CO2capture ability of polyester:A comparative study,J.Ind.Eng.Chem.21(2015)1373–1378.
 Chinese Journal of Chemical Engineering2016年7期
Chinese Journal of Chemical Engineering2016年7期
- Chinese Journal of Chemical Engineering的其它文章
- Vanadium oxide nanotubes for selective catalytic reduction of NO x with NH3
- Optimal design for split-and-recombine-type flow distributors of microreactors based on blockage detection☆
- Theoreticalpredictions ofviscosity ofmethane under confined conditions☆
- Permeabilization of Escherichia coli with ampicillin for a whole cell biocatalyst with enhanced glutamate decarboxylase activity☆
- Formation of crystalline particles from phase change emulsion:In fluence of different parameters
- The effect of SiO2 particle size on iron based F–T synthesis catalysts
