Enhanced performances of polypropylene membranes by molecular layer deposition of polyimide☆
Sen Xiong,Ting Sheng,Liang Kong,Zhaoxiang Zhong,Jun Huang,Yong Wang*
State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
1.Introduction
Molecular layer deposition(MLD),an advanced modification technique which is based on sequential self-limiting surface reactions,has been recently utilized to functionalize a wide variety of materials[1–4].With the advantages such as uniform and conformal deposition on any possible substrate and precise control of deposition layer thickness,MLD is distinguished from other techniques and particularly suited for the homogeneous deposition of porous materials with fine porosities,i.e.,membranes[5–7].With similarprinciplesas Atomic Layer Deposition(ALD),which has been intensively utilized forthe deposition ofinorganic compounds such as oxides,nitrides and sulfides;MLD growth was initially developed forthe deposition oforganic polymers[8–10].The deposition temperatures of ALD for inorganic compounds are normally in the range of 200 to 500°C;however,the deposition of organic compounds by MLD has not been thoroughly investigated as most polymeric materials tend to decompose under such high temperature.It has been recently reported that such deposition technology managed to tune the chemical properties and pore structures of the membrane materials,ranging from polymeric membranes to ceramic membranes[11,12].
So far as we know,the deposition of polymeric materials such as polyamide,polyuria,polythiourea and polyimide has been reported[13–15].Among them,polyimide(PI)materials have found great application in the production of membrane materials for solventresistant nanofiltraton and gas separation due to their resistance to high temperatures,mechanical stress and a variety of chemicals[15–17].Nevertheless,the MLD deposition of PI has rarely been reported due to the relatively low diffusion coefficient of the polymeric precursor pyromellitic dianhydride(PMDA)per se[17].Yoshimura et al.first reported the deposition of PI using precursors PMDA and 2,4-diaminonitrobenzene(DNB)/4,4′-diaminodiphenyl ether(DDE)in 1991[18].Different PI thin films have also been studied using the precursors such as PMDA,1,4-phenylenediamine(PDA),4,4′-oxydianiline(ODA),and ethylenediamine(EDA)etc.at the temperature of 160–200°C[15].Though the MLD deposition temperature has been reduced to 170°C of PMDA-ODA and PMDA-diaminohexane(DAH)precursors for PI,it is still difficult for the polymeric substrates films to survive under such high temperature[19,20].MLD at the temperature of 165°C was then applied for the deposition of PI and for further carbonization to form uniform and continuous carbon nanofilms;nevertheless,the deposition condition was not ideal for polymeric materials[21].
In our previous work,a novel route for the deposition of PI has been successfully introduced on the surface of polyethersulfone(PES)membranes followed by chemical crosslinking to effectively tune the pore size and permeation performance of the membranes.Thermal/mechanical stability and surface hydrophilicity have also been improved upon MLD and crosslinking process[17].Nonetheless,one of the major challengesforthe utilization ofPIisthe deposition conditions.The deposition temperature required as high as 160°C considering the extremely low diffusion coefficient of PMDA and it may condensate during the MLD process.However,most polymeric membranes such as polypropylene(PP)and polycarbonate cannot withstand such high temperature and a disappearance of the pore structure is inevitable.To apply polymeric membrane materials in the area of surface functionalization,particularly molecular layer deposition,it is of great significance to optimize the deposition conditions and ultimately a low deposition temperature can be realized for the deposition of PI on polymeric substrates.
In this work,we have successfully decreased the MLDdeposition temperature of polymeric materials to 110°C,and the deposition parameters have been optimized to ensure the MLD process.Pristine PP membranes without any pre-treatment were deposited with PI for various cycles.Moreover,the performances of the PP membranes have been effectively improved via MLD deposition of PI in multiple aspects,for instance the permeation,surface hydrophilicity and thermal stability.The selectivity of the PP membranes as well as the integrity of the porous structure has also been examined to investigate the in fluence of PI deposition.
2.Experimental
2.1.Materials
Porous PP membranes(Celgard 2400)with a thickness of 25μm and an average pore size of43 nm were used as substrate membranes in this work.Pyromellitic dianhydride(PMDA,99.5%,J&K Scientific)and ethylenedaime(EDA,≥99.5%,Sigma-Aldrich)were used as precursors for polyimide MLD process.Nitrogen with high purity(99.99%)was used as precursor carrier and purging gas.Monodispersed colloidal silica nanospheres with a diameter of 12 nm were purchased from Sigma-Aldrich with an initial concentration of 30%.Si wafers were obtained from local suppliers.
2.2.MLD of PI on PP membranes
Si wafers were first placed and deposited in a laboratory-made ALD chamber to optimize the deposition conditions.PP membranes were then placed in the ALD reactor with the chamber pre-heated to 110°C and pumped to~2 Torr(1 Torr=133.322 Pa).PMDA and EDA vapors were alternatively pulsed into the chamber for the duration of 1.5 and 0.05 s,with the exposure time for 25 and 5 s,respectively to ensure the homogeneous diffusion of PMDA,as well as sufficient adsorption and reaction between the precursors and the substrate membranes.The purge time for PMDA and EDA was 210 and 120 s,respectively.High purity nitrogen with a flow rate of 50 ml·min−1was used to thoroughly sweep the by-product and unabsorbed/unreacted precursors out of the reactor.One typical MLD cycle can be described as PMDA pulse/exposure/N2purge/EDA pulse/exposure/N2purge.PP membranes were deposited for different cycles ranging from 50 to 250.Both Si wafers and nonporous dense PP membranes obtained by hot press were deposited with PI at the same temperature and then used as references.
2.3.Characterizations
Surface morphology of the PI-deposited PP membranes was performed on a HitachiS4800 Field Emission Scanning Electron Microscopy(FESEM)operated at the power of 5 kV and the current of 10 μA.A thin layer of Pt/Pd alloy sputtering was pre-coated on the samples to avoid surface charging.A spectroscopic ellipsometer(Compete EASEM-2000 U,J.A.Woollam)with the wavelength ranged from 246.1 to 999.8 nm in an incident angle of 65°was applied to measure the thickness of the PI films deposited on the surface of Si wafers.The initial SiO2layer was determined with a thickness of 2.5 nm and then subtracted.More than 5 spots on the surface of Si wafer were selected and the thickness was correspondingly measured.Microscopic morphologies of the Si wafer were characterized by Atomic Force Microscopy(AFM,Park Systems XE-100).The Fourier Transformation Infrared(FTIR)spectra were obtained from a Nicolet 8700 FTIR spectrometer in the mode of attenuated total re flection(ATR).Thermal analysis was performed on a TA TG440F thermal gravimetric analyzer(TGA)in nitrogen atmosphere with the temperature from 30 to 600°C with a heating rate of 10 °C·min−1.Surface contact angle goniometer(Dropmeter A-100,Maist)was utilized to obtain the dynamic water contact angle.For each sample,at least 3 sites were tested and the average water contactangle was acquired.To testthe mechanicalstability of the PI-deposited PP membranes,ultrasonication treatment was applied for 10 min at the power of 120 W,and the quantity of the particles on the surface were compared via the SEM images.
2.4.Permeation performance of PI-deposited PP membranes
Pure water flux and the retention rate towards monodispersed silica nanospheres ofthe pristine,PI-deposited PP membranes were performed on a stirred filtration cell(Amicon 8010,Millipore Co.,Billerica,MA)atthe pressure of 0.05 MPa.The membranes were pre-wetted in ethanol solution for 1 min prior to permeation test,and then were circulated for 10 min to obtain a stable flux value.The retention performances were measured against monodispersed silica nanosphere solutions diluted for 2500 folds.The retention rate was calculated by comparing the concentrations of Si element in the feed and permeates solutions.An inductive coupled plasma emission spectrometer(ICP,Optima 7000DV,Perkin-Elmer)was used to measure the concentration of Si element in the solutions.
3.Results and Discussion
3.1.MLD growth of PI films on Si wafers
Previous reports revealed thatthe polymeric precursor PMDAvaporis prone to condensation in the ALD chamber and pipelines attemperatures lower than 150°C due to its relatively large molecular weight(218 g·mol−1),bulky molecular size and extremely low saturated vapor pressure(4×10−3MPa at 305°C),therefore it might cause clogging problems[15,17].To avoid chemical vapor deposition(CVD)and to guarantee the surface reaction ofMLDprocess,PES membranes with considerably higher glass transition temperature(230°C)have been applied as the substrates as they can endure the MLD deposition temperature in our previous work[17].The results also confirmed the maintenance of the structural stability at the deposition temperature of 160°C.However,here in this work,PP membranes with a relatively lower decomposition temperature of 110–120 °C were chosen as the substrates and thus the deposition parameters shall be adapted correspondingly to meet the requirement of lower temperature for MLD process.Take pulse time as example,shorter pulse time is essential as fewer amounts of PMDA precursors will be pulsed into the chamber,indicating it is less difficult for PMDA to evaporate.Prolonging the purge time will ensure the complete removal of the unreacted/unadsorbed precursors and by-product.From thermodynamic perspective,thermal motion of the molecules will be slow down atlowertemperature;therefore longerexposure time is required for the precursors to diffuse homogeneously in the ALD chamber.
To guarantee the proper MLD deposition on the PP membranes,nonporous planar Si wafers were first selected to deposit PI via MLD to optimize the deposition parameters.The thickness of the PI films on the surface of Si wafers as a function of deposition cycle numbers was shown in Fig.1.It can be seen that the thickness of the deposition layer increased along with the growing number of MLD cycles.For instance,the thickness of the PI layer increased to 19 and 41 nm after deposited for 50 and 100 cycles,respectively.The layer thickness further enhanced to 85 and 219 nm when the deposition cycle number reached 250 and 750,respectively.Generally the thickness of the deposition layer increases linearly with the MLD cycle number with a growth rate of 0.35 nmpercycle,which indicates the deposition ofPIon PP membranes is in consistence with the traditional cyclic sequential self-limiting growth mechanism of ALD/MLD technique[3,5].
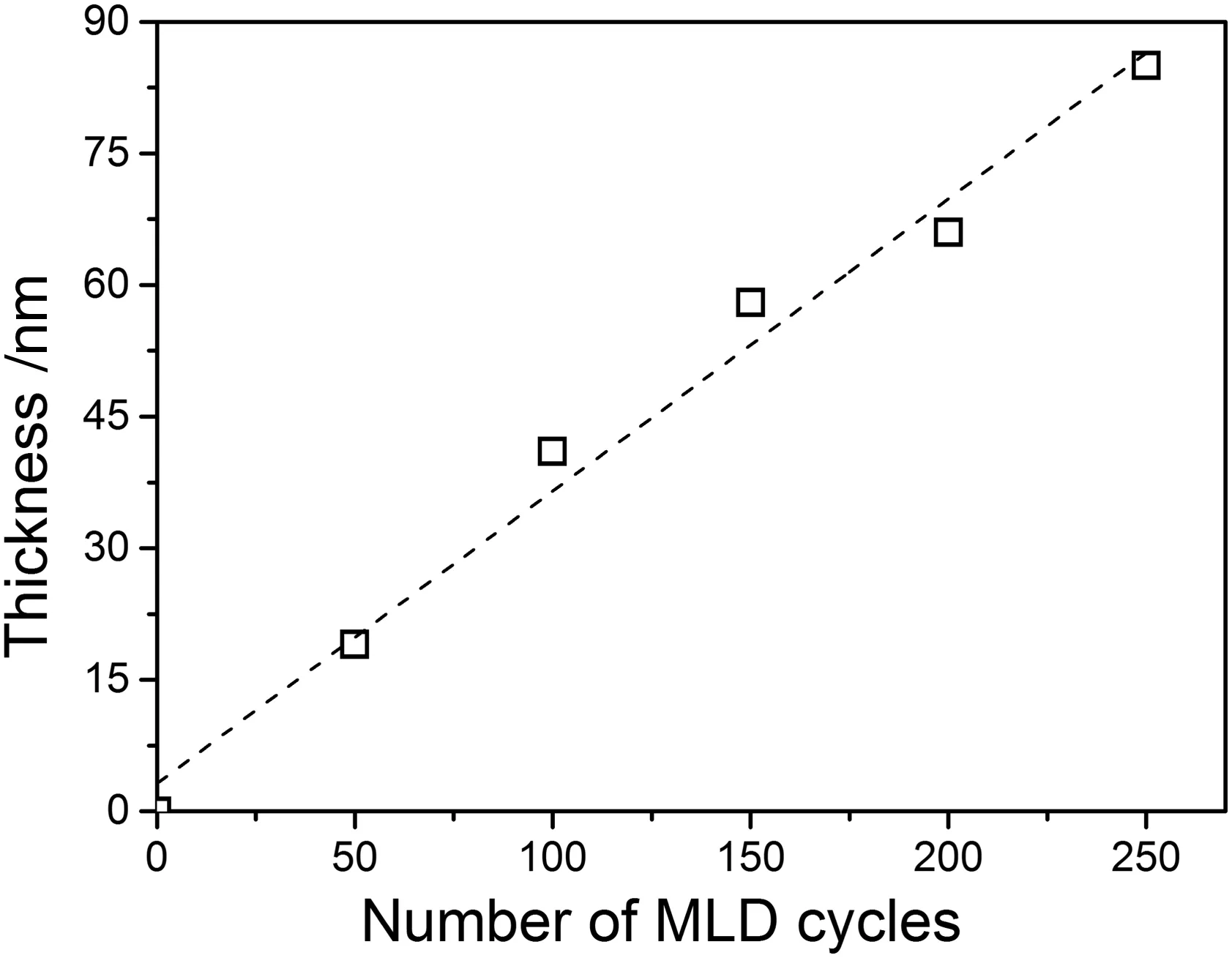
Fig.1.Thickness of PI deposition film as a function of MLD cycle numbers.
SEM was applied to observe the surface and cross-sectional morphologies of the deposition layer on non-porous substrates,Si wafers.As shown in Fig.2,the PI deposition films were conformal and pin-hole free on the surface of Si wafers even after 750 cycles of MLDprocess and no significant change in the morphology of the PI deposition layer can be observed regardless of the deposition cycles.Such phenomenon is in agreement with the MLD growth pattern on the non-porous substrates[3,22].
Microscopic analysis was also carried out by using Atomic Force Microscopy;we managed to determine the surface roughness of an initial SiO2layeron Siwaferas 0.269 nm(Fig.3).After deposited for500 cycles,the Si wafer displayed a surface roughness as 0.285 nm,suggesting the smooth surface of the deposition layer was successfully maintained on the Siwaferduring MLDprocess,which once again confirmsthe conformal and uniform nature of deposition layer obtained by MLD technique.
3.2.PI film composition on PP membranes via MLD deposition
It has been previously reported that PI deposition layer can be directly obtained via MLD technique rather than the generation of polyamic acid(PAA)[15,20].To investigate the in fluence on functional groups after MLD,infrared analysis of the pristine PP membranes and the PI-deposited membranes being deposited for different cycles are measured and the spectra are shown in Fig.4.The peaks located at the wavenumber of 1770 and 1710 cm−1can be designated to the symmetric and asymmetric stretching vibrations of imide C=O bond,respectively;which indicates the appearance of imide bond after the MLD deposition of polyimide(marked with asterisk)[17,23].Further increase in the deposition cycle number to 150 and 250 gives rise to the intensified peak located at 1710 cm−1.The peak appeared at the wavenumber of 725 cm−1(marked with asterisk)represents the characteristic peak of imide ring,which confirms the successful deposition of PI on PP membranes[24,25].However,the peaks centered at 1640 and 1560 cm−1(marked with dot)of PAA cannot be detected in any spectrum of PP membranes subjected to different MLD cycle numbers until it reached 250.It is worth mentioning that the characteristic peaks of PI cannot be observed in the spectrum of 100 MLD cycles PI-deposited PP membrane.The spectrum without obvious changes might be attributed to the ultrathin layer of PI deposited on PP membranes,which is even lower than the instrumental detection limit.Additionally,no trace of the characteristic peaks of anhydride(1806 and 1860 cm−1)nor amine(3300–3500 cm−1)can be detected in the spectra despite the deposition cycles,which eliminates the existence of any unreacted monomers of PI on the PI-deposited PP membranes[15,24].These results suggested thatthe deposition of PI layers on the surface of PP membranes at low temperature of 110°C is an approachable route for the polymeric substrate materials and it is PI rather than PAA that formed in the PP membranes via MLD process.As for the characteristic peaks of PP membranes themselves,the asymmetric stretching vibration peaksof–CHbond in–CH3groupslocated in the region between 2800 and 2970 cm−1,and the bending vibration peaks of–CH bond in –CH2– groups centered in the range of 1380 to 1480 cm−1progressively weakened with growing MLD cycle numbers,which might be attributed to the deposition layer blocking these absorption peaks(marked with circles)[26].It is an indirect indicator that higher MLD cycle number leads to thicker layer of PI films whose shielding effect deteriorated the penetration of IR signals into the PP membranes.
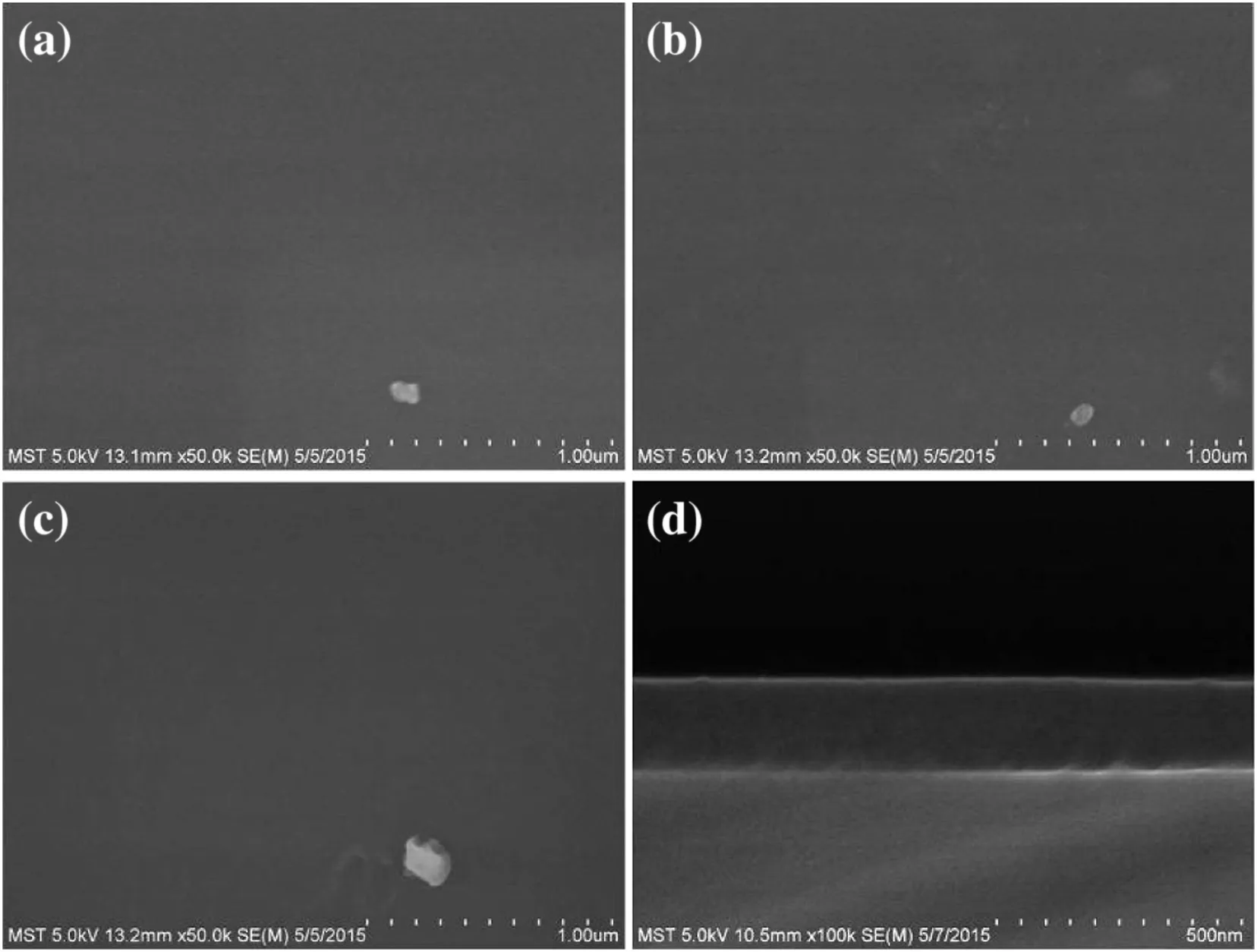
Fig.2.SEM images of Si wafer after PI deposition for 150(a),250(b),and 750(c)MLD cycles and the cross-sectional image(d)subjected to 750 MLD cycles.
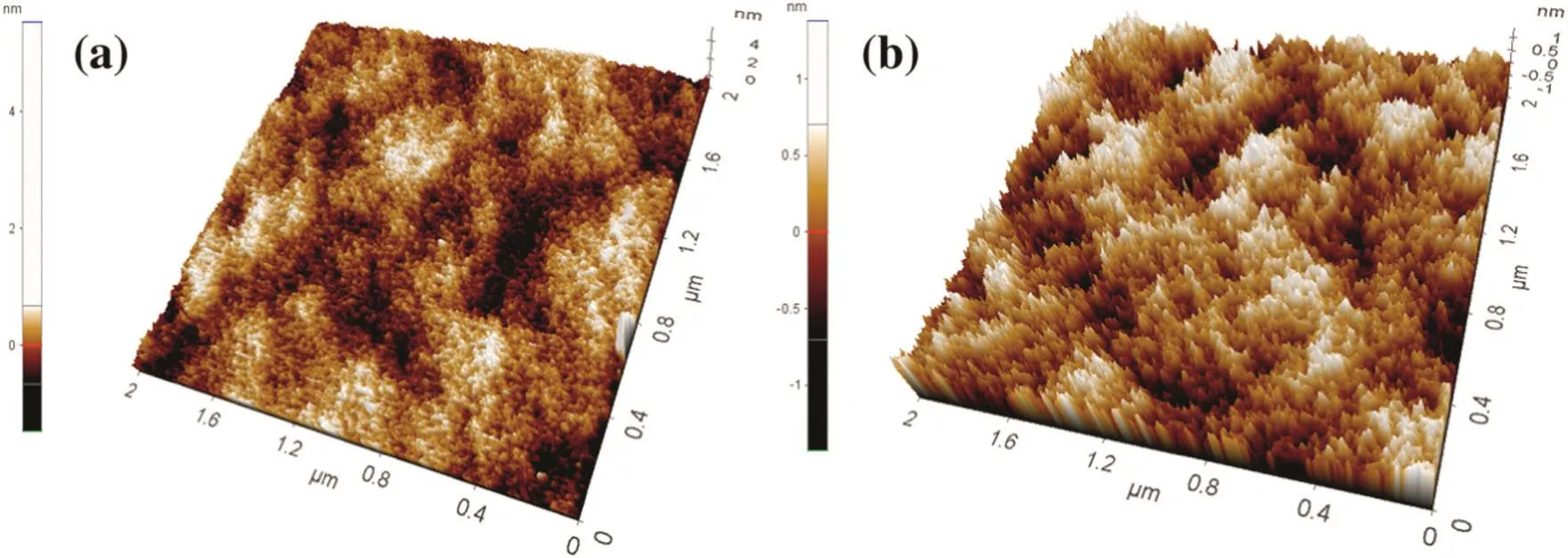
Fig.3.AFM images of pristine Si wafer(a)and after 500 MLD cycles(b).
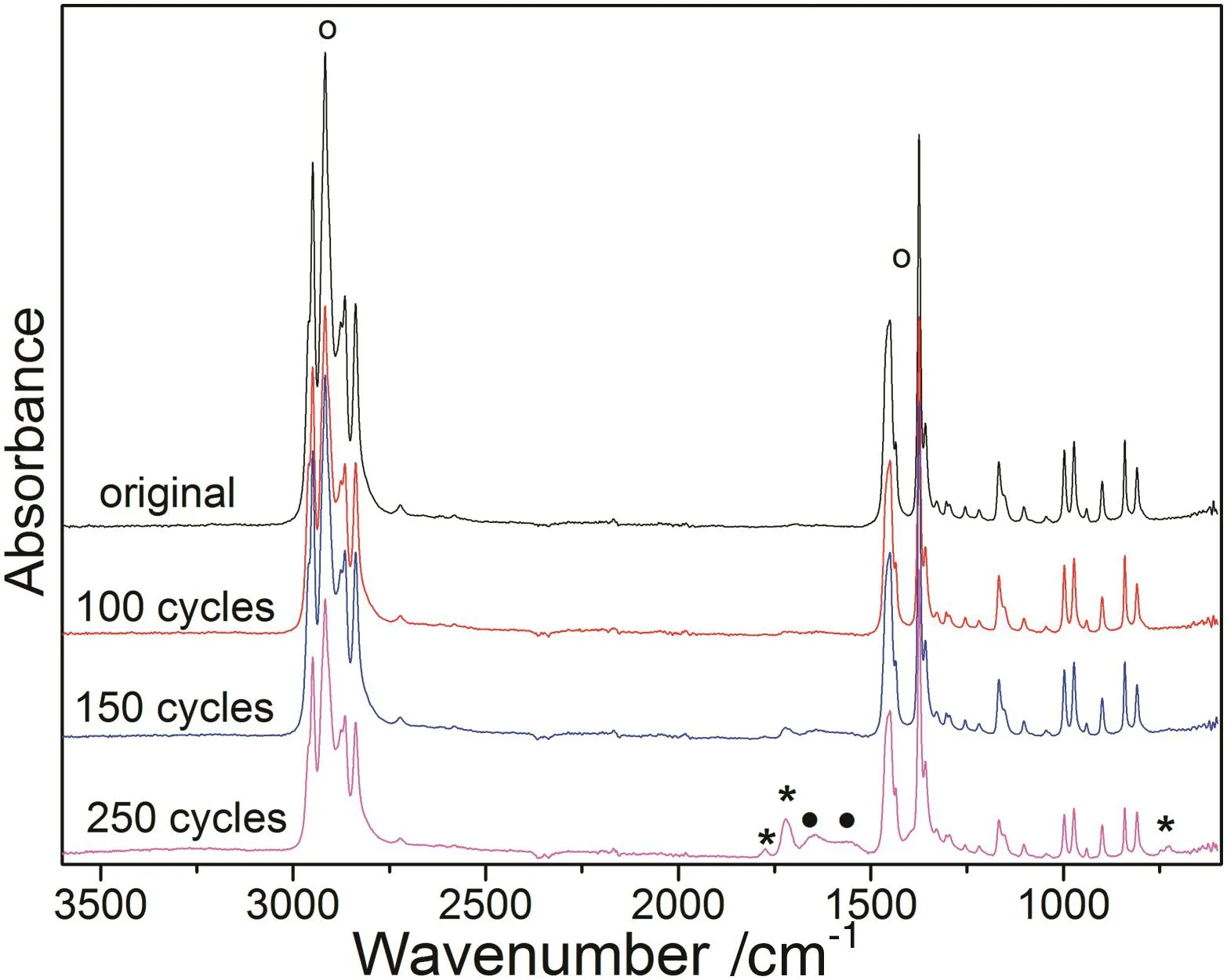
Fig.4.FTIR spectra of PI-deposited PP membranes subjected to different MLD cycles.
3.3.Surface morphology and hydrophilicity of PI-deposited PP membranes
Mostpolymeric materials are in shortage ofactive groups due to their inert surface,the deposition mechanism for MLD on polymeric substrate materials is henceforth significantly different from that on the inorganic materials[11,27].For inorganic substrate materials with abundant hydroxyl groups on the surface,a smooth yet highly conformal deposition layer with great uniformity can be obtained via MLD process.However,the deposition layer will grow as isolated island-like particles on the surface ofpolymeric substrates,such as PP,PES and polytetra fluoroethylene membranes,etc.due to some preferential nucleation sites on the subsurface regions[6,28].In ourwork here,one ofthe precursors was pulsed into the chamber and physicaladsorbed on the surface ofPPmembranes,followed by permeation onto the sub-surface regions in the duration of exposure mode.The globular particulates were then generated on the surface of PP membranes after the reactions with the other precursor pulsed into the chamber.Surface morphologies ofthe PP membranes before and after MLD deposition were then investigated by SEM as shown in Fig.5.It can be seen from Fig.5a that the pristine PP membranes showed a porous structure with stripe-shaped pores.Surface morphology displayed insignificant change and the PI-deposited PP membranes maintained the original smooth surface when the deposition cycles are less than 150 due to the ultralow amount of deposited PI on the surface ofPP membranes(Fig.5b–d).Smallparticulates appeared when the MLD cycle number reached 200;moreover,the quantity of the particles as well as the particle size further grew along with the increasing MLD cycle number(Fig.5e).The particulates entirely covered the skeleton of PP membranes with an average diameter of 25 nm when the deposition cycle reached 250(Fig.5f).The results suggested that the growth mechanism of deposition layers via MLD on inert porous polymeric surfaces is different from that on Si wafers.Dense,nonporous PP membranes obtained from hot press were also deposited for 250 cycles to compare the surface morphology(Fig.5g–h).The results indicated that the PI layers grew as globular particulates on the surface of dense PP membranes.Therefore it can be concluded that the growth pattern,i.e.,the island-shaped particles or smooth conformal layers produced on the substrates are mainly determined by the nature of the substrates rather than the surface roughness properties.
To further characterize the surface wettability of the PI-deposited PP membranes,water contact angles of PI-deposited PP membranes with different MLD cycles are presented in Fig.6.The initial contact angle of 111°implied strong hydrophobicity of the pristine PP membranes.Considering PI displays a relatively stronger hydrophilicity than that of PP membranes,a decrease in contact angle can be expected after the deposition ofPIfor differentcycles on the surface ofPP membranes.In the initial stage during MLD deposition of PI,i.e.,less than 150 cycles,the pore size barely changed due to the ultrathin layer;indicating that the in fluence on the surface wettability of the PP membranes by PI-deposition is negligible.After 200 MLD cycles of PI deposited on PP membranes,the water contact angle moderately dropped to 97°.Such change might be assigned to the ultrathin nature of PI deposition films on the surface of hydrophobic PP membranes when the MLD cycle number was less than 200,the amountofPIlayerwas insignificantto affectthe surface hydrophilicity,which was also confirmed by the SEM images.Gradually with the continuous growth of PI layer,a conformal layer appeared with great uniformity which was thick enough to thoroughly cover the membrane surface as well as the pore walls,and ultimately the more prominentdrop in contactangle can be realized.The relatively enhanced surface hydrophilicity of the deposited membranes can be primarily ascribed to the inherent hydrophilic nature of PI covering the membranes.
3.4.Stability of PI-deposited PP membranes
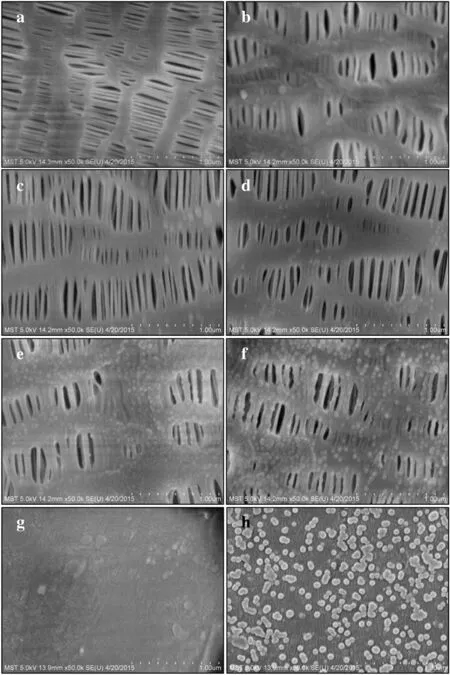
Fig.5.SEM images of porous(a–f)and nonporous(g–h)PP membranes before(a,and g)and after PI deposition with 50(b),100(c),150(d),200(e)and 250(f,h)MLD cycles.
Thermal stability is one of the most important factors in the evaluation of the performances of membrane materials.Composite membranes originating from PI with superior thermal stability and other polymeric materials could enhance the stability of membranes against harsh atmosphere.TGA curves of PI-deposited PP membranes as well as pristine PP membranes were compared and shown in Fig.7.The original PP membranes decomposed at the temperature of 435°C in the atmosphere of nitrogen.However,the PI-deposited PP membranes extended the decomposition temperature to 445 and 450°C after deposited for 100 and 250 cycles,respectively.The results suggested that thermal stability successfully developed after MLD deposition of PI on the surface of PP membranes comparing to the pristine membranes.The coating of PI will protect the PP membranes from thermal decomposition;moreover,larger quantity of PI results in better thermal resistance ofPI-deposited PP membranes.The residualmass percentage ofthe PI-deposited PP membranes also increased comparing to the pristine PP membranes as shown in the insetof Fig.7.PIwill carbonize during the thermal treatment;for instance,the residual mass percentage increased from 0.14%of the pristine PP membrane to 0.72%after the MLD deposition for 100 cycles[21].Further increment of MLD cycles to 250 results in the 0.96%residual mass percentage,which once again confirmed the deposition amount of PI on the surface of PP membranes increased with the MLD cycles.PP membranes have been widely applied in the field of lithium-ion battery separators;and the development of thermal stability will greatly improve the safety performances of the lithium-ion battery separators.

Fig.6.Water contact angle of PI-deposited PP membranes as a function of MLD cycle numbers.

Fig.7.TGA curves of PP membranes subjected to differentMLD cycles,the inset is the TGA curves in the temperature range of 480–600 C.
Furthermore,surface morphology of the PP membranes before and afterthe ultrasonication treatmentwascompared to investigate the stability of the PI-deposited PP membranes(Fig.8).The quantity of the particles on the surface was analyzed via Nanomeasurer and the results showed a slightdecline from 135 per μm2to 110 perμm2.Such insignificant decrease indicated that the mechanical stability of the PI-deposited PP membranes was successfully maintained even under harsh circumstances.Both the thermal and mechanical stability characterization confirmed that the PI-deposited PP membranes could withstand the environment with harsh conditions,which may thus find large scale applications in the field such as water treatment and pervaporation.
3.5.Permeation and separation performancesofPI-deposited PPmembranes
Pure water flux and retention of the PI-deposited PP membranes were then measured and the pristine PP membranes were used as references.Fig.9 shows the permeation and rejection of SiO2nanoparticles for PI-deposited PP membranes as a function ofMLD cycles ofPI.Surface hydrophilicity of the porous materials is one of the most important factors in determining the permeability of the membranes[29].As discussed in Section 3.3,the deposition of PI on the surface of PP membranes could improve the hydrophilicity ofPP membranes as a resultofthe moderately hydrophilic nature ofPIitself.Itcan be expected thatthe permeation of PI-deposited PP membranes would be somehow improved.As can be seen,the generalwater flux ofPI-deposited PP membranes displays a noticeably increment comparing to the pristine PP membranes with an original flux of 2440 L·m−2·h−1·MPa−1.For instance,the permeation enhanced 12%after PI deposited for merely 50 cycles.Water flux further increased 30%and maximized at 3150 L·m−2·h−1·MPa−1with the deposition cycle of150.With the prolonging ofMLDcycle repetition,namely more than 150,the increment of surface hydrophilicity slowed down(as shown in Fig.6),which is in consistence with the slightly decreased water flux after it reached peak value.As the continuously increasing in MLD cycle numbers,i.e.,200 and 250,water flux slightly dropped though it maintained an increment of 25%in a comparison of original PP membranes;for instance,200 and 250 cycles of PI deposition led to an overall water flux higher than 3000 L·m−2·h−1·MPa−1.Such trend in water permeability can be ascribed from the competing effects of the enhanced surface hydrophilicity and the declining effective pore size with the growing MLD cycles.Both effective pore size and surface hydrophilicity are crucial in determining the water permeation of the membranes[30].In our case here,for membranes with low MLD cycles,the surface hydrophilicity plays the major role whilst for membranes with high MLD cycles,the decline in pore size dominantly in fluences the permeation of the membranes compared to the increase in surface hydrophilicity.Nevertheless,the pore size of the PI-deposited PP membranes cannot provide a reliable conclusion due to the highly porous nature of the PP membranes.

Fig.9.Pure water flux and retention of PI-deposited PP membranes as a function of MLD cycle numbers.

Fig.8.SEM images of PP membranes before(a)and after(b)ultrasonication treatment.
The selectivity of the PI-deposited PP membranes was also investigated and it can be found from Fig.9 that the retention rate of PI-deposited membranes towards monodispersed 12-nm-SiO2nanosphereswasgenerally above 85%regardlessofthe PIMLDcycle number.Such high separation efficiency can be attributed to the ultrathin layerof PIdeposited films even athigh MLD cycles;more importantly,the effective pore size did not reduce to the adequate degree to evidently diminish the retention of 12-nm-SiO2comparing to the initial pore size of the pristine PP membranes.[29].
4.Conclusions
We demonstrate that molecular layer deposition(MLD)technique can be applied in the surface modification of polypropylene membranes at low temperature of 110°C.PP membranes without pre-treatment were utilized for the deposition of polyimide;surface hydrophilicity was therefore enhanced along with the growing number of MLD cycles.Increasing thickness ofPIdeposition layergave rise to the 30%increment of permeation of PI-deposited PP membranes.Meanwhile the overall separation efficiency of PI-deposited PP membranes was maintained higher than 85%towards monodispersed 12-nm-SiO2nanospheres.From practical point of view,the deposition of PI at lower temperature will enhance the thermal stability of the PP membranes in safety performances,which provides future application in the field oflithium-ion battery separators.
[1]J.S.Jur,J.C.Spagnola,K.Lee,B.Gong,Q.Peng,G.N.Parsons,Temperature-dependent subsurface growth during atomic layer deposition on polypropylene and cellulose fibers,Langmuir 26(2010)8239–8244.
[2]A.M.Schwartzberg,D.Olynick,Complex materials by atomic layer deposition,Adv.Mater.(2015).
[3]C.Detavernier,J.Dendooven,S.P.Sree,K.F.Ludwig,J.A.Martens,Tailoring nanoporous materials by atomic layer deposition,Chem.Soc.Rev.40(2011)5242–5253.
[4]C.A.Wilson,R.K.Grubbs,S.M.George,Nucleation and growth during Al2O3atomic layer deposition on polymers,Chem.Mater.17(2005)5625–5634.
[5]S.M.George,A.W.Ott,J.W.Klaus,Surface chemistry for atomic layer growth,J.Phys.Chem.100(1996)13121–13131.
[6]Q.Xu,Y.Yang,X.Wang,Z.Wang,W.Jin,J.Huang,Y.Wang,Atomic layer deposition of alumina on porous polytetra fluoroethylene membranes for enhanced hydrophilicity and separation performances,J.Membr.Sci.415(2012)435–443.
[7]Y.S.Jung,A.S.Cavanagh,L.Gedvilas,N.E.Widjonarko,I.D.Scott,S.H.Lee,G.H.Kim,S.M.George,A.C.Dillon,Improved functionality of lithium-ion batteries enabled by atomic layer deposition on the porous microstructure of polymer separators and coating electrodes,Adv.Energy Mater.2(2012)1022–1027.
[8]H.Chen,Q.Lin,Q.Xu,Y.Yang,Z.Shao,Y.Wang,Plasma activation and atomic layer deposition of TiO2on polypropylene membranes for improved performances of lithium-ion batteries,J.Membr.Sci.458(2014)217–224.
[9]D.K.Nandi,U.K.Sen,S.Sinha,A.Dhara,S.Mitra,S.K.Sarkar,Atomic layer deposited tungsten nitride thin films as a new lithium-ion battery anode,Phys.Chem.Chem.Phys.17(2015)17445–17453.
[10]C.Bugot,N.Schneider,M.Bouttemy,A.Etcheberry,D.Lincot,F.Donsanti,Study of atomic layer deposition of indium oxy-sulfidefilms for Cu(In,Ga)Se2solar cells,Thin Solid Films 582(2015)340–344.
[11]Q.Xu,J.Yang,J.Dai,Y.Yang,X.Chen,Y.Wang,Hydrophilization of porous polypropylene membranes by atomic layer deposition of TiO2for simultaneously improved permeability and selectivity,J.Membr.Sci.448(2013)215–222.
[12]F.B.Li,Y.Yang,Y.Q.Fan,W.H.Xing,Y.Wang,Modification of ceramic membranes for pore structure tailoring:The atomic layer deposition route,J.Membr.Sci.397(2012)17–23.
[13]P.W.Loscutoff,H.-B.-R.Lee,S.F.Bent,Deposition of ultrathin polythiourea films by molecular layer deposition,Chem.Mater.22(2010)5563–5569.
[14]C.Prasittichai,H.Zhou,S.F.Bent,Area selective molecular layer deposition of polyurea films,ACS Appl.Mater.Interfaces 5(2013)13391–13396.
[15]M.Putkonen,J.Harjuoja,T.Sajavaara,L.Niinisto,Atomic layer deposition of polyimide thin films,J.Mater.Chem.17(2007)664–669.
[16]B.T.Low,N.Widjojo,T.S.Chung,Polyimide/polyethersulfone dual-layer hollow fiber membranes for hydrogen enrichment,Ind.Eng.Chem.Res.49(2010)8778–8786.
[17]T.Sheng,H.Chen,S.Xiong,X.Q.Chen,Y.Wang,Atomic layer deposition of polyimide on microporous polyethersulfone membranes for enhanced and tunable performances,AIChE J.60(2014)3614–3622.
[18]T.Yoshimura,S.Tatsuura,W.Sotoyama,Polymer- films formed with monolayer growth steps by molecular layer deposition,Appl.Phys.Lett.59(1991)482–484.
[19]S.Yoshida,T.Ono,M.Esashi,Local electrical modification of a conductivityswitching polyimidefilm formed by molecular layer deposition,Nanotechnology 22(2011)9.
[20]L.D.Salmi,E.Puukilainen,M.Vehkamaki,M.Heikkila,M.Ritala,Atomic layer deposition of Ta2O5/polyimide nanolaminates,Chem.Vap.Depos.15(2009)221–226.
[21]P.Yang,G.Wang,Z.Gao,H.Chen,Y.Wang,Y.Qin,Uniform and conformal carbon nanofilms produced based on molecular layer deposition,Materials 6(2013)5602–5612.
[22]S.K.Panda,H.Shin,Step coverage in ALD,Atomic layer deposition of nanostructured materials,Wiley-VCH Verlag GmbH&Co.KGaA 2011,pp.23–40.
[23]Y.Du,S.M.George,Molecular layer deposition of nylon 66 films examined using in situ FTIR spectroscopy,J.Phys.Chem.C 111(2007)8509–8517.
[24]H.Yao,Y.Zhang,Y.Liu,K.You,S.Liu,B.Liu,S.Guan,Synthesis and properties of cross-linkable high molecular weight fluorinated copolyimides,J.Polym.Sci.A Polym.Chem.52(2014)349–359.
[25]X.D.Huang,S.M.Bhangale,P.M.Moran,N.L.Yakovlev,J.S.Pan,Surface modification studies of Kapton(R)HN polyimidefilms,Polym.Int.52(2003)1064–1069.
[26]Y.Tapiero,B.L.Rivas,J.Sanchez,M.Bryjak,N.Kabay,Polypropylene membranes modified with interpenetrating polymer networks for the removal of chromium ions,J.Appl.Polym.Sci.132(2015).
[27]J.D.Ferguson,A.W.Weimer,S.M.George,Atomic layer deposition of Al2O3films on polyethylene particles,Chem.Mater.16(2004)5602–5609.
[28]Q.Xu,Y.Yang,J.Yang,X.Wang,Z.Wang,Y.Wang,Plasma activation of porous polytetra fluoroethylene membranes for superior hydrophilicity and separation performances via atomic layer deposition of TiO2,J.Membr.Sci.443(2013)62–68.
[29]H.Chen,L.Kong,Y.Wang,Enhancing the hydrophilicity and water permeability of polypropylene membranes by nitric acid activation and metal oxide deposition,J.Membr.Sci.487(2015)109–116.
[30]Q.Wang,X.Wang,Z.Wang,J.Huang,Y.Wang,PVDF membranes with simultaneously enhanced permeability and selectivity by breaking the tradeoff effect via atomic layer deposition of TiO2,J.Membr.Sci.442(2013)57–64.
 Chinese Journal of Chemical Engineering2016年7期
Chinese Journal of Chemical Engineering2016年7期
- Chinese Journal of Chemical Engineering的其它文章
- Vanadium oxide nanotubes for selective catalytic reduction of NO x with NH3
- Optimal design for split-and-recombine-type flow distributors of microreactors based on blockage detection☆
- Theoreticalpredictions ofviscosity ofmethane under confined conditions☆
- Permeabilization of Escherichia coli with ampicillin for a whole cell biocatalyst with enhanced glutamate decarboxylase activity☆
- Formation of crystalline particles from phase change emulsion:In fluence of different parameters
- The effect of SiO2 particle size on iron based F–T synthesis catalysts
