低氧性肺动脉高压小鼠体内脂质水平的变化*
范风云, 沈婉婷, 郑青青, 高 媛, 胡凯媛, 范小芳, 毛孙忠, 龚永生
(温州医科大学低氧医学研究所, 浙江 温州 325035)
低氧性肺动脉高压小鼠体内脂质水平的变化*
范风云, 沈婉婷, 郑青青, 高 媛, 胡凯媛, 范小芳, 毛孙忠, 龚永生Δ
(温州医科大学低氧医学研究所, 浙江 温州 325035)
目的:观察低氧性肺动脉高压小鼠体内脂质代谢的变化,探讨脂质代谢异常在低氧性肺动脉高压发生发展中的意义。方法:SPF级雄性C57BL/6小鼠20只,随机分为2组(n=10):常氧组和低氧组。常压连续低氧3周(9%~11% O2,23 h/d)复制慢性低氧性肺动脉高压模型,测定小鼠右心室压(RVSP)和右心室与左心室加室间隔重量比[RV/(LV+S)],Elisa法检测血浆中总胆固醇(TC)、低密度脂蛋白(LDL)、高密度脂蛋白(HDL)的含量;real-time PCR法检测肝组织中3-羟基-3-甲基戊二酸单酰辅酶A还原酶(HMGCR)、低密度脂蛋白受体(LDLR)、清道夫受体B1(SR-B1)、固醇调节元件结合因子2(SREBF2)等基因的表达。结果:低氧组小鼠RVSP、RV/(LV+S)显著高于常氧组(P<0.05),血浆中HDL含量及HDL/LDL比值较常氧组显著降低(P<0.05),肝组织中LDLR、SR-B1基因表达较常氧组显著下调(P<0.05);RVSP与HDL/LDL比值及LDLR、SR-B1基因表达呈显著负相关(P<0.05)。结论:脂质代谢异常参与小鼠低氧性肺动脉高压的形成。
脂质代谢;肺动脉高压;低氧;小鼠
近年来,脂质代谢异常在肺动脉高压和肺血管重塑进程中的作用倍受关注。研究表明:低氧或高脂膳食更容易诱发脂质代谢相关基因如载脂蛋白E(apolipoprotein E,apoE)[1]、脂联素(adiponectin,APN)[2]、过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptor gamma,PPARγ)[3]等基因敲除小鼠肺血管重塑和肺动脉高压的形成,而应用APN[4]、PPARγ激动剂[5]可降低肺动脉高压和改善肺血管重塑,提示脂质代谢异常参与肺动脉高压的形成。但脂质代谢相关基因敲除小鼠本身就已存在脂质代谢异常,高脂膳食喂养亦可导致小鼠脂质代谢异常,有关单纯低氧诱导的肺动脉高压形成中是否存在脂质代谢异常的问题尚未明了。
本实验复制小鼠低氧性肺动脉高压模型,观察其血浆脂质含量及肝组织脂质代谢相关基因表达的变化,旨在探讨低氧性肺动脉高压的形成中脂质代谢的变化及可能的意义。
1 材料与方法
1.1 实验动物与试剂
SPF级雄性C57BL/6小鼠,体重20~25 g,由上海斯莱克实验动物有限责任公司提供,动物许可证号SCXK(沪2007-0005)。小鼠血浆总胆固醇(total cholesterol,TC)、低密度脂蛋白(low density lipoprotein,LDL)、高密度脂蛋白(high density lipoprotein,HDL)等Elisa试剂盒购自美国Phoenix Pharm Inc.;Quantscript RT Kit和Real Master Mix (Probe)购自天根生化科技(北京)有限公司。
1.2 动物模型的制备
SPF级雄性C57BL/6小鼠20只,随机分为2组(n=10):常氧组和低氧组。将低氧组小鼠置于常压低氧舱内(9%~11% O2,23 h/d),连续3周,常规饲料喂养;常氧组置于舱外,自由呼吸空气,其它饲养条件与低氧组相同。
1.3 动物处理与标本留取
动物饲养3周后,用异氟烷吸入麻醉,右心导管法经右侧颈外静脉插管至右心室,经PowerLab生理信号处理系统(AD Instruments Inc.,Australia)记录右心室收缩压(right ventricular systolic pressure,RVSP),RVSP可间接反映小鼠肺动脉压。然后,经腹动脉取血,装入预冷含肝素试管内,离心后取上清,-70℃保存待测血脂含量。
放血处死动物立即取出心肺,分别称取右心室(right ventricle,RV)和左心室加室间隔(left ventricle plus septum,LV+S)的重量,并计算出RV/(LV+S)的重量比作为反映右室肥大的指标。每组取6只小鼠的右肝组织约100 mg,用于提取总RNA,待测脂质代谢相关基因mRNA的表达水平。
1.4 血浆TC、LDL、HDL含量检测(Elisa法)
血浆TC、LDL、HDL含量通过Elisa试剂盒检测,步骤完全按照试剂盒说明书操作。
1.5 肝组织脂质代谢相关基因mRNA的表达水平检测(real-time PCR法)
Trizol一步法提取肝组织中总RNA,Quant Reverse Transcriptase一步法逆转录成cDNA,TaqMan探针法行实时定量PCR扩增。PCR反应条件:95℃预变性15 min,95℃变性10 s,60℃退火20 s,40个循环。每个样本做3个复孔,TaqMan探针由ABI公司提供。以10倍稀释模板梯度样本进行实时定量PCR反应,目标基因与内参照基因扩增效率接近100%情况下,以内参照基因β-actin基因作为标准进行相对定量,结果采用ΔΔCT法分析,计算低密度脂蛋白受体(low density lipoprotein receptor,LDLR),3-羟基-3-甲基戊二酸单酰辅酶A 还原酶(HMG-CoA reductase,HMGCR)、清道夫受体B1(scavenger receptor class B1,SR-B1)、固醇调节元件结合因子2(sterol regulatory element-binding factor-2,SREBF2)等基因的相对表达水平。
1.6 统计学处理
2 结果
2.1 低氧性肺动脉高压小鼠RVSP和RV/(LV+S)的变化
低氧小鼠组RVSP与RV/(LV+S)分别较常氧组高69.8%和50.7%(P<0.05,表1),提示低氧性肺动脉高压小鼠复制成功。
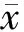
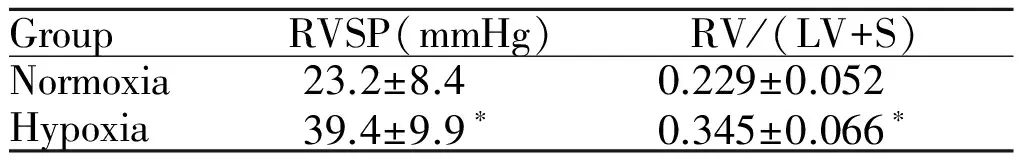
GroupRVSP(mmHg)RV/(LV+S)Normoxia23.2±8.40.229±0.052Hypoxia39.4±9.9∗0.345±0.066∗
RVSP: Right ventricular systolic pressure; RV/(LV+S): The weight ratio of right ventricle (RV) to left ventricle plus septum (LV+S)
*P<0.05vsnormoxia
2.2 低氧性肺动脉高压小鼠血脂含量的变化
低氧组小鼠血浆中TC、LDL含量与常氧组之间无显著差异(P>0.05);低氧组小鼠HDL含量、HDL/LDL比值较常氧组分别低17%和38%(P<0.05,表2)。
Tab. 2 Comparison of the concentrations of lipids in mice plasma between the two groups(±s,n=10)
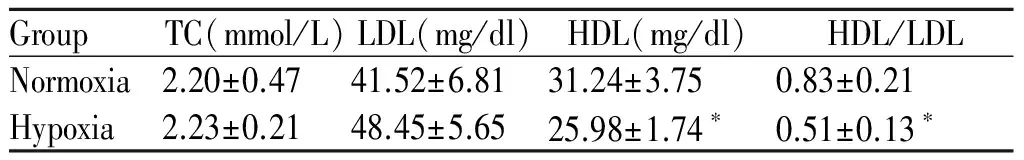
GroupTC(mmol/L)LDL(mg/dl)HDL(mg/dl)HDL/LDLNormoxia2.20±0.4741.52±6.8131.24±3.750.83±0.21Hypoxia2.23±0.2148.45±5.6525.98±1.74∗0.51±0.13∗
TC: Total cholesterol; LDL: Low density lipoprotein; HDL: High density lipoprotein
*P<0.05vsnormoxia
2.3 低氧性肺动脉高压小鼠肝组织脂质代谢相关基因表达的变化
低氧组小鼠肝组织中HMGCR、SREBF2基因表达与常氧组无显著性差异(P>0.05);与常氧组比较,低氧组小鼠肝组织中LDLR、SR-B1基因表达分别下调41%、53%(P<0.05,表3)。
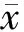
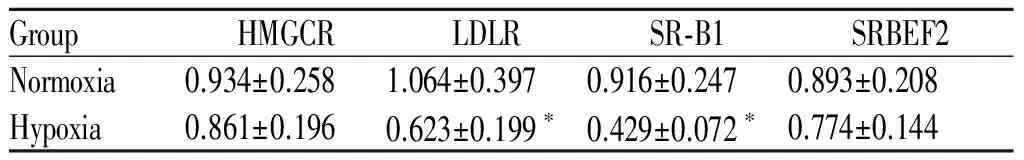
GroupHMGCRLDLRSR⁃B1SRBEF2Normoxia0.934±0.2581.064±0.3970.916±0.2470.893±0.208Hypoxia0.861±0.1960.623±0.199∗0.429±0.072∗0.774±0.144
HMGCR: HMG-CoA reductase; LDLR: Low density lipoprotein receptor; SR-B1: Scavenger receptor class B1; SREBF2: Sterol regulatory element-binding factor-2
*P<0.05vsnormoxia
2.4 相关性分析
RVSP与血浆中HDL/LDL比值以及肝组织中HMGCR,LDLR,SR-B1,SREBF2 mRNA表达的相关性分析表明:RVSP与HDL/LDL(r=-0.627,P=0.026)及LDLR(r=-0.757,P=0.006)、SR-B1(r=-0.934,P=0.001)基因表达呈显著负相关(P<0.05,图1),而与HMGCR(r=-0.32,P=0.168)、SREBF2(r=-0.38,P=0.122)基因表达无显著相关性。
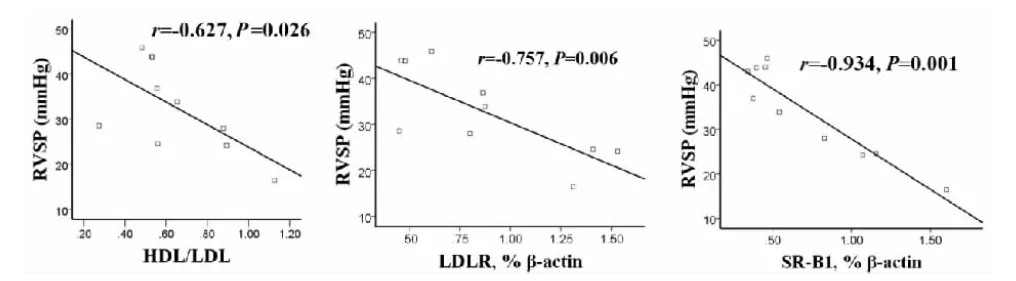
Fig. 1 Correlation analysis of RVSP and HDL/LDL, LDLR and SR-B1 between the two groups
3 讨论
临床和动物实验表明:肥胖、高脂血症、胰岛素抵抗与肺动脉高压、肺血管重塑的发生发展密切相关[6,7]。目前常在基因敲除小鼠水平探讨脂质代谢异常与肺动脉高压发生发展的关系,APN、PPARγ、apoE是常见的脂质代谢相关基因,研究发现APN或PPARγ基因敲除小鼠更容易诱发肺血管重塑、肺动脉高压,而外源性APN可以直接抑制肺血管平滑肌细胞增殖,在低氧和炎症诱导的肺动脉高压中具有抗炎和抗血管重塑的作用[4],PPARγ激动剂可降低肺动脉高压和改善肺血管重塑等作用[5];另外,Allan Lawrie等人用高脂膳食喂养apoE基因敲除小鼠发现该小鼠右心室收缩压显著增高、肺血管重塑明显[1],这些都提示脂质代谢异常参与肺动脉高压的发生发展。但脂质代谢相关基因敲除小鼠本身就存在脂质代谢异常,高脂膳食本身亦可导致小鼠脂质代谢异常,并不能真实反映脂质代谢异常在低氧性肺动脉高压形成中的作用。
实验结果显示:低氧小鼠组RVSP与RV/(LV+S)较常氧组显著升高,提示低氧性肺动脉高压小鼠复制成功[8]。HDL被认为是动脉粥样硬化的保护因素,血浆中HDL的含量与冠心病的发生呈负相关,因为HDL具有逆转运胆固醇、抗氧化、促纤溶、抗血栓等作用[9,10]。提示低氧性肺动脉高压小鼠血浆中脂质含量发生变化,HDL/LDL比值下调可能是引发低氧性肺动脉的因素之一。
血浆胆固醇的来源途径主要有两种:一是从食物中吸收的外源性胆固醇;另一种是肝脏中合成的内源性胆固醇。HMGCR是体内催化胆固醇内源性合成的关键酶,是一些抗动脉粥样硬化药物治疗靶点[11]。SRECF2是一类核转录因子,主要参与调节HMGCR等基因的转录[12],在胆固醇的合成途径中发挥重要作用。LDLR为一种膜镶嵌式蛋白质,该受体介导和调控LDL的胞吞作用,是摄取和清除LDL的关键受体,上调LDLR基因表达,可使血中LDL水平降低[13]。SR-B1是高密度脂蛋白受体,在肝脏中表达丰富,SR-B1介导HDL的胆固醇逆转运,并且具有清除氧化型LDL(ox-LDL)的作用[14]。
低氧组SR-B1、LDLR基因表达下调,提示胆固醇的清除减少,预示低氧肺动脉高压小鼠血浆LDL含量应该是上升的,但实验结果却显示:低氧组小鼠血浆LDL含量与常氧组相比虽然有少量升高但无显著性差异,这或许与低氧妨碍LDL的形成[15],或低氧诱导LDL氧化修饰为ox-LDL增加而LDL本身含量无明显变化[16]等因素有关。有关低氧对机体内LDL的形成、LDLR介导的胆固醇摄取与清除的影响及意义值得进一步深入研究。
综上所述,在低氧性肺动脉高压形成中小鼠体内脂质含量水平及脂质代谢相关基因表达水平发生变化,提示脂质代谢异常可能参与小鼠低氧性肺动脉高压的形成。
[1] Lawrie A, Hameed AG, Chamberlain J,etal. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner [J].AmJPathol, 2011, 179(4): 1693-1705.
[2] Summer R, Fiack CA, Ikeda Y,etal. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease [J].AmJPhysiolLungCellMolPhysiol, 2009, 297(3): L432-438.
[3] Hansmann G, Wagner RA, Schellong S,etal. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation [J].Circulation, 2007, 115(10): 1275-1284.
[4] Weng M, Raher MJ, Leyton P,etal. Adiponectin decreases pulmonary arterial remodeling in murine models of pulmonary hypertension [J].AmJRespirCellMolBiol, 2011, 45(2): 340-347.
[5] Nisbet RE, Bland JM, Kleinhenz DJ,etal. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model [J].AmJRespirCellMolBiol, 2010, 42(4): 482-490.
[6] Green DE, Sutliff RL, Hart CM. Is peroxisome proliferator-activated receptor gamma (PPARγ) a therapeutic target for the treatment of pulmonary hypertension [J]?PulmCirc, 2011, 1(1): 33-47.
[7] Zamanian RT, Hansmann G, Snook S,etal. Insulin resistance in pulmonary arterial hypertension [J].EurRespirJ, 2009, 33(2): 318-324.
[8] 李先伟, 杜 洁, 李元建. 降钙素基因相关肽对低氧性肺动脉高压肺动脉胶原沉积的影响[J]. 中国应用生理学杂志, 2013, 29(2): 182-186.
[9] Rohatgi A, Khera A, Berry JD,etal. HDL cholesterol efflux capacity and incident cardiovascular events [J].NEnglJMed, 2014, 371(25): 2383-2393.
[10]丁 宇, 司全金. 重组人生长激素对老年男性慢性心力衰竭患者血脂代谢影响的研究[J]. 中国应用生理学杂志, 2014, 30(3): 247-250.
[11]Peng P, Wang L, Yang X,etal. A preliminary study of the relationship between promoter methylation of the ABCG1, GALNT2 and HMGCR genes and coronary heart disease [J].PLoSOne, 2014, 9(8): e102265.
[12]Musso G, Cassader M, Bo S,etal. Sterol regulatory element-binding factor 2 (SREBF-2) predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism [J].Diabetes, 2013, 62(4):1109-1120.
[13]Martinelli N, Girelli D, Lunghi B,etal. Polymorphisms at LDLR locus may be associated with coronary artery disease through modulation of coagulation factor VIII activity and independently from lipid profile[J].Blood, 2010, 116(25):5688-5697.
[14]Gao M, Zhao D, Schouteden S,etal. Regulation of high-density lipoprotein on hematopoietic stem/progenitor cells in atherosclerosis requires scavenger receptor type BI expression[J].ArteriosclerThrombVascBiol, 2014, 34(9): 1900-1909.
[15]Douglas RM, Bowden K, Pattison J,etal. Intermittent hypoxia and hypercapnia induce pulmonary artery atherosclerosis and ventricular dysfunction in low density lipoprotein receptor deficient mice[J].JApplPhysiol(1985), 2013, 115(11): 1694-1704.
[16]Rydberg EK, Krettek A, Ullstrøm C,etal. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages [J].ArteriosclerThrombVascBiol, 2004, 24(11): 2040-2045.
Changes of lipid levels in mice with hypoxic pulmonary arterial hypertension
FAN Feng-yun, SHEN Wan-ting, ZHENG Qing-qing, GAO Yuan, HU Kai-yuan, FAN Xiao-fang, MAO Sun-zhong, GONG Yong-shengΔ
(Institute of Hypoxia Medicine, Wenzhou Medical University, Wenzhou 325035, China)
Objective: To observe the changes of lipid levels in mice with pulmonary hypertension induced by hypoxia. Methods: The animal model of hypoxic pulmonary hypertension was established by exposing the mice to isobaric hypoxic chamber for 3 weeks (23 h/d, regular chow feed). Twenty male C57BL/6 mice were randomly divided into normoxia group and hypoxia group (n=10). The concentrations of total cholesterol, low density lipoprotein (LDL)and high density lipoprotein (HDL) in plasma were detected by Elisa method.The mRNA levels of HMG-CoA reductase (HMGCR), low density lipoprotein receptor (LDLR), scavenger receptor class B1 (SR-B1), and sterol regulatory element-binding factor-2 (SREBF2) in liver were measured by real-time PCR. Results: ① The right ventricular systolic pressure (RVSP) and the weight ratio of right ventricle (RV) to left ventricle plus septum (LV+S) of hypoxia group were significantly higher than those of normoxia group (P<0.05).② The concentrations of HDL and HDL/LDL in plasma were significantly higher in hypoxia group, compared with normoxia group (P<0.05).③The mRNA levels of LDLR and SR-B1in liver were significantly down-regulated in hypoxia group (P<0.05).④RVSP were significantly negative correlated with HDL/LDL, the gene expression of LDLR and SR-B1 (P<0.05). Conclusion: Abnormal lipid metabolism participates in the pathological proceeding of pulmonary hypertension induced by hypoxia.
lipid metabolism; pulmonary hypertension; hypoxia; mice
浙江省自然基金(LY14H010005);浙江省大学生科技创新项目(2014R413029)
2015-06-23
2016-05-12
R363.2
A
1000-6834(2016)05-463-04
10.13459/j.cnki.cjap.2016.05.020
△【通讯作者】Tel: 0577-86699521; E-mail: fxbwzmc@126.com

