粪便菌群移植治疗艰难梭菌感染有效性和安全性的Meta分析
郑晗晗,江学良
作者单位:250000山东省济南市,济南军区总医院消化科
粪便菌群移植治疗艰难梭菌感染有效性和安全性的Meta分析
郑晗晗,江学良
作者单位:250000山东省济南市,济南军区总医院消化科
【摘要】目的探讨粪便菌群移植(FMT)治疗复发性、难治性、危重性艰难梭菌感染(CDI)的有效性和安全性。方法计算机检索PubMed、EMBase、Cochrane Library、中国期刊全文数据库,检索时间从建库至2015年5月。按照PICOS原则制定检索策略,对检索词同时进行主题词和自由词检索。提取文献相关信息,包括样本量、CDI类型、是否合并炎症性肠病(IBD)、年龄、捐赠者、移植方式、粪便制剂、随访时间等。采用英国国立临床优化研究所(NICE)推荐的对病例系列的质量评价清单评价纳入文献质量。结果纳入24篇文献,共743例患者,663例获得临床缓解,临床缓解率为89.2%,合并缓解率为87.6%〔95%CI(84.0%,90.4%)〕。亚组分析显示,经上消化道实施FMT的患者合并缓解率为81.5%〔95%CI(75.3%,86.5%)〕,低于经下消化道实施FMT患者的合并缓解率89.7%〔95%CI(85.5%,92.7%)〕,差异有统计学意义(P<0.05);采用冷冻制剂实施FMT的患者合并缓解率为85.5%〔95%CI(78.4%,90.6%)〕,与采用新鲜粪便制剂实施FMT患者的合并缓解率为88.3%〔95%CI(84.1%,91.5%)〕比较,差异无统计学意义(P>0.05);合并IBD患者合并缓解率为72.7%〔95%CI(53.1%,86.3%)〕,与未合并IBD患者的合并缓解率87.8%〔95%CI(84.6%,90.4%)〕比较,差异无统计学意义(P>0.05)。11篇文献发现了可能与FMT治疗相关的不良反应,如呕吐、发热、腹胀等,多为自限性,可在短时间内缓解。接受FMT治疗后死亡的患者中,死亡原因与患者本身其他疾病有关,没有证据证明与FMT治疗相关。结论FMT治疗复发性、难治性、危重CDI安全有效,且经下消化道实施FMT较上消化道有更高的临床缓解率。 1.3资料提取文献的审查和评价由2位作者独立完成,通过阅读文献题目及,排除无关文献,对其余文献进一步阅读和分析,决定最终是否能够入选,最后将纳入的文献是否合格进行交叉核对,出现分歧时讨论决定是否纳入。文献提取内容包括:题目、第一作者、发表时间、样本量、CDI类型、是否合并IBD、年龄、捐赠者、移植方式、粪便制剂、随访时间等。 2.1文献检索及质量评价检索出FMT治疗CDI的相关文献5 281篇,通过阅读文献题目及排除5 237篇,阅读全文后,纳入24篇[26-31,36-53]英文文献,国内尚无符合纳入标准的文献。文献筛选流程图见图1。纳入的24篇文献中,3篇文献[31,37,42]是多中心研究,4篇[27,28,36,51]没有清楚明确地描述研究的假说或目的、目标,4篇[31,36,41,51]没有清楚地报告纳入和排除标准,5篇[29,44,47,50-51]没有对测量的结局做出明确的定义,4篇[29,44-45,52]前瞻性地收集数据,9篇[26,28-29,38,41,44-45,47,52]准确地描述患者是连续招募的,只有1篇[36]文献没有清楚明确地描述研究的主要发现,没有文献对结局进行分层分析。多数文献NICE评分≥4分,属于高质量文献,5篇[27,36,39,50-51]在4分以下,属于低质量文献。纳入文献的基本特征见表1。
全球范围内,复发性和难治性艰难梭菌感染(CDI)发病率、病死率不断增加[1-4]。CDI初次发作后,30%的患者会复发,多次复发的患者,60%会再次复发[5-7]。危重CDI患者的病死率可高达58%[8-10]。而高毒菌株(NAP1/B1/027)的出现,使治疗的失败率继续增加[11]。目前,传统的治疗方案效果并不理想,非达霉素虽能降低复发率,但其费用较高,且对多次复发患者的疗效尚不明确[12-13]。
粪便菌群移植(FMT)能够重塑肠道菌群的多样性,使肠道功能得以恢复,从而抵御艰难梭菌及其产生的毒素[14- 15]。FMT可追溯至中国的东晋时期,距今约有1 700多年的历史[16-17],著名医药学家葛洪成功地用人粪清治疗食物中毒、腹泻、发热并濒临死亡的患者[18]。受药品安全及卫生的相关管理规定,FMT并未得到广泛应用及发展。直到1958年Eiseman等[19]报道了FMT成功治疗伪膜性肠炎患者,FMT的价值才逐渐得到认可。在随后的50多年中,不断有FMT成功治疗CDI的报道[20-31]。
目前FMT治疗CDI的相关研究有限,缺乏设计合理的大规模、多中心的随机对照试验,对FMT的长期有效性和安全性研究较少,对合适患者、粪便制剂与剂量、移植方式与次数的选择等缺乏统一的标准。仅有的循证医学证据[32]也存在主要结局指标定义不明确、样本量较少等缺陷。因此,本研究增大样本量,并对主要结局指标做出定义,进一步探讨FMT治疗CDI的有效性和安全性,并对不同移植方式、不同粪便制剂、是否合并炎症性肠病(IBD)的患者疗效进行亚组分析,为FMT的临床应用提供参考。
1资料与方法
1.1文献纳入与排除标准
1.1.1文献纳入标准(1)研究设计:接受FMT治疗的复发性或难治性或危重CDI患者;未设有对照组;≥10例的病例系列;(2)研究对象:复发性或难治性或危重CDI患者;伴或不伴感染(如IBD),有明确的患者临床特征及结局;(3)干预措施:FMT治疗,包括任何形式的FMT,如结肠镜、鼻胃管、灌肠等,不限制FMT次数和粪便剂型;(4)结局指标:以腹泻的临床缓解作为主要结局指标[33]。
FMT治疗后,患者的临床症状一般在治疗当时或48 h内出现缓解,未缓解的患者在3~7 d再次给予FMT治疗,因此,本研究综合以上原因并结合临床相关指南[33-34],将腹泻的临床缓解定义为:FMT治疗后大便次数减少或性状改善,且在7 d内不需其他干预治疗。在FMT治疗7 d后,缓解的患者出现症状复发或需要联合其他药物治疗才能控制症状或使用其他抗生素后再次出现症状者,也认为已获得了临床缓解。
1.1.2文献排除标准(1)使用非粪便来源的肠道细菌进行移植;(2)FMT治疗CDI阴性的抗生素相关性腹泻;(3)未报告FMT治疗结果;(4)会议摘要、读者来信、问卷调查;(5)联合其他药物治疗CDI。
1.2文献检索策略计算机检索PubMed、EMBase、Cochrane Library、中国期刊全文数据库,检索时间从建库至2015年5月。按照PICOS(Patients,Intervention,Comparisons,Outcomes,Study)原则制定检索策略,对检索词同时进行主题词和自由词检索。英文检索词为“clostridium”“clostridium difficile”“fecal microbiota transplant”;中文检索词为“艰难梭菌感染”“粪便菌群移植”。语种限制为中、英文。
1.4文献质量评价采用英国国立临床优化研究所(NICE)推荐的对病例系列的质量评价清单[32]评价纳入文献质量,包括以下内容:(1)为提高研究结果的代表性,病例系列中的病例最好来自不同级别的医疗机构,开展多中心研究;(2)清楚明确地描述研究的假说或目的、目标;(3)清楚地报告纳入和排除标准;(4)对测量的结局做出明确定义;(5)前瞻性收集数据;(6)准确地描述患者为连续招募;(7)清楚明确地描述研究的主要发现;(8)将结局进行分层分析及报告,如按照疾病分期、化验结果异常、患者的特征等。每项1分,≥4分的文献为高质量文献,否则为低质量文献。
1.5统计学方法采用MetaAnalyst Beta 3.13进行统计分析,采用I2和Q检验评价各文献异质性,当I2=0时,表明各文献的变异仅由抽样误差引起;I2<25%表明各文献存在轻度异质性,25%~50%表明有中度异质性,I2>50%或P<0.10时,认为各文献有较大的异质性。以腹泻的临床缓解作为主要的结局指标,采用随机效应模型[35]计算临床缓解率、合并缓解率。以P<0.05为差异有统计学意义。
2结果
2.2Meta分析结果
2.2.1合并缓解率各文献存在轻度异质性(I2=23%,P=0.156)。纳入文献共743例患者,663例获得临床缓解,临床缓解率为89.2%,合并缓解率为87.6%〔95%CI(84.0%,90.4%),见图2〕。
2.2.2不同移植方式的亚组分析9篇文献[26-27,30,36,43,47,49,51-52]经上消化道(胃镜、鼻胃管、鼻十二指肠管、内镜引导下经皮胃造瘘管、口服胶囊制剂)实施FMT,各文献无异质性(I2=0,P=0.933)。共192例患者,159例获得临床缓解,临床缓解率为82.8%,合并缓解率为81.5%〔95%CI(75.3%,86.5%),见图3〕。19篇文献[28-31,36-42,44-46,48-51,53]经下消化道(肠镜、灌肠)实施FMT,各文献有轻度异质性(I2=24%,P=0.170)。共551例患者,504例获得临床缓解,临床缓解率为91.5%,合并缓解率为89.7%〔95%CI(85.5%,92.7%),见图4〕。经下消化道实施FMT的合并缓解率高于经上消化道,合并缓解率之差为-8.2%〔95%CI(-14.2%,-2.2%)〕,差异有统计学意义(P<0.05)。

图1 文献筛选流程图

第一作者发表时间样本量(例)合并IBD(例)CDI类型平均年龄(岁)捐赠者移植方式粪便制剂随访时间NICE评分(分)Aas[26]2003180复发性73±9亲属、非亲属鼻胃管新鲜9年5Macconnachie[27]2009150复发性81.5非亲属鼻胃管新鲜平均16周2Yoon[38]2010120复发性、难治性66亲属结肠镜新鲜3周~8年5Rohlke[37]2010190复发性49亲属、非亲属结肠镜新鲜平均27.2个月5Garborg[36]2010402复发性75亲属胃镜、结肠镜新鲜80d1Mellow[28]2011130复发性、难治性63.5亲属结肠镜新鲜平均5.3个月4Jorup-Ronstrom[39]2012321复发性75非亲属灌肠冷冻1~68个月3Kelly[41]2012260复发性59亲属结肠镜新鲜平均10.7个月4Hamilton[29]20124314复发性NR非亲属结肠镜冷冻、新鲜2个月5Kassam[40]2012270复发性、难治性69.4非亲属灌肠新鲜平均427d4Mattila[42]2012700复发性73亲属、非亲属结肠镜新鲜12周~12个月5Rubin[43]2013750复发性63非亲属鼻胃管、胃镜、PEG新鲜60d4Pathak[49]2014121复发性NR亲属、非亲属结肠镜、NDT新鲜2~30个月4Russell[51]2014103复发性NR亲属鼻胃管、结肠镜新鲜1个月~4年1Youngster[52]2014200复发性、难治性64.5非亲属口服胶囊制剂冷冻2~6个月6Zainah[30]2014140复发性、危重性73.4±11.9亲属、非亲属鼻胃管、结肠镜新鲜7~100d4Ray[50]2014201复发性、难治性62亲属结肠镜新鲜3.2个月3Kronman[47]2014103复发性5.4亲属、非亲属鼻胃管新鲜平均44d4Lee[48]2014940复发性、难治性71.8非亲属灌肠新鲜6~24个月4Dutta[45]2014270复发性64.5亲属、非亲属结肠镜、小肠镜新鲜9.7~34.0个月6Allegretti[44]2014229复发性、难治性NR亲属、非亲属结肠镜新鲜平均3个月5Khan[46]2014200复发性、难治性NR亲属、非亲属结肠镜新鲜3~6个月4Satokari[53]2015490复发性NR亲属、非亲属结肠镜新鲜、冷冻12周~1年4Tvede[31]2015550复发性65.6非亲属灌肠冷冻30d4
注:CDI=艰难梭菌感染,IBD=炎症性肠病,NR=没有报道,PEG=内镜引导下经皮胃造瘘管,NDT=鼻十二指肠管,NICE=英国国立临床优化研究所
Figure 2Forest plot for clinical remission rates of CDI patients treated by FMT

图3 经上消化道实施FMT治疗CDI临床缓解率的森林图
Figure 3Forest plot of clinical remission rates of CDI patients treated by upper gastrointestinal FMT

图4 经下消化道实施FMT治疗CDI临床缓解率的森林图
Figure 4Forest plot of clinical remission rates of CDI pateints treated by lower gastrointestinal FMT
2.2.3不同粪便制剂的亚组分析5篇文献[29,31,39,52-53]采用冷冻制剂(冷冻的粪便混悬液、冷冻的胶囊制剂、冷冻培养的粪便菌株)实施FMT,各文献无异质性(I2=0,P=0.566)。共142例患者,123例获得临床缓解,临床缓解率为86.6%,合并缓解率为85.5%〔95%CI(78.4%,90.6%),见图5〕。21篇文献[26-30,36-38,40-51,53]采用新鲜粪便制剂实施FMT,各文献有中度异质性(I2=26%,P=0.131)。共601例患者,540例获得临床缓解,合并缓解率为88.3%〔95%CI(84.1%,91.5%),见图6〕。采用冷冻制剂与新鲜制剂实施FMT的合并缓解率之差为-2.8%〔95%CI(-3.4%,9.0%)〕,差异无统计学意义(P>0.05)。

图5 冷冻制剂治疗CDI临床缓解率的森林图
Figure 5Forest plot of clinical remission rates of CDI pateints treated by frozen feces preparation

图6 新鲜制剂治疗CDI临床缓解率的森林图
Figure 6Forest plot of clinical remission rates of CDI patients treated by fresh feces preparation
2.2.4是否合并IBD的亚组分析8篇文献[29,36,39,44,47,49-51]纳入的部分患者合并IBD,各文献有轻度异质性(I2=2%,P=0.413)。共34例患者,27例患者获得临床缓解,临床缓解率为79.4%,合并缓解率为72.7%〔95%CI(53.1%,86.3%),见图7〕。24篇文献[26-31,36-53]纳入的部分患者未合并IBD,各文献有轻度异质性(I2=11%,P=0.310)。共纳入709例患者,636例获得临床缓解,临床缓解率为89.7%,合并缓解率为87.8%〔95%CI(84.6%,90.4%),见图8〕。合并IBD与未合并IBD患者合并缓解率之差为-15.1%〔95%CI(-1.0%,30.3%)〕,差异无统计学意义(P>0.05)。
2.3不良反应11篇文献[26-27,29,31,44-45,47-48,50,52-53]发现了可能与FMT治疗相关的不良反应,如呕吐、发热、腹胀等,多为自限性,可在短时间内缓解(见表2)。接受FMT治疗后死亡的患者中,死亡原因与患者本身其他疾病有关,如恶性肿瘤、心血管疾病、器官功能衰竭等,没有证据证明与FMT治疗相关。在Aas等[26]研究中,1例患者经FMT治疗后,症状没有改善,在FMT治疗后的第3天发展为腹膜炎,并且很快死亡。该例患者在接受FMT时已患有严重的终末期肾病,需要腹膜透析治疗,因此难以说明其死亡与FMT有关。

图7 FMT治疗CDI合并IBD的临床缓解率的森林图
Figure 7Forest plot of clinical remission rates of CDI patients combined with IBD treated by FMT
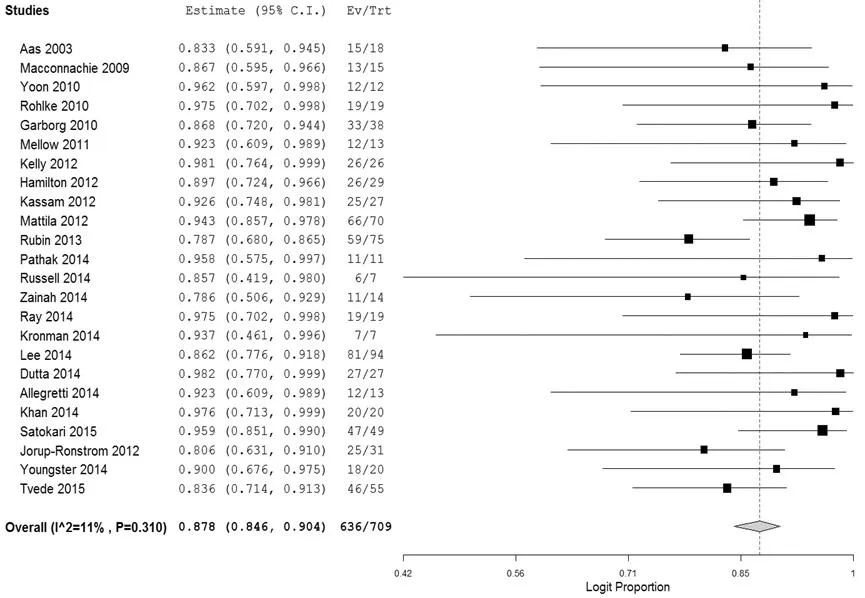
图8 FMT治疗CDI未合并IBD的临床缓解率的森林图
Figure 8Forest plot of clinical remission rates of CDI patients without IBD treated by FMT

表2 FMT治疗CDI的不良反应
注:NR=没有报道
3讨论
本研究采用NICE质量评价工具对纳入的病例系列进行质量评价,虽然病例系列研究在治疗疗效评价中的证据级别低于随机对照试验、类试验,但本研究纳入的多数文献质量评分在4分及以上,为高质量研究,仅5篇低于4分,文献总体质量较高。
FMT治疗复发性、难治性或危重性CDI患者是有效的。本研究发现,FMT治疗CDI的临床缓解率为89.2%,合并缓解率为87.6%,与Kassam等[32]Meta分析结果(临床缓解率为89.7%,合并缓解率为89.1%)相近,低于Sofi等[54]研究的合并缓解率91.2%,分析原因可能为本研究纳入的样本量大,部分患者合并其他胃肠道疾病等危险因素有关。
FMT治疗复发性、难治性或危重CDI患者是安全的。FMT通过将健康者的粪便悬液或粪便菌群注入患者胃肠道,以达到修复患者受损的肠道菌群的目的,虽有一些轻度不良反应的发生,如腹痛、发热、恶心、呕吐等,但均在短时间内得到缓解,未发生与FMT治疗相关的严重不良反应。患者对FMT的耐受性好,未见因FMT传播疾病的报道。
本研究发现,经下消化道实施FMT较上消化道有更高的临床缓解率。分析可能的原因为:(1)若患者病情较重,通过结肠镜实施FMT有诱发肠穿孔的风险,因此会选择经上消化道实施FMT[30]。故经上消化道实施FMT治疗的患者病情通常较经下消化道实施患者严重;(2)经上消化道注入的粪便制剂量较少;(3)经下消化道实施FMT,可以使粪便菌群免受胃酸及胰酶的破坏,且直接作用于病变的肠道黏膜,使移植的粪便菌群最大限度发挥作用。近期一项研究发现,通过结肠镜实施FMT治疗CDI比万古霉素或非达霉素更为经济有效[55]。
本研究未发现使用冷冻制剂与新鲜制剂的患者临床缓解率存在差异,分析可能的原因为冷冻制剂和新鲜制剂有相同的有效菌株,虽然冷冻制剂经过冷冻储存,但其有效菌株并没有被破坏。FMT治疗CDI的有效菌株尚不明确,仍需进一步研究。
本研究同样未发现CDI合并IBD与未合并IBD患者的临床缓解率存在差异,分析可能的原因为CDI和IBD均涉及肠道菌群结构的改变,FMT对CDI和IBD均发挥作用。最近发表的FMT治疗活动性溃疡性结肠炎的随机对照试验显示,FMT治疗组比安慰剂组有更高的临床缓解率,并且差异有统计学意义,经FMT治疗后患者的肠道菌群多样性较治疗前增加[56]。
本研究局限性:(1)纳入文献均来自国外,研究对象以欧美人群居多,使研究结论在我国患者的推广受到限制;(2)病例系列研究论证强度低于随机对照试验,在一定程度上增加了偏倚,可能会使缓解率增大。本研究纳入文献的患者均为10例及以上,减少个案报道和小病例系列的偏倚,且是目前样本量最大的FMT治疗CDI有效性的Meta分析,首次探讨了不同制剂、是否合并IBD患者临床缓解率的差异。在FMT广泛应用于治疗CDI之前,期待设计合理的大样本随机对照试验进行进一步研究。
作者贡献:郑晗晗进行课题设计、资料收集整理、数据分析、撰写论文;江学良参与课题设计,进行质量控制及审校。各作者均对文章负责。
本文无利益冲突。
参考文献
[1]Wendt JM,Cohen JA,Mu Yi,et al.Clostridium difficile infection among children across diverse US geographic locations[J].Pediatrics,2014,133(4):651-658.
[2]Burke KE,Lamont JT.Clostridium difficile infection:a worldwide disease[J].Gut and Liver,2014,8(1):1-6.
[3]Khanna S,Baddour L,Huskins WC,et al.The epidemiology of clostridium difficile infection in children:a population-based study[J].Clin Infect Dis,2013,56(10):1401-1406.
[4]Rupnik M,Wilcox MH,Gerding DN.Clostridium difficile infection:new developments in epidemiology and pathogenesis[J].Nat Rev Microbiol,2009,7(7):526-536.
[5]Louie TJ,Miller MA,Mullane KM,et al.Fidaxomicin versus vancomycin for Clostridium difficile infection[J].N Engl J Med,2011,364(5):422-431.
[6]Pépin J,Valiquette L,Gagnon S,et al.Outcomes of clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027[J].Am J Gastroenterol,2007,102(12):2781-2788.
[7]Vardakas KZ,Polyzos KA,Patouni K,et al.Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole:a systematic review of the evidence[J].Int J Antimicrob Agents,2012,40(1):1-8.
[8]Ramaswamy R,Grover H,Corpuz M,et al.Prognostic criteria in Clostridium difficile colitis[J].Am J Gastroenterol,1996,91(3):460-464.
[9]Rubin MS,Bodenstein LE,Kent KC.Severe clostridium difficile colitis[J].Dis Colon Rectum,1995,38(4):350-354.
[10]Lamontagne F,Labbé AC,Haeck O,et al.Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain[J].Ann Surg,2007,245(2):267-272.
[11]Petrella LA,Sambol SP,Cheknis A,et al.Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C.difficile BI strain[J].Clin Infect Dis,2012,55(3):351-357.
[12]Crook DW,Walker AS,Kean Yin,et al.Fidaxomicin versus vancomycin for Clostridium difficile infection:meta-analysis of pivotal randomized controlled trials[J].Clin Infect Dis,2012,55(S2):S93-103.
[13]Bartsch SM,Umscheid CA,Fishman N,et al.Is fidaxomicin worth the cost? An economic analysis[J].Clin Infect Dis,2013,57(4):555-561.
[14]Chang JY,Antonopoulos DA,Kalra A,et al.Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea[J].J Infect Dis,2008,197(3):435-438.
[15]Khoruts A,Dicksved J,Jansson JK,et al.Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea[J].J Clin Gastroenterol,2010,44(5):354-360.
[16]Zhang F,Luo W,Shi Y,et al.Should we standardize the 1,700-year-old fecal microbiota transplantation?[J].Am J Gastroenterol,2012,107(11):1755.
[17]张发明.粪菌移植的概念、历史、现状和未来[J].中国内镜杂志,2012,18(9):930-934.
[18]葛洪(东晋).肘后备急方[M].天津:天津科技出版社,2000:36.
[19]Eiseman B,Silen W,Bascom GS,et al.Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis[J].Surgery,1958,44(5):854-859.
[20]Bowden TA,Mansberger AR,Lykins LE.Pseudomembraneous enterocolitis:mechanism for restoring floral homeostasis[J].Am Surg,1981,47(4):178-183.
[21]Schwan A,Sjölin S,Trottestam U,et al.Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces[J].Lancet,1983,2(8354):845.
[22]Russell G,Kaplan J,Ferraro M,et al.Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child:a proposed treatment protocol[J].Pediatrics,2010,126(1):e239-242.
[23]Zhang FM,Wang HG,Wang M,et al.Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn′s disease[J].World J Gastroenterol,2013,19(41):7213-7216.
[24]余超,周秀华.粪菌灌肠治疗重症监护病房艰难梭菌感染腹泻1例报告并文献复习[J].中国中西医结合急救杂志,2013,20(4):309-310.
[25]Xiao YM,Wang JY,Che YR,et al.One case of pediatric severepseudomembranous enteritis treated with fecal microbiota transplantation and literature review[J].Chinese Journal of Evidence Based Pediatrics,2014,9(1):37-40.(in Chinese)
肖咏梅,王佳怡,车艳然,等.粪便微生物移植治疗幼儿重症伪膜性肠炎1例并文献复习[J].中国循证儿科杂志,2014,9(1):37-40.
[26]Aas J,Gessert CE,Bakken JS.Recurrent clostridium difficile colitis:case series involving 18 patients treated with donor stool administered via a nasogastric tube[J].Clin Infect Dis,2003,36(5):580-585.
[27]Macconnachie AA,Fox R,Kennedy DR,et al.Faecal transplant for recurrent Clostridium difficile-associated diarrhoea:a UK case series[J].QJM,2009,102(11):781-784.
[28]Mellow MH,Kanatzar A.Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection-results and follow-up[J].J Okla State Med Assoc,2011,104(3):89-91.
[29]Hamilton MJ,Weingarden AR,Sadowsky MJ,et al.Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection[J].Am J Gastroenterol,2012,107(5):761-767.
[30]Zainah H,Hassan M,Shiekh-Sroujieh L,et al.Intestinal microbiota transplantation,a simple and effective treatment for severe and refractory Clostridium difficile infection[J].Dig Dis Sci,2014,60(1):181-185.
[31]Tvede M,Tinggaard M,Helms M.Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea:results from a case series of 55 patients in Denmark 2000-2012[J].Clin Microbiol Infect,2015,21(1):48-53.
[32]Kassam Z,Lee CH,Yuan Y,et al.Fecal microbiota transplantation for Clostridium difficile infection:systematic review and meta-analysis[J].Am J Gastroenterol,2013,108(4):500-508.
[33]Debast SB,Bauer MP,Kuijper EJ,et al.European society of clinical microbiology and infectious diseases:update of the treatment guidance document for clostridium difficile infection[J].Clin Microbiol Infect,2014,20(S2):1-26.
[34]Crobach MJ,Dekkers OM,Wilcox MH,et al.European society of clinical microbiology and infectious diseases (ESCMID):data review and recommendations for diagnosing clostridium difficile-infection (CDI)[J].Clin Microbiol Infect,2009,15(12):1053-1066.
[35]DerSimonian R,Laird N.Meta-analysis in clinical trials [J].Control Clin Trials,1986,7(3) :177-188.
[36]Garborg K,Waagsbø B,Stallemo A,et al.Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea[J].Scand J Infect Dis,2010,42(11/12):857-861.
[37]Rohlke F,Surawicz CM,Stollman N.Fecal flora reconstitution for recurrent Clostridium difficile infection:results and methodology[J].J Clin Gastroenterol,2010,44(8):567-570.
[38]Yoon SS,Brandt LJ.Treatment of refractory/recurrent C.difficile-associated disease by donated stool transplanted via colonoscopy:a case series of 12 patients[J].J Clin Gastroenterol,2010,44(8):562-566.
[39]Jorup-Ronstrom C,Håkanson A,Sandell S,et al.Fecal transplant against relapsing Clostridium difficile-associated diarrhea in 32 patients[J].Scand J Gastroenterol,2012,47(5):548-552.
[40]Kassam Z,Hundal R,Marshall JK,et al.Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection[J].Arch Intern Med,2012,172(2):191-193.
[41]Kelly CR,De Leon L,Jasutkar N.Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients:methodology and results[J].J Clin Gastroenterol,2012,46(2):145-149.
[42]Mattila E,Uusitalo-Seppälä R,Wuorela M,et al.Fecal transplantation,through colonoscopy,is effective therapy for recurrent Clostridium difficile infection[J].Gastroenterology,2012,142(3):490-496.
[43]Rubin TA,Gessert CE,Aas J,et al.Fecal microbiome transplantation for recurrent Clostridium difficile infection:report on a case series[J].Anaerobe,2013,19:22-26.
[44]Allegretti JR,Korzenik JR,Hamilton MJ.Fecal microbiota transplantation via colonoscopy for recurrent C.difficile infection[J].J Vis Exp,2014(94).doi:10.3791/52154.
[45]Dutta SK,Girotra M,Garg S,et al.Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection[J].Clin Gastroenterol Hepatol,2014,12(9):1572-1576.
[46]Khan MA,Sofi AA,Ahmad U,et al.Efficacy and safety of,and patient satisfaction with,colonoscopic-administered fecal microbiota transplantation in relapsing and refractory community- and hospital-acquired Clostridium difficile infection[J].Can J Gastroenterol Hepatol,2014,28(8):434-438.
[47]Kronman MP,Nielson HJ,Adler AL,et al.Fecal microbiota transplantation via nasogastric tube for recurrent clostridium difficile infection in pediatric patients[J].J Pediatr Gastroenterol Nutr,2014,60(1):23-26.
[48]Lee CH,Belanger JE,Kassam Z,et al.The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema[J].Eur J Clin Microbiol Infect Dis,2014,33(8):1425-1428.
[49]Pathak R,Enuh HA,Patel A,et al.Treatment of relapsing Clostridium difficile infection using fecal microbiota transplantation[J].Clin Exp Gastroenterol,2014,7(1):1-6.
[50]Ray A,Smith R,Breaux J.Fecal microbiota transplantation for clostridium difficile infection:the ochsner experience[J].Ochsner J,2014,14(4):538-544.
[51]Russell GH,Kaplan JL,Youngster I,et al.Fecal transplant for recurrent Clostridium difficile infection in children with and without inflammatory bowel disease[J].J Pediatr Gastroenterol Nutr,2014,58(5):588-592.
[52]Youngster I,Russell GH,Pindar C,et al.Oral,capsulized,frozen fecal microbiota transplantation for relapsing Clostridium difficile infection[J].JAMA,2014,312(17):1772-1778.
[53]Satokari R,Mattila E,Kainulainen V,et al.Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection-an observational cohort study[J].Aliment Pharmacol Ther,2015,41(1):46-53.
[54]Sofi AA,Silverman AL,Khuder S,et al.Relationship of symptom duration and fecal bacteriotherapy in Clostridium difficile infection-pooled data analysis and a systematic review[J].Scand J Gastroenterol,2013,48(3):266-273.
[55]Konijeti GG,Sauk J,Shrime MG,et al.Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection:a decision analysis[J].Clin Infect Dis,2014,58(11):1507-1514.
[56]Moayyedi P,Surette MG,Kim PT,et al.Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial[J].Gastroenterology,2015,149(1):102-109.
(本文编辑:吴立波)
·医学循证·
【关键词】难辨梭菌;粪便菌群移植;有效性研究;Meta分析
郑晗晗,江学良.粪便菌群移植治疗艰难梭菌感染有效性和安全性的Meta分析[J].中国全科医学,2016,19(2):199-205.[www.chinagp.net]
Zheng HH,Jiang XL.Fecal microbiota transplantation for clostridium difficile infection:a meta-analysis[J].Chinese General Practice,2016,19(2):199-205.
Fecal Microbiota Transplantation for Clostridium Difficile Infection:A Meta-analysisZHENGHan-han,JIANGXue-liang.DepartmentofGastroenterology,theGeneralHospitalofJi′nanMilitaryCommand,Ji′nan250000,China
【Abstract】ObjectiveTo investigate the efficacy and safety of fecal microbiota transplantation(FMT) in recurrent,refractory and severe clostridium difficile infection(CDI).MethodsA computer-based research was conducted on PubMed,EMBase,Cochrane Library and CNKI,with the research time set from database establishment to May 2015.Searching strategy was made based on PICOS principle,and keywords were searched as both subject term and free term.Relevant information was extracted from literatures,including sample volume,CDI type,whether combined with IBD,age,donator,transplanting methods,feces preparation,follow-up period,etc.The quality evaluation inventory for cases recommended by National Institute for Health and Clinical Excellence (NICE) of Britain was employed in the literature quality evaluation.ResultsA total of 24 pieces of literature were included,and there were 743 patients concerned,of which 663 patients had clinical remission with a clinical remission rate of 89.2% and a merged remission rate of 87.6%〔95%CI(84.0%,90.4%)〕.The subgroup analysis showed that the merging remission rate of patients who received FMT through upper gastrointestinal tract was 81.5%〔95%CI(75.3%,86.5%)〕,lower (P<0.05) than that of patients who received FMT through lower gastrointestinal tract,which was 89.7%〔95%CI(85.5%,92.7%)〕;the merging remission rate of patients who underwent FMT using frozen feces preparation was 85.5%〔95%CI(78.4%,90.6%)〕,and the merging remission rate of patients who underwent FMT using fresh feces preparation was 88.3%〔95%CI(84.1%,91.5%)〕,without significant difference between them(P>0.05).The merging remission rate of patients combined with IBD was 72.7%〔95%CI(53.1%,86.3%)〕,and that of patients without IBD combined was 87.8%〔95%CI(84.6%,90.4%)〕,without significant differences between them (P>0.05).Adverse reactions that may be related with FMT treatment were found in 11 pieces of literatures,such as vomit,fever,abdominal distension,and most symptoms were self-confined and could remit soon.Among patients who died after FMT treatment,the cause of death was related with other diseases of these patients,and there were no evidences of the relation between FMT treatment and their death.ConclusionFMT is safe and effective for treating recurrent,refractory,and severe CDI.Lower gastrointestinal FMT delivery lead to higher clinical remission rate.
【Key words】Clostridium difficile;Fecal microbiota transplantation;Validation studies;Meta-analysis
收稿日期:(2015-06-23;修回日期:2015-11-25)
【中图分类号】R 378.8
【文献标识码】A
doi:10.3969/j.issn.1007-9572.2016.02.016
通信作者:江学良,250000山东省济南市,济南军区总医院消化科;E-mail:jiangxueliang678@126.com

