以硝基对苯二甲酸和4,4′-联吡啶为配体的四个金属Ag配合物的合成、结构多样性和电子光谱
马爱青 朱龙观
(1浙江大学化学系,杭州310027)
(2广东医学院药学院,东莞523808)
以硝基对苯二甲酸和4,4′-联吡啶为配体的四个金属Ag配合物的合成、结构多样性和电子光谱
马爱青1,2朱龙观*,1
(1浙江大学化学系,杭州310027)
(2广东医学院药学院,东莞523808)
由2-硝基-1,4-对苯二甲酸和4,4′-联吡啶作为起始原料合成了4个金属银的配位聚合物,{[Ag(4,4′-bipy)]·2-Hnbdc·2H2O ·CH3OH}n(1),{[Ag(4,4′-bipy)(2-Hnbdc)]}n(2),{[Ag2(4,4′-bipy)2(2-nbdc)]·2H2O}n(3),和{[Ag2(4,4′-bipy)2(2-nbdc)(H2O)]·2H2O}n(4)。通过IR、元素分析、TG、UV和荧光光谱以及粉末衍射等手段,对配合物进行了表征和性质研究。单晶衍射分析显示,配合物1为1D阴-阳离子型聚合物,配合物2为1D双链结构,且结构中不存在溶剂分子。配合物3和4均为1D链状结构。结构的多样性主要是由配体构象、硝基对苯二甲酸的配位模式以及弱作用(如π-π堆积、Ag…Ag作用以及氢键等)导致的。结构的不同也使得它们的稳定性、紫外吸收以及荧光光谱存在着差异。
配位模式;结构分析;银(Ⅱ)配合物;硝基对苯二甲酸
0Introduction
Phenyldicarboxylate metal complexes in recent years have received much attention due to their potential applications in catalysis[1-3],adsorption[4-10], luminescent[11],and chemical sensors[12-14].With the deepen of the investigation in the 1,4-benzenedicarboxylate metal complexes,people have recognized that the functional groups on benzene ring are very important in the assembly of the structures and properties.The contribution of several functional groups is not simply linear sums of the pure components[15].In the large number of 1,4-benzenedicarboxylate derivatives,seldom metal complexes with 2-nitro-1,4-benzenedicarboxylic acid(2-H2nbdc)have been explored. Totally only 17 transition complexes with nbdc ligand including Zn,Cd,Cu,Bi,In,Pb,Mn,Sn,and U (CSD,Version 5.36-Feb 2015)[16]were synthesized.The 2-nbdc ligand can be used to form cation-cation interaction dimer[17],enhance the hydrophobic interaction in the cavities of MOF,and form hysteretic single-crystal to single crystal transform cycle[18].
In addition,silver coordination polymers have attracted much attention not only because of the diverse coordination arrangement of the Ag(Ⅱ)ion varying from 2 to 6,but also the fascinating structural diversities(linearity,triangle,tetrahedron and trigonal -pyramid with occasional instances of square and octahedron)[19]and potential applications in many areas such as optics or electrical conductivity,magnetism, host-guest chemistry,and catalyst[20-21].The supramolecular chemistry of Ag(Ⅱ)coordination polymers represents a dynamic and thriving field which abounds with various supramolecular forces such as metal-ligand, metal-π,and metal-metal interactions,hydrogen bonds, π-π stacking,and anion interactions[22-23].Therefore the crystallization of Ag(Ⅱ)complexes would depend on the delicate balance of thermodynamic and kinetic contributionsconcerningsynergeticsupramolecular interactions,which may account for the fact that the structures and topologies of Ag(Ⅱ)complexes can be astonishingly varied even with same ligands.
However,the investigation about silver-complexes is still very limited.So far there is no any report on the silver complex with nbdc ligand,and only one complex of the M/4,4-bipyridine(4,4′-bipy)/2-H2nbdc system was reported in our lab[24].In coordination chemistry,complexes with diverse structures can be easily synthesized due to the variable coordination modes of metal ions and ligands,while structures are in general strongly related to their properties.Therefore, the directional or rational synthesis is very important forthepotential applications of coordination compounds. Herein we present the synthesis,structures,and properties of four silver complexes with same components, namely{[Ag(4,4′-bipy)]·2-Hnbdc·2H2O·CH3OH)}n(1), {[Ag(4,4′-bipy)(2-Hnbdc)]}n(2),{[Ag2(4,4′-bipy)2(2-nbdc)]·2H2O}n(3),and{[Ag2(4,4′-bipy)2(2-nbdc)(H2O)] ·2H2O}n(4).
1Experimental
1.1 General information
Chemicals and solvents were of analytical grade and purchased from commercial sources,used as obtainedwithoutfurtherpurification.Elemental analyses(C,H and N)were performed on a Elementar Vario EL III Elemental Analyzer.The infrared spectra (KBr pellets)were recorded on a Bruker VERTEX 70 spectrophotometer in the range of 400~4 000 cm-1. Thermal analyses were performed on a NETZSCH 409 F3 thermal analyzer at a heating rate of 10℃·min-1in flowing nitrogen atmosphere in the temperature range of 30~800℃using Al2O3crucibles.The UVVis spectra were recorded in methanol at room temperature with a Thermo Evolution 300 spectrophotometer.The fluorescence spectroscopic studies were carried out in methanol and in the solid state at room temperature with a RF-5301pc spectrophotometer(Shimadzu,Kyoto,Japan).The powder X-ray diffractions were measured by Rigaku D/MaX 2550PC with Cu Kα radiation.The simulated powder XRD patterns of complexes 1~4 were derived from single crystal X-ray data by Mercury program.
1.2 Syntheses of complexes 1~4
1.2.1 Synthesis of{[Ag(4,4′-bipy)]·2-Hnbdc·2H2O· CH3OH)}n(1)
Complex 1 was synthesized by layered-solution method in a tube with the diameter of 0.7 cm.The bottom layer was 5 mL aqueous solution containing 0.022 mol ·L-1of AgNO3and the upper layer was 10 mL methanol solution containing 0.011 mol·L-1of H2nbdc and 0.011 mol·L-1of 4,4′-bipy.After six days later,colorless block crystals were grown on the layered interface and were collected by filtration.Yield:68.3%.Anal.Calcd. for C19H20N3O9Ag(%):C 42.09,H 3.72,N 7.75.Found (%):C 42.21,H 3.66,N 7.72.IR(KBr,cm-1):1 702(s), 1 617(s),1 600(s),1 529(s),1 486(s),1 380(s),1 353(s), 1 310(s),1 288(s),1 253(s),1 219(m),1 153(m),1 064 (w),1 027(m),933(w),840(w),806(s),771(s),702(m), 623(w),515(w).
1.2.2 Synthesis of{[Ag(4,4′-bipy)(2-Hnbdc)]}n(2)
Complex 2 was synthesized by hydrothermal method.A mixture of AgNO3(0.050 0 g,0.29 mmol), 4,4′-bipy(0.038 g,0.24 mmol),and 2-H2nbdc(0.053 g 0.25 mmol)in water(15 mL)was sealed in a 25 mL Teflon-lined autoclave and heated to 130℃for 24 h, then cooled to room temperature.Resulting pale yellow block crystals were obtained.Yield:62.5%.Anal. Calcd.for C18H12N3O6Ag(%):C 45.59,H 2.55,N 8.86. Found(%):C 45.32,H 2.69,N 8.86.IR(KBr,cm-1): 1 676(m),1 605(s),1 523(s),1 488(s),1 382(s),1 339 (s),1 310(s),1 275(s),1 240(s),1 119(m),1 073(s), 1 058(m),911(w),864(w),828(m),815(s),780(s),738 (m),713(w),666(w),640(w),600(w),510(w),463(w).
1.2.3 Synthesis of{[Ag2(4,4′-bipy)2(2-nbdc)]·2H2O}n(3)
A mixture of AgNO3(0.083 g),H2nbdc(0.104 g), and 4,4′-bipy(0.080 g)in CH3OH(15 mL)and water (10 mL)was stirred and white precipitate occurred, then three drops of ammonia were added and most of white precipitate was dissolved,filtered and set aside at room temperature in dark,after about five weeks pale yellow block crystals were obtained.Yield: 56.2%.Anal.Calcd.for C28H23N5O8Ag2(%):C 43.49,H 3.00,N 9.06.Found(%):C 42.59,H 3.09,N 8.88.IR (KBr,cm-1):1 598(s),1 529(s),1 484(m),1 411(m), 1 355(s),1 219(w),1 126(w),1 070(w),1 006(w),913 (w),812(s),805(s),729(w),639(w),574(w),487(m).
1.2.4 Synthesis of{[Ag2(4,4′-bipy)2(2-nbdc)(H2O)]· 2H2O}n(4)
The synthesis of 4 was similar to that described for 3.The only difference was that we changed CH3OH in 3 to CH3CN(20 mL).After about five weeks,colorless block crystals were obtained.Yield:63.5%.Anal. Calcd.for C28H25N5O9Ag2(%):C 42.50,H 3.18,N 8.85. Found(%):C 42.61,H 2.97,N 8.81.IR(KBr,cm-1): 1 598(s),1 529(s),1 484(m),1 412(s),1 354(s),1 220 (m),1 069(m),1 004(w),912(w),815(s),727(w),635 (m),576(w),485(m).
1.3 X-ray crystallographic determination
Crystallographic data were collected at 296 K on an Oxford Diffraction Xcalibur CCD diffractometer using graphite monochromated Mo Kα radiation(λ= 0.071 073 nm).The frames were integrated with the CrysAlisPro package[25]and the data were corrected for absorptionusingtheprogramCrysAlisPro.The structures were solved by direct methods and refined byfull-matrixleast-squarestechniquesusingthe SHELXL-97 programs[26].All the non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms on carbons were put at the calculated positions,while other hydrogen atoms were found in the Fourier maps.All H atoms were refined with isotropicthermalparameters.Thegraphicswere drawn by the ORTEP and Olex2[27-28].Details of crystal data and structure refinements for the four complexes are listed in Table1 .
CCDC:945166,1;945167,2;945168,3;945169, 4.
2Results and discussion
2.1 Synthesis
Four complexes with structural diversity have been achieved by the different synthetic methods and variable solvents.Complex 1 was synthesized by the layered-solution method in a slender tube.Complex 2 was obtained using hydrothermal synthesis.Complexes 3 and 4 were synthesized in different mixed solvents through slow evaporation.Considering their structural diversity these complexes may be transformed under external stimulation.Therefore,complex 1 was heated at 130℃three hours and crystals were changed intopowder sample.The IR showed that the heated sample for 1 lost all solvents,while its spectrum is different from that of 2,indicating the simple heating did not transform the complex 1 into 2.The 2-Hnbdc ligands in 1 and 2 have largely different coordination modes and simple heating could not make these chemical bonding rearrangement.Similar experimental was done for complexes 3 and 4.And the desolvated 3 and 4 have same IR spectra,indicating the small different coordination modes in complexes 3 and 4 can be transformed due to their structural diversity is only controlled by the solvents.
Based on the above information,we can conclude that solvents,temperature and synthetic methods are very important for structural diversity and thermal stability,since external stimuli may influence the coordination properties and abilities of the H2nbdc ligand,and also have effect on the participation of the solvents,which are helpful for the formation of weak bonds and supramolecular structure.
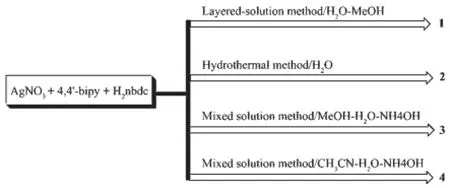
Scheme 1Synthetic route of complexes 1~4
2.2 Analysis of the structures of 1~4
The powder XRD patterns of complexes 1~4 and their simulative patterns calculated by Mercury using single crystal data agree well with each other(see Supportinginformation),indicatingthebulksof samples are purity.
In an asymmetrical unit for complex 1,the silver ion is four-coordinated with two longer distances (0.285 4(2)and 0.306 7(2)nm)of Ag-O representedby open bonds in Fig.1 .The geometry of the silver ion is a planar completed by two N atoms from two 4,4′-bipy and two O atoms from one water molecule and one nitro group(Table2 ).The 2-Hnbdc ligand is partlydeprotonatedanditscarboxylatesarenot coordinated to metal ion but form weak bond with Ag+using the nitro group.In general the nitro group on benzene ring is rare to coordinate with metal ion.Theunits form 1D chain structure(Fig.2 ) and the shortest Ag…Ag distance between chains is 0.532 9 nm,indicating there is no interaction between chains.
In complex 2,the silver ion is four-coordinated with a longer distance(0.292 2(2)nm)between the silver ion and nitro group.The deprotonated carboxylate coordinates to the silver ion.The coordination geometry of the silver ion is a planar environment completed by two N atoms from two 4,4′-bipy and twoO atoms from the carboxylate and nitro groups(Fig.3 and Table2 ).Complex 2 is a 1D double chain structure(Fig.4 ),which is different from complex 1.In 2,the shortest distance of Ag…Ag is 0.379 02(6)nm. Though it is shorter than that in 1,it is still longer than the van der Waals diameter of silver(0.344 nm), indicating no obvious interaction exists between them.
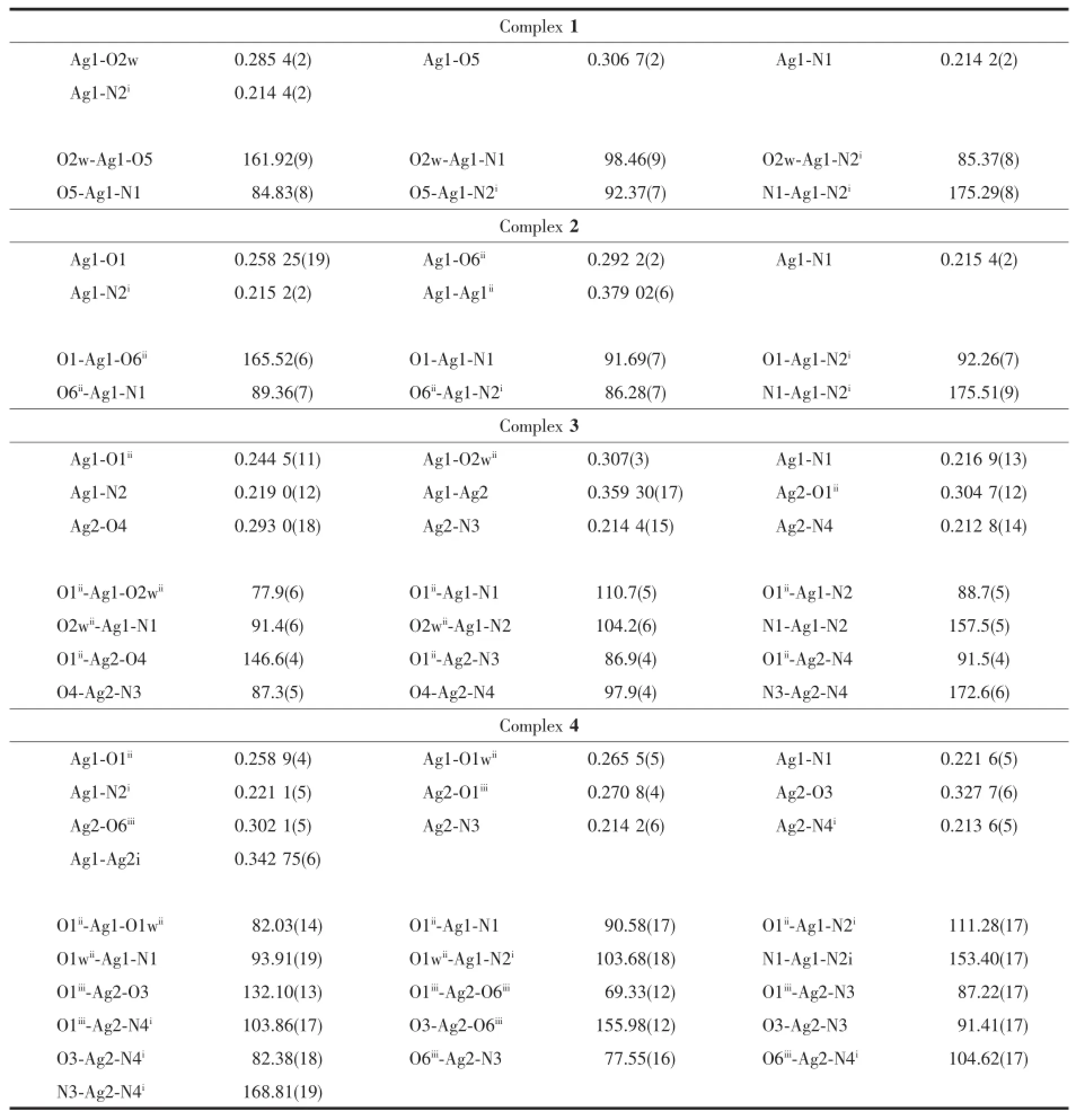
Table2 Selected bond lengths(nm)and angles(°)for complexes 1~4
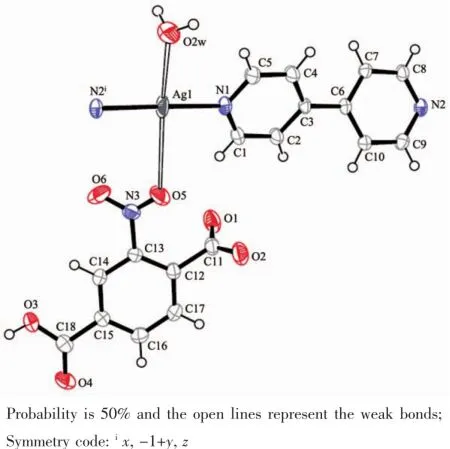
Fig.1 View of the coordination environment in 1 with the numbering scheme

Fig.2 View of the 1D chain formed by[Ag(4,4′-bipy)]+in 1
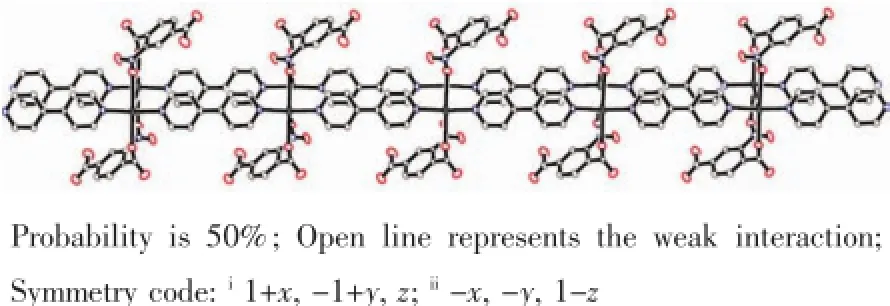
Fig.3 View of the coordination environment in 2 with the numbering scheme
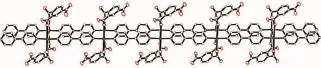
Fig.4 View of the 1D double chain in 2
In 3,the Ag1 is four coordinated with a longer Ag1-O2w being 0.307(3)nm(Fig.5 ).The Ag2 is also four coordinated with two longer distances of 0.293 0(18) and 0.304 7(12)nm.The 2-nbdc ligand is monodentately coordinated to silver ion as a terminal ligand.If we ignore the bond lengths over 0.30 nm,the molecular structure of 3 is a 1D chain(Fig.6 );if we consider all bonds,the molecular structure is a 2D layer and further interpenetrates into a 3D network.The Ag…Ag distance in the double chain is 0.359 30(17)nm, which is shorter than those of in 1 and 2.
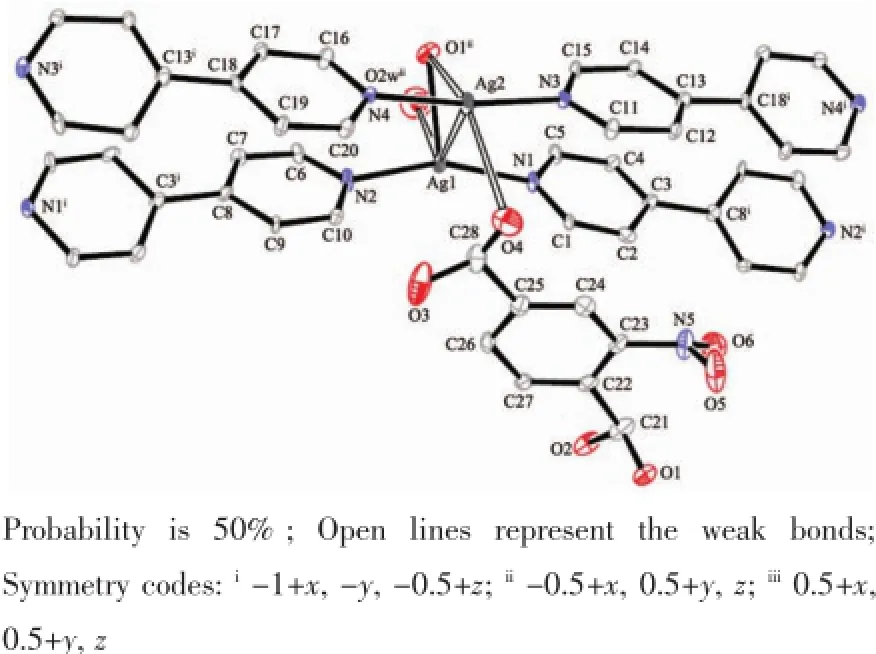
Fig.5 View of the coordination environment for complex 3 with the numbering scheme
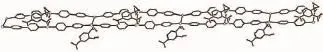
Fig.6 View of 1D chain for 3
In 4,the Ag1 is four coordinated and the Ag2 is five coordinated with two longer bond lengths(Fig.7 ). The distance of Ag1…Ag2iis 0.342 75(6)nm,which is similar to the sum of van der Waals radius of two silver ions.The molecular structure of 4 is a 1D molecular ladder combined by Ag-Ag interaction in adjacentchains,which is different from 2 and 3(Fig.8 ).
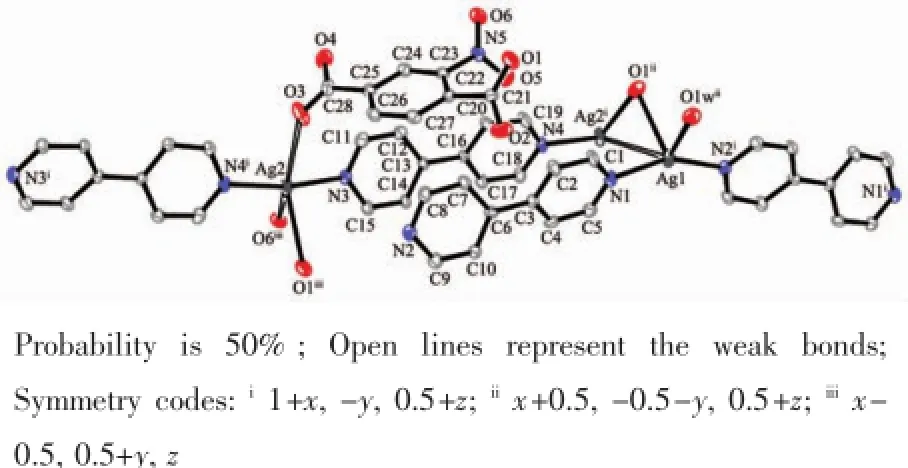
Fig.7 View of the coordination environment in 4 with the numbering scheme
Fromaboveinformation,wecanknowthat complex 2 has no any lattice solvent or coordinated water molecule,while other three contain coordinatedwater molecules or solvents.The basic structures for these four complexes are 1D architectures formed by [Ag(bipy)]+unit.Under the considering of the weak interactions,complex 1 is a 1D chain,1D double chain for 2,1D chain or extended 2D layer for 3,1D ladder chain for 4.

Fig.8 View of 1D chain for 4
In 1,solvents and anionic 2-Hnbdc form 1D hydrogen-bonding structure(Fig.9 ).Complex 2 is a 2D hydrogen-bondinglayerassembledthroughthe connection of 1D chains between 2-Hnbdc ligands.In 3,lattice water molecules and carboxylates form hydrogen bonds,generating a 2D hydrogen-bonding layer.For 4,water molecules and 2-Hnbdc form a 2D hydrogen-bonding layer.
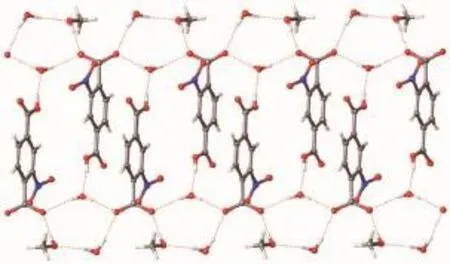
Fig.9 1D hydrogen bonding structure formed by solvents and anionic 2-Hnbdc in 1
In these complexes there are some π-π interactions between pyridine rings of 4,4′-bipyridine ligands. In 1,the interactions are strong with the centroid to centroid distances of 0.349 75(13)nm and 0.345 16(13) nm between rings of N2C6~C10.In 2 the centroid to centroiddistancesare0.370 94(18)nm and 0.387 85(18) nm between Cg1 and Cg2 where Cg1 is N1C1~5 and Cg2 is N2C6~C10.In 3 the centroid-to-centroid distances are 0.376 4(8)nm between Cg1 and Cg2, 0.369 4(9)nm between Cg1 and Cg3,and 0.372 7(8) nm between Cg3 and Cg4 where Cg1 is N1C1~C5, Cg2 is N3C11~C15,Cg 3 is N4C16~C20,and Cg4 is N2C6~C10.In 4,the centroid-to-centroid distances is 0.369 4(4)nm between Cg1 and Cg2,0.370 9(4)nm between Cg3 and Cg4,0.388 6(4)nm between Cg2 and Cg3 where Cg1 is N1C1~C5,Cg2 is N4C16~C20,Cg3 is N2C6~C10,and Cg4 is N3C11~C15.In 1 there is the shortest stacking interaction distance and complexes 2 and 4 are weaker than those of complexes 1 and 3.
These four complexes exhibit structural diversity with same components of Ag+,nbdc,and 4,4′-bipy. The solvents,interaction between silver ion and nitro group,and the positions of two pyridine rings in each 4,4′-bipy influence the assembly of the structures. The conformations of 4,4′-bipy ligands in 1~4 are listed in Table3 .In the free ligand the dihedral angles are 17°and 34°[25].
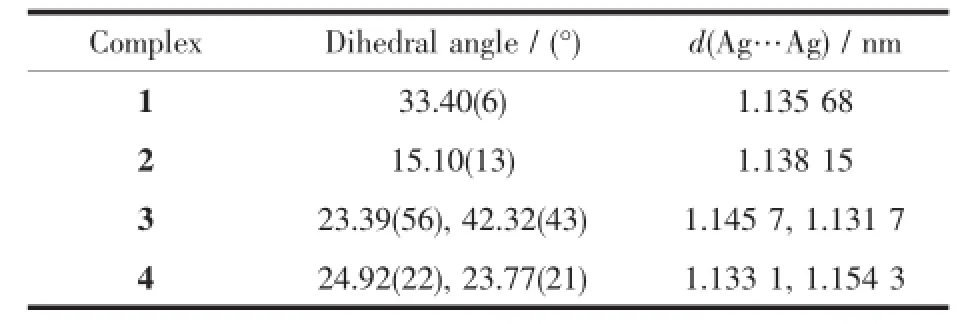
Table3 Dihedral angles of pyridine rings in each 4,4′-bipyridine
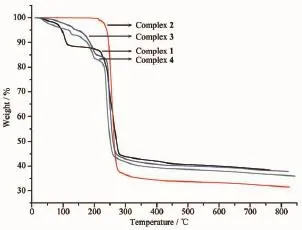
Fig.1 0TG curves of complexes 1~4
2.3 Analysis of TG
To understand the thermal stabilities of the four silver(Ⅱ)coordination polymers,the samples were analyzed by TGA,as shown in Fig.1 0.The curves of complexes 1,3 and 4 all show instability from room temperature,since they have solvent molecules in the structures.And complexes 3 and 4 have similar weight loss way since they show same structures after losing the solvent molecules.TG analysis for1 showed that the weight loss of two water molecules and one methanol from room temperature to 110℃is 11.56%(Calcd.12.56%).With a short platform,complex 1 rapidly disrupts at 220℃.For complex 3, the weight loss of 4.83%from 35 to 139℃corresponds to the release of two water molecules(Calcd.4.66%). Without a clear platform,complex 3 began to decompose slowly from 156 to 230℃,and then the structure quickly collapsed.Similar to complex 3,complex 4 released three water molecules in the range of room temperature to 139℃(Calcd.6.83%,Obsd.6.84%), and the decomposition temperature is also 156℃. Different from complexes 1 and 3~4,complex 2 shows good thermal stability.It is characterized by only one abrupt weight loss step(64.81%)from 220~350℃and then no obvious change occurred till 800℃.
Upon comparison to complexes 1~4,the products of complexes 1 and 2 after losing their solvents are more stable than those of 3 and 4.The reason maybe that in complex 1 the carboxylate does not coordinate to the silver,the coordination of the nitro group is less strong than that of the carboxylate.In complex 2,in the double chain Ag…Ag distance is longer than those of complexes 3 and 4.All the above information clearly reflects the structural stability of complex 2.
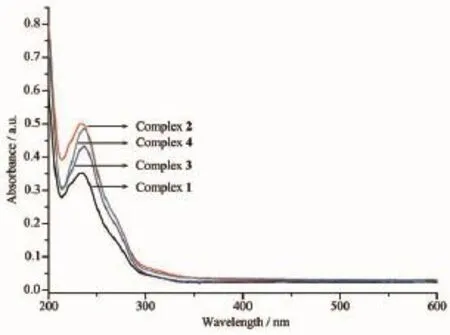
Fig.1 1UV spectra of complexes 1~4 at room temperature in methanol
2.4 UV-Vis property
UV-Vis spectra for complexes 1~4 and corresponding ligands were measured in methanol with the concentration of 1.015×10-5mol·L-1at room temperature and shown in Fig.1 1.As they are composed by the same ligands,the complexes show similar absorption peaks at about 235 nm and 200 nm,which mainly come from the H2nbdc and 4,4′-bipyridine(Table4 ). Though the UV-Vis absorption bands for these complexes are ascribed to π-π*intraligand(IL)transition, the absorptions are slightly stronger than those of ligands,suggesting the coordination can strengthen the absorption.The strongest absorption occurs in the complex 2 and the absorption strength order is 2〉4〉3〉1,indicating that configuration ofcomplexes, coordinating modes of ligands and solvents all can influence the absorptions.
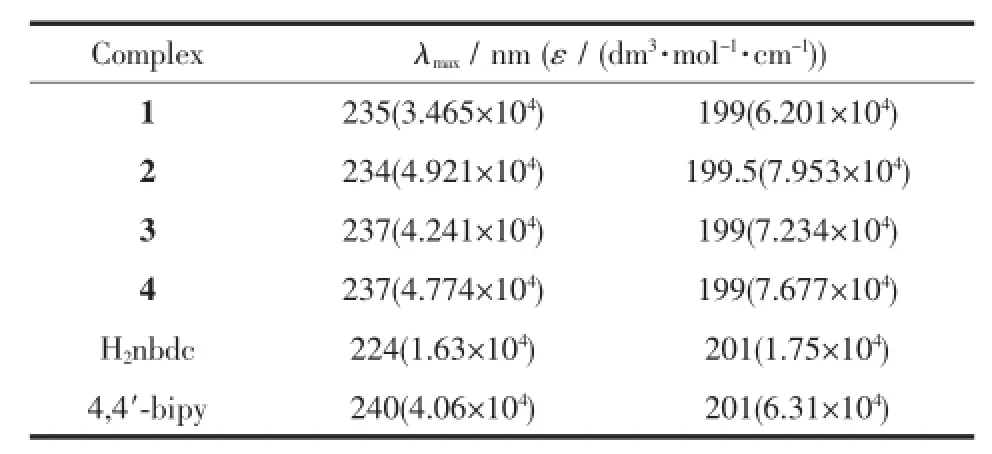
Table4 UV-Vis absorption spectral data of complexes 1~4 in CH3OH(C=1.015×10-5mol·L-1)
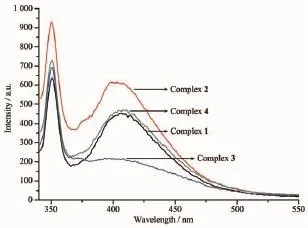
Fig.1 2Fluorescent spectra of complexes 1~4 in the methanol at room temperature(λex=315 nm)
2.5 Fluorescence property
Both fluorescence spectra in methanol and in solid state were measured at room temperature as shown in Fig.1 2 and 13,respectively.In methanol,the λexis 315 nm and they show two extinct emission peaks at about 350 and 400 nm,which are from the H2nbdc ligand(351 nm and 407 nm).Therefore the solution spectra showed the ligand emission character. In the solid state,the λexis 240 nm,and they also showed two emission peaks.They are located at about 290 and 390 nm,somewhat blue-shift compared to the 416 nm for H2nbdc[17].The stronger peaks for four complexes are about 390 nm,and solvation may take a role for the maximum emission location,since 10nm blue-shift occurs in solid state,compared with the spectra in methanol.In the reference the existence of solventmoleculescanpromotethefluorescence emission intensity[26],but for our system both kinds of spectra show that complex 2 has the strongest emission peak,while complex 2 has no solvent but with the most co-planar 4,4′-bipyridine ligand,probably leading to the intense emission.
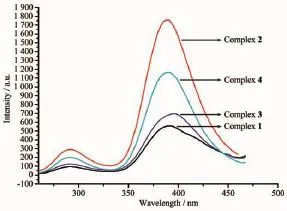
Fig.1 3Fluorescent spectra of complexes 1~4 in solid state at room temperature(λex=240 nm)
3Conclusions
We have synthesized four diverse silver coordination polymers with same components.The structural transform between complexes 1 and 2 can not be achieved by simple heating due to in complex 1 the 2-Hnbdc coordinates to the silver ions.However, desolvated complexes 3 and 4 have same structural character,indicating the solvents can mediate the conversion of complexes 3 and 4.In these four complexes there are abundant weak interactions,such as weak bond,π-π aromatic stacking effect,and hydrogen bonding interactions.In complexes 1,2,and 4,the nitro groups of 2-Hnbdc ligands weakly coordinate with silver ions,which is rarely reported.An another interesting structural information is that in some transition metal complexes with nbdc the nitro group exists as disordered form,while in our four silver complexes all nitro groups are normal without any disorder.Complex 2 has the highest thermal stability,the strongest electronic absorption,and the strongest fluorescence emission.
Acknowledgements:The authors thank the National Natural Science Foundation of China(No.21073157).
Supporting information is available at http://www.wjhxxb.cn
[1]Kim S N,Kim H Y,Cho H Y,et al.Catal.Today,2013,204: 85-93
[2]Opanasenko M,Shamzhy M,Lamac M,et al.Catal.Today, 2013,204:94-100
[3]Wan Y,Chen C,Xiao W M,et al.Microporous Mesoporous Mater.,2013,171:9-13
[4]Hu X F,Lu Y K,Dai F N,et al.Microporous Mesoporous Mater.,2013,170:36-44
[5]Mendes P A P,Ragon F,Rodrigues A E,et al.Microporous Mesoporous Mater.,2013,170:251-258
[6]Brand S K,Colon Y J,Getman R B,et al.Microporous Mesoporous Mater.,2013,171:103-109
[7]Yang J,Grzech A,Mulder F M,et al.Microporous Mesoporous Mater.,2013,171:65-71
[8]Duan L H,Dong X Y,Wu Y Y,et al.J.Porous Mater.,2013, 20:431-440
[9]Liu H,Zhao Y G,Zhang Z J,et al.Chem.Asian J.,2013,8: 778-785
[10]Zheng B S,Yun R R,Bai J F,et al.Inorg.Chem.,2013,52: 2823-2829
[11](a)Chen Y M,Cao Q,Gao D D,et al.J.Coord.Chem.,2013, 66:3829-3838
(b)Allendorf M D,Bauer C A,Bhakta R K,et al.Chem. Soc.Rev.,2009,38:1330-1352
[12]Wang Y,Wu Y C,Xie J,et al.Sens.Actuators B,2013, 177:1161-1166
[13]Robinson A L,Stavila V,Zeitler T R,et al.Anal.Chem., 2012,84:7043-7051
[14]Kreno L E,Leong K,Farha O K,et al.Chem.Rev.,2012, 112:1105-1125
[15]Deng H X,Doonan C J,Furukawa H,et al.M.Science, 2010,327:846-850
[16]Allen F H.Acta Crystallogr.,Sect.B:Struct.Sci.,2002,58: 380-388
[17]Severance R C,Smith M D,zur Loye H C.Inorg.Chem., 2011,50:7931-7933
[18]Wang X F,Wang Y,Zhang Y B,et al.Chem.Commun., 2012,48:133-135
[19](a)Chen C L,Kang B S,Su C Y.Aust.J.Chem.,2006,59:3-18
(b)Ma A Q,Zhu L G.RSC Adv.,2014,4:14691-14699
(c)Hakimi M,Moeini K,Mardani Z,et al.J.Coord.Chem.,2013,66:1129-1141
[20](a)Uchida S,Kawamoto R,Tagami H,et al.J.Am.Chem. Soc.,2008,130:12370-12376
(b)Coleman K S,Chamberlayne H T,Turberville S,et al. Dalton Trans.,2003,14:2917-2922
[21](a)Fujii Y,Terao J,Kambe N.Chem.Commun.,2009,9: 1115-1117
(b)Genuis E D,Kelly J A,Patel M,et al.Inorg.Chem.,
2008,47:6184-6194
(c)Seward C,Chan J,Song D,et al.Inorg.Chem.,2003,42: 1112-1120
[22]Akhbari K,Morsali A,Zhu L G.J.Mol.Struct.,2008,891: 132-137
[23]Khlobystov A N,Blake A J,Champness N R,et al.Coord. Chem.Rev.,2001,222:155-192
[24]He H Y,Zhu L G,Ng S W.Acta Crystallogr.,2005,E61: m601-m602
[25]CrysAlisPro,Version 1.171.33.52,Oxford Diffraction Ltd., 2009.
[26]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[27]Farrugia L J.J.Appl.Cryst.,1999,32:837-838
[28]Dolomanov O V,Bourhis L J,Gildea R J,et al.J.Appl. Cryst.,2009,42:339-341
Structural Diversity,Supramolecular Assembly,and Electronic Spectra of Four Silver Coordination Polymers with Same Components of 2-Nitro-1,4-benzenedicarboxylate and 4,4′-Bipyridine
MA Ai-Qing1,2ZHU Long-Guan*,1
(1Department of Chemistry,Zhejiang University,Hangzhou 310027,China)
(2School of Pharmacy,Guangdong Medical University,Dongguan,Guangdong 523808,China)
Four diverse silver coordination polymers with the same components of silver,4,4′-bipyridine(4,4′-bipy), and 2-nitro-1,4-benzenedicarboxylic acid(2-H2nbdc)have been synthesized,namely{[Ag(4,4′-bipy)]·2-Hnbdc· 2H2O·CH3OH}n(1),{[Ag(4,4′-bipy)(2-Hnbdc)]}n(2),{[Ag2(4,4′-bipy)2(2-nbdc)]·2H2O}n(3),and{[Ag2(4,4′-bipy)2(2-nbdc)(H2O)]·2H2O}n(4),and characterized by IR,elemental analysis,TG,UV,fluorescence spectra,and powder X-ray analysis.The single crystal X-ray analysis showed that complex 1 is a 1D cation-anionic polymer,complex 2 is a 1D double chain without any solvent,and complexes 3 and 4 are 1D chain structures.Diverse structures differ with respect to molecular conformation,coordination modes of 2-Hnbdc,and weak interactions.In these complexes there are weak bonds,π-π aromatic stacking interactions,Ag…Ag interaction,and hydrogen bonding. The diverse structures are related to the thermal stability,UV absorptions,and fluorescence emissions.CCDC: 945166,1;945167,2;945168,3;945169,4.
coordination modes;structure elucidation;silver coordination compound;nitrobenzenedicarboxylate
O614.122
A
1001-4861(2015)08-1651-10
10.11862/CJIC.2015.227
2015-05-04。收修改稿日期:2015-06-04。
国家自然科学基金(No.21073157)资助项目。
*通讯联系人。E-mail:chezlg@zju.edu.cn;会员登记号:S06N0578M1207。

