PMBP缩4-甲基水杨酰肼铜、锌配合物:合成、结构及锌配合物的荧光性质
李晓静 蔡红新 吴伟娜侯莹 王震
(河南理工大学物理化学学院,焦作454000)
PMBP缩4-甲基水杨酰肼铜、锌配合物:合成、结构及锌配合物的荧光性质
李晓静 蔡红新*吴伟娜*侯莹 王震
(河南理工大学物理化学学院,焦作454000)
合成并通过单晶衍射、元素分析、红外光谱表征了配合物[(Cu)(L)(Cl)]·0.5EtOH·1.5H2O(1)和{[Zn(L)(NO3)]·2CH3CN}n(2)的结构(HL为PMBP缩4-甲基水杨酰肼;PMBP=1-phenyl-3-methyl-4-benzoyl-5-pyrazolone)。单晶衍射结果表明,配合物1中,Cu(Ⅱ)离子与来自烯醇化脱质子配体L-的2个O原子和1个N原子,及1个氯离子配位,采取扭曲的平面正方形配位构型。而配合物2中,Zn(Ⅱ)离子采取扭曲的三角双锥配位构型,与来自L-的NO2电子供体,1个单齿配位的硝酸根和相邻配体吡唑啉酮N原子配位,形成沿b轴方向的一维链状结构。在310 nm紫外光激发下,配合物2在434和459 nm处有很强的荧光发射,而配体的荧光发射峰在521 nm,强度明显弱于配合物。此外,固态配体和配合物2的荧光寿命分别为7.352 8和7.755 6 μs。
酰腙;Zn(Ⅱ)配合物;Cu(Ⅱ)配合物;荧光;晶体结构
It is well known that Schiff bases are an important class of ligands in coordination chemistry and have been found extensive application in different fields[1-5]. Among them,the Schiff base derivatives of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone(PMBP)and their metal complexes have been widely investigated due to their high biological and pharmaceutical activities,such as antibacterial,antitumor,antivirus enzyme-inhibitor[6-8]. Although some PMBP Schiff bases have been reported toshowexcellentphotochromicandfluorescence properties[9-10],studies on the fluorescence properties of the metal complexes with such series of ligands are relatively few.
Generally,zincioniscloselyrelatedto biochemistry,clinicaldiagnosticsaswellas environmental pollution[11-15].Furthermore,a large amount of Zn(Ⅱ)acylhydrazones have been reported for their fluorescence properties[11,14-15].Therefore,in this paper,Cu(Ⅱ)and Zn(Ⅱ)complexeswithan acylhydrazone ligand derived from PMBP and 4-methyl salicylic hydrazidehavebeensynthesizedandstructural determined by single-crystalX-raydiffraction.In addition,the fluorescence properties of the ligand and its Zn(Ⅱ)complex were discussed in detail.

Scheme 1Reaction scheme for the synthesis of HL
1Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchasedcommerciallyandusedasreceived. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FTIR spectrophotometer.1H NMR spectra ofHLwasacquiredwithBrukerAV400NMR instrument in DMSO-d6solution with TMS as internal standard.Fluorescence spectra were determined on an Edinburgh FLS980 spectrophotometer.
1.2 Preparations of the ligand and complexes
As shown in Scheme 1,the ligand HL was prepared by condensation of PMBP(2.78 g,10 mmol) and 4-methyl salicylic hydrazide(1.66 g,10 mmol)in ethanol solution(30 mL)under reflux condition for 5 h.The yellow solid was filtered and washed three times with ethanol.Crystals of HL·0.5EtOH suitable forX-raydiffractionanalysiswereobtainedby recrystallization of HL from ethanol solution.Yield: 3.02g(71%).m.p.138~142℃.Elemental analysis Calcd.for HL(C25H22N4O3)(%):C:70.41;H:5.20;N: 13.14;Found:C:70.34;H:5.21;N:13.24.FTIR (cm-1):ν(O-H)3 404,ν(O=C pyrazolone)1 634,ν(O= C acylhydrazone)1 596,ν(C=C)1 580,ν(C=N pyrazolone)1 536.1H NMR(400 MHz,DMSO-d6)δ: 6.798~6.833(2H),7.278~7.750(10H),8.022~8.046 (1H)for Ar-H,2.302(3H,s,CH3of benzene ring), 1.276(3H,s,-CH3of pyrazolone ring).
The complex 1 and 2 were generated by reaction of HL(5mmol)with equal molar of CuCl2·2H2O in ethanol and Zn(NO3)2·6H2O(1:1 molar ratio)in acetonitrile solution,respectively.Crystals of 1 and 2 suitable for X-ray diffraction analysis were obtained byevaporatingthereactionsolutionsatroom temperature.
1:greenneedles.Anal.Calcd.for C26H27N4O5CuCl(%):C:54.36;H:4.74;N:9.75. Found(%):C:54.18;H:4.42;N:10.13.FTIR(cm-1): ν(O-H)3415,ν(O=C-N pyrazolone)1 613,ν(C=C) 1 571,ν(C=N pyrazolone)1 531,ν(C=N)1 486.
2:colorless blocks.Anal.Calcd.for C29H27N7O6Zn (%):C:54.86;H:4.29;N:15.44.Found(%):C: 54.81;H:4.18;N:15.52.FTIR(cm-1):ν(O-H)3 405,ν(O=C-N pyrazolone)1 618,ν(C=C)1 586,ν(C=N pyrazolone)1 548,ν(C=N)1 489.
1.3.1 X-ray crystallography
The X-ray diffraction measurement for HL· 0.5EtOH,complexes 1 and 2 was performed on a BrukerSMARTAPEXⅡCCDdiffractometer equipped with a graphite monochromatized Mo-Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode. Semi-empirical absorption correction was applied to the intensity data using the SADABS program[16].The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[17].All non-hydrogen atoms were refined anisotropically.AllHatomswerepositioned geometricallyandrefinedusingaridingmodel. SQUEEZE procedure was applied to deal with the crystal solvent molecules of complexes 1 and 2. Details of the crystal parameters,data collection and refinements for three compounds are summarized in Table1 .
CCDC:1058420,HL·0.5EtOH;1058421,1; 1058422,2.

Table1 Crystal data and structure refinement for the HL·0.5EtOH,1 and 2
2Results and discussion
2.1 Crystal structures description
Selected bond distances and angles of threecompounds are listed in Table2 .As shown in Fig.1 a, HL in the crystal structure of HL·0.5EtOH is in a ketone form,in which the bond lengths of carbonyl C7-O1(0.126 4(2)nm)and C18-O2(0.125 1(2)nm) are comparable to those of some reported Schiff base ligands derived from PMBP[9].
Oncecoordinatedwithmetalion,the acylhydrazone ligand HL is deprotonated.In addition, the distances of the enolized C-O and imine C-N bands in both complexes are intermediate between singleand doublebond,suggesting anextended conjugation in anionic ligand after complexation.The structural analysis reveals that the asymmetric unit of 1(Fig.1 b)is build of two similar neutral mononuclear complex units,a half crystal ethanol and a half crystal water.Each copper(Ⅱ)center with distorted square planar geometry is four-coordinated as[Cu(OON)Cl], with one nitrogen and two oxygen atoms provided by the enolizated ligand L-and one chloride anion.The coordination bond lengths around both Cu(Ⅱ)center are in the normal range,with Cu-O being 0.189 6(5)~0.197 5(5)nm,Cu-N being 0.196 6(6)and 0.197 7(7) nm,Cu-Cl being 0.221 5(3)and 0.222 7(3)nm,respectively.

Fig.1 ORTEP drawing of HL·0.5EtOH(a),1(b)and 2(c)with 10%thermal ellipsoids;(d)Chain-like structurealong b axis in complex 2
However,the zinc(Ⅱ)center in 2(Fig.1 (c))is surrounded by one nitrate anion,one NO2donor set of an enolizated ligand L-and one pyrazoline nitrogen atom from another adjacent acylhydrazone ligand,thus forming one dimension chain-like framework along b axis(Fig.1 (d)).According to the Addison rule[18],the geometric index τ is 0.526 2,indicating that the coordination geometry of Zn(Ⅱ)ion is best described as a distorted trigonal biyramid rather than tetragonal pyramid.The equatorial plane of the trigonal biyramid is made up of N2i,N3 and O4 atoms(Symmetry code:i0.5-x,-0.5+y,0.5-z),while O1 and O2 atoms occupy the axial positions in trans manner.
2.2 IR spectra
The IR spectra for both complexes are more or less similar due to the similarity in coordination modes of the ligands with the metal centre.ν(O=C pyrazolone)vibrations of the free ligand is at 1 634 cm-1,it shifts to 1 613 and 1 618 cm-1in complexes 1 and 2,respectively,showing the pyrazolone O=C bond participates in the coordination in each complex.The O=C-N characteristic stretching vibration absorption of the acylhydrazone group in the free ligand is at 1 597 cm-1,while it is absent in both complexes.Meanwhile, new(N=C-O)stretching vibration absorption are observed at 1 486 and 1 489 cm-1in complexes 1 and2,respectively,whichrevealingthatinboth complexes the acylhydrazone C=O in O=C-N moiety has enolizated and the oxygen atom coordinates to the central metal ion[8].The peak at 1 536 cm-1should be assigned to the ν(C=N pyrazolone)vibration,it appears at 1 531 and 1 548 cm-1in complexes 1 and 2,respectively,clearly indicating that the nitrogen atom of pyrazolone ring takes part in the coordination with Zn(Ⅱ)ion in complex 2,while does not in complex 1.Itis in accordance withtheX-ray diffraction analysis result.

Table2 Selected bond lengths(nm)and angles(°)in HL·0.5EtOH,1 and 2
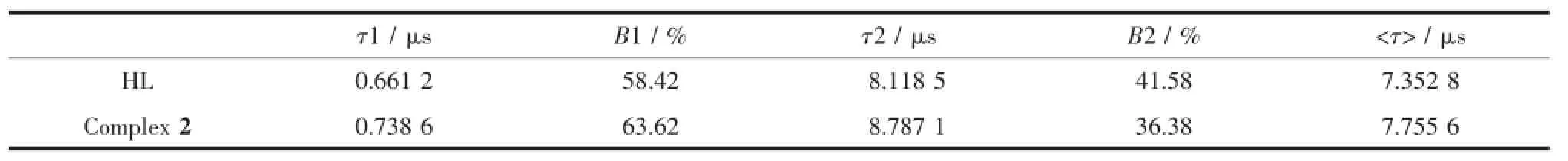
Table3 Luminescent decay data of HL and complex 2 in the solid state
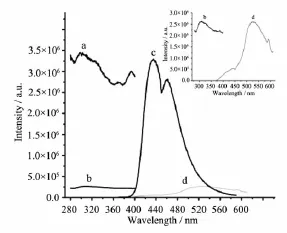
Fig.2 Fluorescence excitation spectra of complex 2(a) and free ligand HL(b);emission spectra of complex 2(c)and free ligand HL(d)in the solid state at room temperature
2.3 Fluorescence spectra
In the solid state,the fluorescence intensity of 1 is much too weak,thus is not discussed in this work. Fig.2 shows the excitation and emission spectra of the acylhydrazone ligand and 2 in solid state.When excited at 310 nm,complex 2 exhibits two strong emissions at 434 and 459 nm,while the ligand showsrelatively weak emission at 521 nm.This is probably due to the energy transferring from the ligand to the Zn(Ⅱ)ion[19].The behavior of Zn2+coordinated to the ligand is regarded as that of emissive species resulted in a CHEF effect(chelation enhancement of the fluorescence emission)[20].Luminescent decay data of HL and complex 2 in solid state are shown in Table3 , where τ1and τ2are short-and long-decay components, separately.The lifetime values(μs)are determined to be 0.661 2 and 8.118 5 for HL,while 0.738 6 and 8.787 1 for the complex 2.The mean lifetimes〈τ〉are 7.352 8 μs for HL and 7.7556 μs for the complex 2 calculated by the following equation[21]:,where B1and B2are weight factors.
[1]HUANG Chao(黄超),WU Juan(吴娟),CHEN Dong-Mei (陈冬梅),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31:109-113
[2]CHEN Yan-Min(陈延民),XIE Qin-Fan(解庆范),LIU Jin-Hua(刘金花),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31:74-80
[3]CHEN Yan-Min(陈延民),CHU Zhao-Hua(储召华),HAO Gui-Xia(郝桂霞),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31:317-322
[4]ZHUO Xin(卓馨),PAN Zhao-Rui(潘兆瑞),WANG Zuo-Wei (王作为),et al.Chinese J.Inorg.Chem.(无机化学学报), 2006,22:1847-1851
[5]LI Shi-Xiong(李石雄),LIAO Bei-Ling(廖蓓玲),LUO Pei (罗培),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31:291-296
[6]Yang Z Y,Yang R D,Li F S,et al.Polyhedron,2000,19: 2599-2604
[7]Yang Z Y,Wang B D,Li Y H.J.Organomet.Chem.,2006, 691:4159-4166
[8]Wang Y,Yang Z Y.Transition Met.Chem.,2005,30:902 -906
[9]Wang Y,Yang Z Y.J.Lumin.,2008,128:373-376
[10]ZHANG Shu-Ming(张姝明),LI Pei-Fan(李培凡),YU Ming (郁铭),et al.Chinese J.Inorg.Chem.(无机化学学报), 2004,20:439-443
[11]Zhou X Y,Li P X,Shi Z H,et al.Inorg.Chem.,2012,51: 9226-9231
[12]Wu Z K,Chen Q Q,Yang G Q,et al.Sens.Actuators B., 2004,99:511-515
[13]Zhang G Q,Yang G Q,Zhu L N,et al.Sens.Actuators B., 2006,114:995-1000
[14]Sali S,Grabchev I,Chovelon J M,et al.Spectrochim.Acta A,2006,65:591-597
[15]Kulatilleke C P,Silva S A,Eliav Y.Polyhedron,2006,25: 2593-2596
[16]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[17]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen, Germany,1997.
[18]Addison A W,Rao T N.J.Chem.Soc.Dalton Trans., 1984,1349-1356
[19]CHENG Mei-Ling(程美令),CAO Xiang-Qian(曹向前), WANG Chun-Lan(王春兰),et al.Chinese J.Inorg.Chem. (无机化学学报),2006,22:1222-1226
[20]Vicente M,Bastida R,Lodeiro C,et al.Inorg.Chem., 2003,42:6768-6779
[21]Buddhudu S,Morita M,Murakami S,et al.J.Lumin., 1999,83-84:199-203
Cu(Ⅱ)and Zn(Ⅱ)Complexes with an Acylhydrazone Derived from 4-Methyl Salicylic Hydrazide and PMBP:Crystal Structures and Fluorescence Property of Zn(Ⅱ)Complex
LI Xiao-JingCAI Hong-Xin*WU Wei-Na*HOU YingWANG Zhen
(Department of Physics and Chemistry,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Two complexes[(Cu)(L)(Cl)]·0.5EtOH·1.5H2O and{[Zn(L)(NO3)]·2CH3CN}n(HL is the acylhydrazone ligand derived from 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone(PMBP)and 4-methyl salicylic hydrazide)have been synthesized and characterized by single-crystal X-ray diffraction,elemental analysis and IR spectroscopy.X-ray diffraction analysis results show that the coordination geometry of the Cu(Ⅱ)ion in 1 is a distorted square planar geometry with nitrogen and two oxygen atoms provided by the enolizated ligand L-1and one chloride anion.However, in complex 2,the Zn(Ⅱ)ion with a distorted trigonal biyramid coordination geometry is five-coordinated,involving one nitrate anion,one NO2donor set of an enolizated ligand L-and one pyrazoline nitrogen atom from another adjacent acylhydrazone ligand,thus forming one dimension chain-like framework along b axis.When excited at 310 nm,complex 2 exhibits two strong emissions at 434 and 459 nm,while the ligand shows relatively weak emission at 521 nm.In addition,luminescent decay data show that the mean lifetime〈τ〉are 7.352 8 and 7.755 6 μs for HL and complex 2,respectively.CCDC:1058420,HL·0.5EtOH;1058421,1;1058422,2.
hydrazone;Zn(Ⅱ)complex;Cu(Ⅱ)complex;fluorescence;crystal structure
O614.121;O614.24+1
A
1001-4861(2015)08-1661-06
10.11862/CJIC.2015.228
2015-04-10。收修改稿日期:2015-06-02。
国家自然科学基金(No.21001040,21404033,21401046),河南省教育厅自然科学基金(No.12B150011,14B150029)资助。
*通讯联系人。E-mail:me2001@hpu.edu.cn;wuwn08@hpu.edu.;会员登记号:S06N6704M112。

