一系列由柔性N,N′-双(3-吡啶甲基)胺构筑的螺旋配合物的合成、结构和性质
岳翠敏 高东昭 王修光
(天津市功能分子结构与性能重点实验室;无机-有机杂化功能材料化学省部共建教育部重点实验室;天津师范大学化学学院,天津300387)
一系列由柔性N,N′-双(3-吡啶甲基)胺构筑的螺旋配合物的合成、结构和性质
岳翠敏 高东昭*王修光
(天津市功能分子结构与性能重点实验室;无机-有机杂化功能材料化学省部共建教育部重点实验室;天津师范大学化学学院,天津300387)
合成和表征了5个螺旋配位聚合物{[Cu(Hbpma)(H2O)4]2(SO4)3·3.5H2O}n(1)、{[Ni(Hbpma)(H2O)4]4(SO4)6·10.75H2O}n(2)、{[Mn (Hbpma)(H2O)4](SO4)1.5·3H2O}n(3)、{[Zn(Hbpma)(H2O)4]4(SO4)6·4H2O·4CH3OH}n(4)和{[Cu(Hbpma)2(H2O)2](SO4)2·9H2O}n(5),其中bpma代表N,N′-双(3-吡啶甲基)胺。晶体结构分析表明配合物1~4为一维链状结构,配合物5为二维层状结构,其中金属离子由质子化的bpma配体桥连。值得注意的是,采取反-反式构象的柔性bpma配体使得配合物1和2为假螺旋链结构,配合物3和4为螺旋链结构,配合物5为螺旋层结构。同时研究了配合物的磁性和热稳定性。
金属配合物;N,N′-双(3-吡啶甲基)胺;螺旋结构;磁性;热稳定性
0Introduction
Over the past decades,helical structures have received much attention in coordination chemistry and materials chemistry,because helicity is an essence of life and is also important in advanced materials such asopticaldevices,enantiomerseparation,chiral synthesis,ligand exchange,and selective catalysis[1-3]. Onepromisingapproachtoconstructabiological helices is the use of coordination chemistry to direct the assembly of small component molecules into extended helical polymeric materials[4-5].The crucial step is the selection of multifunctional organic ligands containing appropriate coordination sites linked by a spacer with specific positional orientation.On the one hand,the ligands should be ditopic and thus capable of bridging metal atoms in certain directions.On the otherhand,theligandsshouldcontainsteric informationthatcanbeinterpretedbythe arrangement of the bound metal centers,resulting in the formation of helical structures.Up to now,the design of desirable helical structures through the selfassembly of ligands and metal ions has achieved great progress.Agreatdealofhelicalcoordination polymers,such as one-dimensional helices[6-8],twodimensional helical layers[9-11],and three-dimensional frameworks containing helical features[12-13],have been systemically investigated in recent years.As the neutralN-donorligands,1,3-bis(4-pyridyl)propane(bpp) has proven to be a good candidate for the organization of helices because of its length and flexibility,which can assume different conformations such as TT,TG, GG,and GG′(considering the relative orientations of the three CH2groups,T=trans,G=gauche)[14-16].Inspired bytheaforementionedconsiderations,weare interested in exploring various interesting helical structures constructed by another flexible N-donor ligand N,N′-bis(3-pyridylmethyl)amine(bpma)(the imine group could be protonated)(Scheme 1),which hasthesimilarcoordinationabilitiesand conformations with the bpp ligand[17-18].In this paper, we herein report the crystal structures and properties offivedifferenthelicalcoordinationpolymers {[Cu(Hbpma)(H2O)4]2(SO4)3·3.5H2O}n(1),{[Ni(Hbpma) (H2O)4]4(SO4)6·10.75H2O}n(2),{[Mn(Hbpma)(H2O)4] (SO4)1.5·3H2O}n(3),{[Zn(Hbpma)(H2O)4]2(SO4)6·4H2O· 4CH3OH}n(4)and{[Cu(Hbpma)2(H2O)2](SO4)2·9H2O}n(5)bridged by the flexible bpma ligands.

Scheme 1bpma ligand
1Experimental
1.1 Material required and instruments
All chemicals were of reagent grade and used without purification.The N,N′-bis(3-pyridylmethyl) amine(bpma)ligand was prepared using the same method as for N,N′-bis(2-pyridylmethyl)amine[19].Elemental analyses for carbon,hydrogen and nitrogen were carried out with a Leeman-Labs CE-440 elemental analyzer.IR spectra were taken with a Nicolet Avatar 370 FT-IR spectrometer in the range of 4 000~400 cm-1by KBr pellet technique.The variable temperaturemagneticsusceptibilitymeasurements were carried out with a Quantum Design MPMS-XL-7 magnetometer in the temperature range 2~300 K at a magnetic field of 1 000 Oe.The molar magnetic susceptibility was corrected from the sample holder and diamagnetic contributions of all constituent atoms by using Pascal′s constants.Thermogravimetric analyses (TGA)were performed on a Perkin-Elmer TGAQ 500 thermogravimetric analyzer under flowing N2atmosphere with a heating rate of 5℃·min-1up to 650℃.
1.2 Preparation of complex{[Cu(Hbpma)(H2O)4]2(SO4)3·3.5H2O}n(1)
At room temperature,CuSO4·6H2O(26.8 mg,0.1 mmol)and bpma ligand(19.9 mg,0.1 mmol)were dissolved in 15 mL distilled water with stirring for 1 h and filtered.After the filtrate was allowed to stand at room temperature for one week,blue block crystals suitable for X-ray structure analysis were grown by slow evaporation.Yield:38%based on CuSO4·6H2O. Anal.Calcd.(%)for C24H51Cu2N6O23.50S3:C 28.2;H 5.0; N 8.2;Found(%):C 28.1;H 5.1;N 8.3.IR(KBr disc, cm-1):3 447(br),1 437(m),1 108(m),814(m),617(m).
1.3 Preparation of complex{[Ni(Hbpma)(H2O)4]4(SO4)6·10.75H2O}n(2)
The reaction was carried out following a similar procedure as described for complex 1,except that NiSO4·6H2O was used instead of CuSO4·6H2O.Light green block crystals were grown by slow evaporation. Yield:36%based on NiSO4·6H2O.Anal.Calcd.(%) for C48H109.5N12Ni4O50.75S6:C 27.5;H 5.2;N 8.0;Found (%):C 27.5;H 5.1;N 8.1.IR(KBr disc,cm-1):3 222 (br),1 437(m),1 079(m),812(m),618(m).
1.4 Preparation of complex{[Mn(Hbpma)(H2O)4] (SO4)1.5·3H2O}n(3)
The reaction was carried out following a similar procedure as described for complex 1,except that MnSO4·6H2O was used instead of CuSO4·6H2O.Colorless block crystals were grown by slow evaporation. Yield:40%based on MnSO4·6H2O.Anal.Calcd.(%) for C12H28MnN3O13S1.50:C 27.4;H 5.3;N 8.0;Found (%):C 27.3;H 5.4;N 7.9.IR(KBr disc,cm-1):3 274 (br),1 436(m),1 117(m),811(m),618(m).
1.5 Preparation of complex{[Zn(Hbpma)(H2O)4]4(SO4)6·4H2O·4CH3OH}n(4)
At room temperature,ZnSO4·6H2O(26.9 mg,0.1 mmol)and bpma ligand(19.9 mg,0.1 mmol)were dissolved in the methanol solution(5 mL methanol and 10 mL distilled water)with stirring for 1 h and filtered.The filtrate was allowed to stand at room temperature for one week,whereupon colorless block crystals suitable for X-ray structure analysis were grown by slow evaporation.Yield:34%based on ZnSO4·6H2O.Anal.Calcd.(%)for C52H112N12O48S6Zn4:C 29.3;H 5.3;N 7.9;Found(%):C 29.4;H 5.3;N 7.8. IR(KBr disc,cm-1):3 199(br),1 438(m),1 111(m), 812(m),618(m).
1.6 Preparation of complex{[Cu(Hbpma)2(H2O)2] (SO4)2·9H2O}n(5)
The reaction was carried out following a similar procedure as described for complex 4,except that CuSO4·6H2O was used instead of ZnSO4·6H2O.Blue block crystals were grown by slow evaporation.Yield: 31%based on CuSO4·6H2O.Anal.Calcd.(%)for C24H50N6O19S2Cu:C 33.7;H 5.9;N 9.8;Found(%):C 33.6;H 6.0;N 9.9.IR(KBr disc,cm-1):3 435(br), 1 437(m),1 112(m),812(m),618(m).
1.7 Crystallographic data collection and structure determination
The X-ray diffraction measurements of complexes 1,4 and 5 were collected with a Bruker CCD area detector diffractometer equipped with a graphitemonochromatic Mo Kα radiation source(λ=0.071 073 nm),while those of complexes 2 and 3 were detained with a Bruker APEX-ⅡCCD diffractometer.The empirical adsorption corrections by SADABS were carried out[20].The structures were solved by direct methods using the SHELXS-97 program[21]and refined with SHELXL-97[21]by full-matrix least-squares techniques on F2.All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms except for some water molecules were generated geometrically and refined isotropically using the riding model.Some free sulfateanionsandsolvatedwatermoleculesare disordered.Crystallographic data and experimental details for structural analyses are summarized in Table1 and 2.Selected bond lengths and angles are listed in Table3 ~7.
CCDC:966929,1;966932,2;966930,3;966931, 4;1054736,5.

Table1 Crystal data and structure refinements for complexes 1 and 2
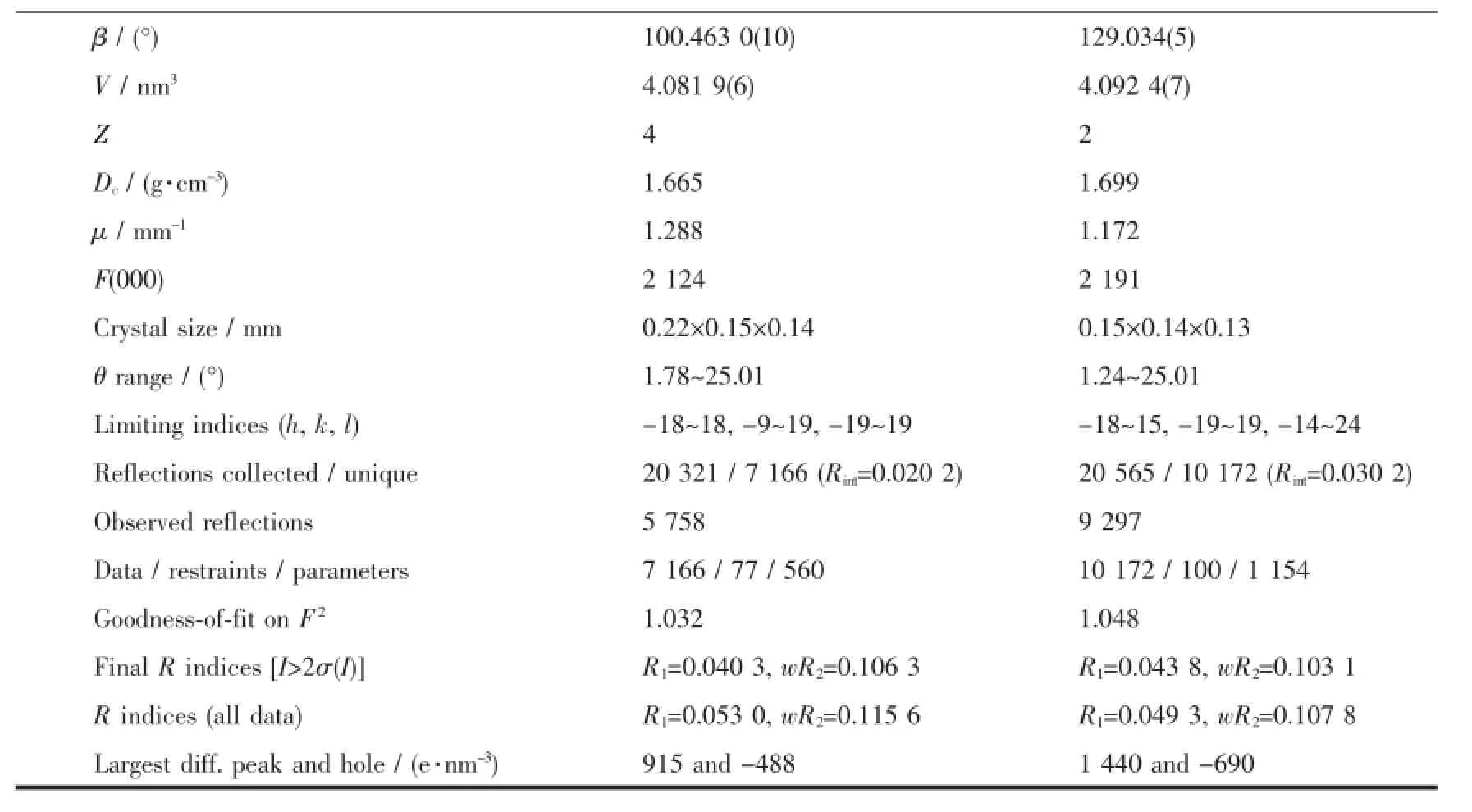
Continued Table1

Table2 Crystal data and structure refinements for complexes 3,4 and 5

Table3 Selected bond lengths(nm)and angles(°)for complex 1
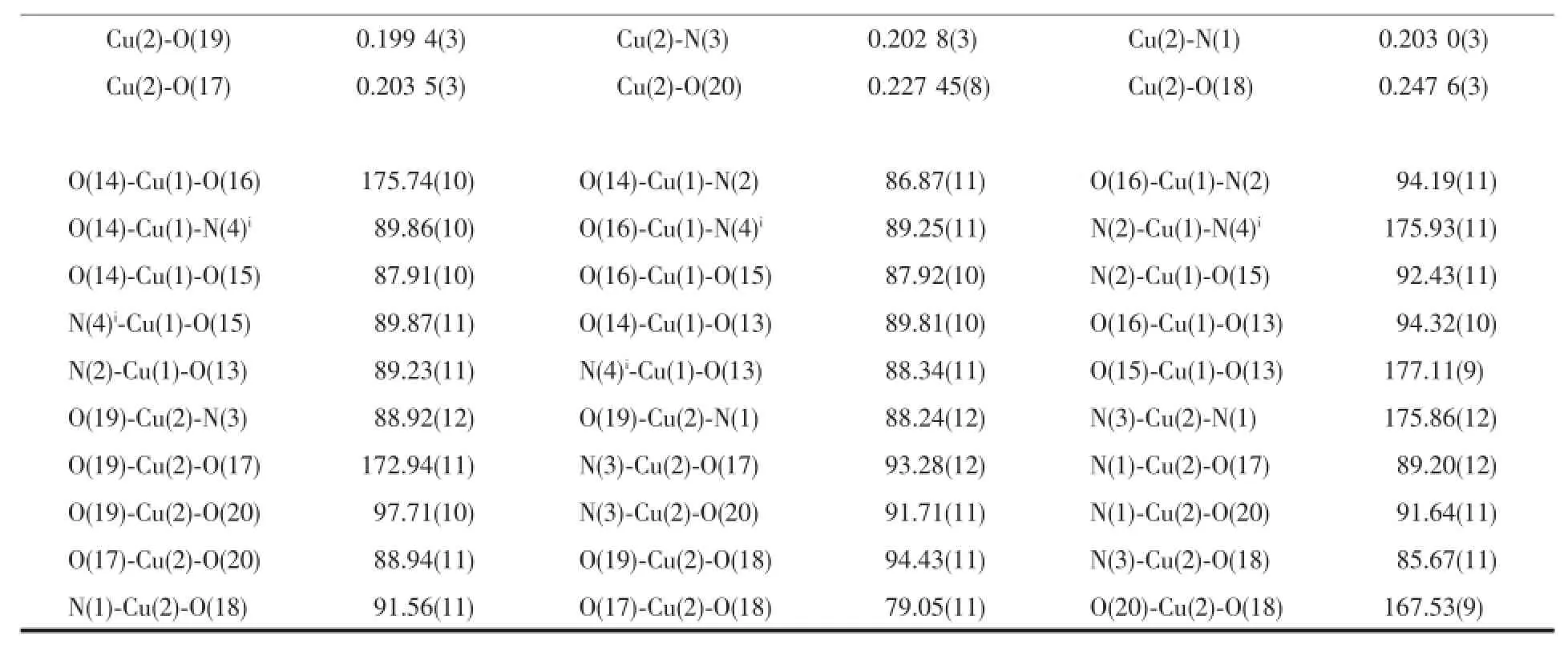
Continued Table2

Table4 Selected bond lengths(nm)and angles(°)for complex 2

Table5 Selected bond lengths(nm)and angles(°)for complex 3

Table6 Selected bond lengths(nm)and angles(°)for complex 4

Table7 Selected bond lengths(nm)and angles(°)for complex 5

Fig.1 View of the coordination environment of the Cu(Ⅱ)centers in complex 1 with limited numbering scheme
2Results and discussion
2.1 Crystal structures of complexes 1 and 2
The X-ray crystal structure analyses reveal that complexes{[Cu(Hbpma)(H2O)4]2(SO4)3·3.5H2O}n(1)and {[Ni(Hbpma)(H2O)4]4(SO4)6·10.75H2O}n(2)are composed of one-dimensional chains bridged by flexible bpma ligands,free sulfate anions and solvated water molecules.In the coordination environments as depicted in Fig.1 and Fig.S1,there are crystallographically two unique Cu(Ⅱ)centers for complex 1 and four unique Ni(Ⅱ)centers for complex 2 in the asymmetric units, which result in one independent chain for complex 1 and three independent chains for complex 2.In two complexes,all metal ions display the same sixcoordinated distorted octahedral coordination geometries with only different bond lengths and angles coordinated by four water oxygen atoms and two pyridine nitrogen atoms of two different bpma ligands.The M-O bond distances fall in the range of 0.198 8(6)~0.247 6(3)nm,while the M-N bond lengths vary from 0.202 4(3)to 0.212 9(6)nm,respectively.Meanwhile, the imine groups of bpma ligands are all protonated adopting the trans-trans conformation and each bpma ligand bridges two metal ions into a one-dimensional chain structure as shown in Fig.2 and Fig.S2.It should be noted that all bpma ligands spiral around the axis composed of the metal ions,thus the onedimensional chain should be described as a pseudohelical structure.Along the chains,the intrachain distances between the successive metalions fall in the range of 1.207 98(10)~1.241 3(1)nm similar to the literature results[22].The packing of complexes 1 and 2 gives the similar 3D supramolecular framework and the sulfate anions occupy the voids between the 1D chains as shown in Fig.3 .

Fig.2 A perspective view of the one-dimensional pseudo-helical chain structure in complex 1
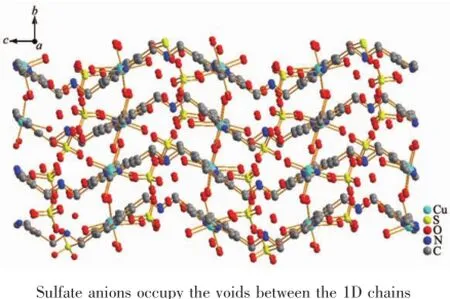
Fig.3 View of the 3D supramolecular framework of complex 1
2.2 Crystal structures of complexes 3 and 4
Complexes{[Mn(Hbpma)(H2O)4](SO4)1.5·3H2O}n(3) and{[Zn(Hbpma)(H2O)4]4(SO4)6·4H2O·4CH3OH}n(4) also consists of one-dimensional chains bridged by flexible bpma ligands,free sulfate anions and solvated molecules.In the coordination environments as depicted in Fig.4 and Fig.S3,there are crystallographically only one unique Mn(Ⅱ)and Zn(Ⅱ)center in the asymmetric units.In two complexes,the metal ions display the same six-coordinated distorted octahedral coordination geometries with only different bond lengths and angles provided by four water oxygen atoms and two pyridine nitrogen atoms of two different bpma ligands.The MO bond distances fall in the range of 0.207 64(18)~0.217 51(17)nm,while the M-N bond lengths vary from 0.217 6(2)to 0.229 66(19)nm,respectively. Furthermore,the imine groups of bpma ligands are all protonated assuming the trans-trans conformation and each bpma ligand bridges two metal ions into a onedimensional chain structure as shown in Fig.5 and Fig.S4.It is noteworthy that both the bpma ligands and the metal ions spiral around a 21-axis,thus the one-dimensional chain should be described as a single -stranded helical structure.Along the helical chains, the helical pitchs are 1.245 73(6)nm for complex 3 and 1.233 09(24)nm for complex 4 corresponding to the c axis.
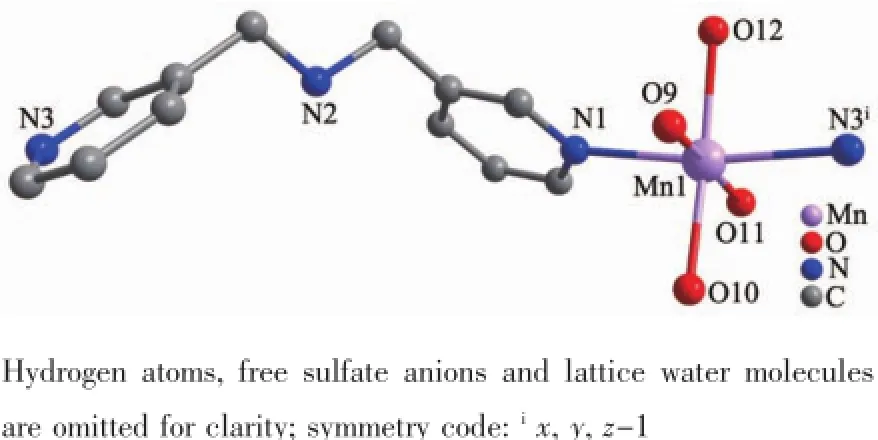
Fig.4 View of the coordination environment of the Mn(Ⅱ)center in complex 3 with limited numbering scheme
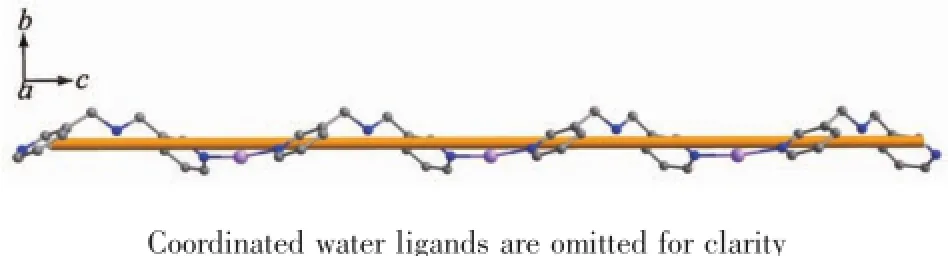
Fig.5 A perspective view of the one-dimensional helical chain structure along the c axis in complex 3
2.3 Crystal structure of complex 5
The X-ray crystal structure analyses reveal that complex{[Cu(Hbpma)2(H2O)2](SO4)2·9H2O}n(5)is composedoftwo-dimensionallayersbridgedby flexible bpma ligands,free sulfate anions and lattice water molecules.In the coordination environment as shown in Fig.6 ,there is crystallographically oneindependent Cu(Ⅱ)center in a distorted six-coordinated octahedral geometry.Four nitrogen atoms from four different protonated bpma ligands form the equatorial plane of the Cu(Ⅱ)ion,while two water ligands occupy the apical positions.In the complex,the Cu-N bond distances vary from 0.202 1(4)to 0.203 6(4)nm and the Cu-O bond lengthsare0.243 5(4)and 0.248 6(4)nm,respectively.Meantime,each bpma ligand binds two Cu(Ⅱ)ions leading to a two-dimensional layer as illustrated in Fig.7 .It is noteworthy that the bpma ligands in the trans-trans conformation form the polymeric helical chains propagating along the crystallographic b axis with a helical pitch of 1.303 52(13) nm.Therefore,the 2D sheet can be described as an achiral helical tubular layer in which the left and right helical chains appear alternatively as shown in Fig.7 .

Fig.6 View of the coordination environment of the Cu(Ⅱ)center in complex 5 with limited numbering scheme
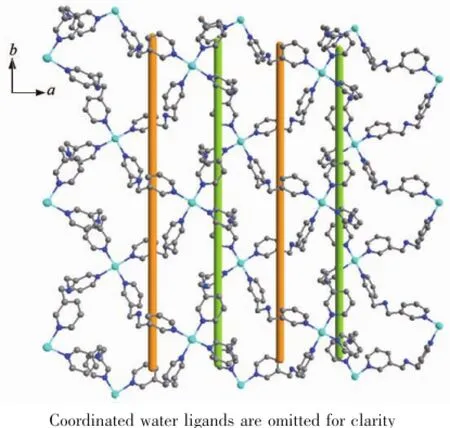
Fig.7 A perspective view of the two-dimensional helical layer structure parallel to the ab plane in complex 5

Fig.8 Plots of χMT(○)and(+)versus T for complex 1
2.4 Magnetic properties
The variable temperature magnetic susceptibilities of complexes 1,3 and 5 were measured and plots of χMT andversus T are shown in Fig.8 ~10,where χMis the magnetic susceptibility per[Cu(Hbpma)(H2O)4]2(SO4)3·3.5H2O unit for complex 1,per[Mn(Hbpma) (H2O)4](SO4)1.5·3H2Ounitforcomplex 3andper [Cu(Hbpma)2(H2O)2](SO4)2·9H2O unit for complex 5. For complex 1,the χMT value at room temperature is 0.740 cm3·K·mol-1,which is a little lower than the spin-only value(0.750 cm3·K·mol-1)expected for two uncorrelated Cu(Ⅱ)centers(S=1/2).For complex 3,the χMT value at room temperature is 4.310 cm3·K·mol-1, which is a little lower than the spin-only value(4.375 cm3·K·mol-1)expected for one high-spin Mn(Ⅱ)center (S=5/2).For complex 5,the χMT value at room temperature is 0.379 cm3·K·mol-1,which is close to the spin-only value(0.375 cm3·K·mol-1)expected for one uncorrelated Cu(Ⅱ)center(S=1/2).On lowering the temperature,the χMT values of three complexes decrease continuously,reaching 0.168 cm3·K·mol-1for complex 1,4.088 cm3·K·mol-1for complex 3 and 0.026 cm3·K·mol-1for complex 5 at 2 K.From the plots ofagainst T,the curves of three complexes follow the Curie-Weiss law χM=C/(T-θ),with fittedparameters θ=-8.70 K and C=0.76 cm3·K·mol-1for complex 1,θ=-0.92 K and C=4.30 cm3·K·mol-1for complex 3 and θ=-25.53 K and C=0.42 cm3·K·mol-1forcomplex5,indicatingthatantiferromagnetic exchange interactions are predominant in three cases. In the crystal structures,the distances between two adjacent Cu(Ⅱ)or Mn(Ⅱ)centers are over 1.1 nm mediated by bpma bridges,so there should be weak antiferromagnetic interactions in three complexes.

Fig.9 Plots of χMT(○)and(+)versus T for complex 3

Fig.1 0Plots of χMT(○)and(+)versus T for complex 5
2.5 Thermal behavior
All the synthesized complexes are air stable. Thermogravimetric analysis(TGA)was carried out in the interest of studying the thermal stability of these helical structure materials and the TGA curves are provided in Fig.1 1.The weight loss curves of five complexes are found to be similar exhibiting a twostep weight loss process.The first step of weight loss in the range of 20~200℃should be corresponding to the partly loss of the free or coordinated water molecules.At about 200℃,five complexes show the weight loss of 7.02%to 19.66%,less than the calculated values(12.02%to 23.98%).Over the range of 200~500℃,the sharp weight loss may be due to the decomposition of the complexes.

Fig.1 1TGA curves for complexes 1~5
3Conclusions
In summary,five helical structure complexes constructed by flexible bpma ligands were synthesized and structurally characterized in which all protonated bpma ligands assume the trans-trans conformation. Complexes 1 and 2 show the pseudo-helical chain structures and complexes 3 and 4 exhibit the singlestranded helical chain structures,whereas complex 5 has an achiral helical tubular layer network.These results are beneficial to form more novel helical structures.
Supporting information is available at http://www.wjhxxb.cn
[1]Leong W L,Vittal J J.Chem.Rev.,2011,111:688-764
[2]Li C P,Guo J,Du M.Inorg.Chem.Commun.,2013,38:70-73
[3]Chamayou A C,Makhloufi G,Nafie L A,et al.Inorg.Chem., 2015,54:2193-2203
[4]Lin M J,Jouaiti A,Kyritsakas N,et al.Chem.Commun.,2010, 46:115-117
[5]He J H,Chen H Y,Xiao D R,et al.CrystEngComm,2011, 13:4841-4845
[6]Xiao D R,Li Y G,Wang E B,et al.Inorg.Chem.,2007,46: 4158-4166
[7]Xin R,Yu X Y,Gao W P,et al.Inorg.Chem.Commun.,2013,35:38-41
[8]SONG Shuang(宋爽),CHA Yu-E(查玉娥),WANG Yu-Feng (王育丰),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(6):1234-1242
[9]Cao X Y,Li Z J,Zhang J,et al.CrystEngComm,2008,10: 1345-1349
[10]Han L,Zhou Y,Zhao W N,et al.Cryst.Growth Des.,2009, 9:660-662
[11]Li Z Z,Du L,Zhang X Z,et al.Inorg.Chem.Commun., 2014,45:20-24
[12]Yuan G Z,Zhu C F,Liu Y,et al.J.Am.Chem.Soc.,2009, 131:10452-10460
[13]Huang F P,Li H Y,Tian J L,et al.Cryst.Growth Des., 2009,9:3191-3196
[14]Zhang J,Chen Y B,Chen S M,et al.Inorg.Chem.,2006,45: 3161-3163
[15]Luan X J,Cai X H,Wang Y Y,et al.Chem.Eur.J.,2006,12: 6281-6289
[16]Sun X L,Wang Z J,Zang S Q,et al.Cryst.Growth Des., 2012,12:4431-4440
[17]Cui Z H,Wang S S,Wang X G,et al.Z.Anorg.Allg.Chem., 2012,638:669-674
[18]Wang S S,Xi P M,Wang X G,et al.Transition Met.Chem., 2013,38:105-112
[19]Larsen S,Michelsen K,Pedersen E.Acta Chem.Scand.Ser. A,1986,40:63-76
[20]Sheldrick G M.SADABS 2.05,University of Göttingen, Germany,2000.
[21]Sheldrick G M.Acta Crystallogr.Sect.A,2008,64:112-122
[22]CUI Zhi-Hua(崔志华),WANG Si-Si(王思思),WANG Xiu-Guang(王修光),et al.Chinese J.Struct.Chem.(结构化学), 2012,31(6):769-776
Syntheses,Crystal Structures and Properties of a Series of Helical Complexes Constructed by Flexible N,N′-Bis(3-pyridylmethyl)amine
YUE Cui-MinGAO Dong-Zhao*WANG Xiu-Guang
(Tianjin Key Laboratory of Structure and Performance for Functional Molecules;Key Laboratory of Inorganic-Organic Hybrid Functional Material Chemistry,Ministry of Education;College of Chemistry,Tianjin Normal University,Tianjin 300387,China)
Five helical coordination polymers{[Cu(Hbpma)(H2O)4]2(SO4)3·3.5H2O}n(1),{[Ni(Hbpma)(H2O)4]4(SO4)6· 10.75H2O}n(2),{[Mn(Hbpma)(H2O)4](SO4)1.5·3H2O}n(3),{[Zn(Hbpma)(H2O)4]4(SO4)6·4H2O·4CH3OH}n(4)and{[Cu (Hbpma)2(H2O)2](SO4)2·9H2O}n(5)(bpma=N,N′-bis(3-pyridylmethyl)amine)were synthesized and structurally characterized.The single-crystal X-ray analyses indicate that complexes 1~4 crystallize in the one-dimensional chain structures and complex 5 shows the two-dimensional layer structure,in which the metal ions are bridged by the protonated bpma ligands.It is noted that the flexible bpma ligands with the trans-trans conformation lead to the pseudo-helical chains for complexes 1~2,the helical chains for complexes 3~4 and a helical layer for complex 5. The magnetic properties and the thermal behavior are investigated.CCDC:966929,1;966932,2;966930,3; 966931,4;1054736,5.
metal complex;N,N′-bis(3-pyridylmethyl)amine;helical structure;magnetic property;thermal behavior
O614.121;O614.24+1;O614.71+1;O614.81+3
A
1001-4861(2015)08-1609-10
10.11862/CJIC.2015.213
2015-04-13。收修改稿日期:2015-05-28。
*通讯联系人。E-mail:tjgaodz@sina.com

