多级结构氧化锌的构筑、形貌调控及其光催化活性
全微雷 张金敏 沈俊海 李良超 李佳佳
(浙江师范大学化学与生命科学学院,金华321004)
多级结构氧化锌的构筑、形貌调控及其光催化活性
全微雷 张金敏 沈俊海 李良超*李佳佳
(浙江师范大学化学与生命科学学院,金华321004)
分别以混合常见二锌盐为锌源,以离子液体和柠檬酸钠为表面活性剂,在绿色温和的条件下采用二次沉积法制备出多级结构氧化锌。用XRD、IR、SEM、UV-Vis、PL等表征了样品的组成、结构、形貌、光致发光性能及光催化性能。探讨了表面活性剂、阴离子、温度等因素对氧化锌形貌的影响。结果表明,由不同表面活性剂所得到样品的多级结构有较大的差异。此外,推测了在多级结构氧化锌形成过程中,阴离子和温度对样品形貌的调控作用,并对比了三种典型样品的光催化性能,其中样品-1的光催化性能最好。
氧化锌;多级结构;形貌调控;光催化
0Introduction
Due to the special microstructure and physicochemical properties,inorganic micro-nano materials with different microscopic sizes and morphologies have been widely applied in optics,electricity,magnetism,biological medicine,catalysis,and other fields[1-4]. Furthermore,with the improvement of application demands,the control on material microscopic morphology has been paid much attention[5-6].As one of the most important semiconductor materials,ZnO has emerged as one of the most promising candidate materials owing to its superior properties and the potential advantage in future electron device[7-10].According to the principle that material properties depend on its structure,researchers have devoted themselvesto the controllable synthesis of materials with different microscopic morphologies and sizes.For example,Ma et al[11]reported three kinds of ZnO with different morphologies and found that they possessed various photocatalytic activities on methyl orange,in which the nanorod-like ZnO has the best photocatalytic activity,the flower-like ZnO is the second,and the nanoparticle ZnO is the worst.In addition,much more different morphologies of ZnO have been reported, such as mesoporous spheres,tubular,four foot shape and fibrous,etc[12-16],in which most researches are about ZnO nanoparticles and nanoplates.However,to the best of our knowledge,there have been no reports onthehierarchicalZnOconstructedbytwo morphologies.
A lot of micro-nano structure in the nature usuallyshowssomeinconceivablefunction.For example,the micro-nano structure on the surface of lotus leaf makes it have super hydrophobic and selfcleaning function.The micro-nano structure on the leg of water flies makes it walk on the water freely[17].So themicro-nanostructurehasinfinitemystery. However,whether the hierarchical ZnO assembled by micro-nano structure has the unusual photocatalytic activity?It is a pity that the reports associated with the above question are very little,the main reason is that it is very difficult to fabricate the well-defined micro-nano structure and to control its morphology effectively.The Chemical Vapor Deposition(CVD) method has been paid attention owing to its high efficiency[18-19].However,the CVD method needs high temperature and can introduce impurities.There are very few reports[20-22]about the simple fabrication of hierarchical ZnO,especially under the green and mild preparation conditions.
Besides,in regard to the regulation of ZnO microstructure,the single zinc salt is often used as the zinc source to fabricate ZnO[23-24],and some common surfactants(e.g.CTAB,PVP,etc.)are used as the template agent or structure-directing agent[25-26].However,these surfactants are difficult to be removed completely in the post-processing,which may have negative effect on ZnO performance,due to the reduction in the polarization plane of ZnO.But the ionic liquid(IL)as a kind of green solvent has been applied in the fabrication of inorganic micro-nano materials including ZnO[27-28],which can work as the co-surfactant and be removed easily.
Thus we choose the mixed zinc salts as the zinc source.Beacuse the anions in zinc salts have various spatial structures and electronegativities,ZnO seed may have different absorption capacities to them,so they can affect the crystal growth to adjust ZnO morphology.On the other hand,the introduction of IL can improve the solubility of inorganics and organics, and IL can play a role as the special surfactant.More importantly,the effect of IL on ZnO morphology can be studied by changing the anion in IL.Herein,we report the ZnO with hierarchical structure by a simple and green secondary deposition method.Based on our previous work[29],it is expected to fabricate the hierarchical ZnO with well-organized micro-nano structures, i.e.,micro-sized ZnO grains are coated by ZnO nanoparticles.In this paper,the precursor solution including the micro-sized ZnO grains is obtained by hydrothermal method.Then the pH value of the precursor solution is adjusted by NaOH solution under the room temperature,the residual Zn2+ions can form ZnO on the surface of micro-sized ZnO grains.As a result,the prepared hierarchical ZnO consists of the micro-sized ZnO grains fabricated by hydrothermal method and the coating layer of nano-sized ZnO particlesobtainedbythesecondarydeposition. Furthermore,someinfluentialfactorsonthe morphology of hierarchical ZnO,such as the kind of anion in zinc salt,surfactants and reaction temperature are studied.In addition,the photocatalytic activity of three kinds of morphological ZnO on methyl orange(MO)is also studied.
1Experimental
1.1 Materials
Zinc acetate(Zn(CH3COO)2·2H2O),Zinc sulfate (ZnSO4·7H2O),Zinc chloride(ZnCl2),Zinc nitrate (Zn(NO3)2·6H2O),NaOH,C3H7NO2,C2H5OH,Sodium citrate(C6H5Na3O7·2H2O)were all of analytical purityprovided by the Shanghai Sinopharm Chemical Reagent Co.,Ltd.and were used as received without further purification.[BMIM]Cl,[BMIM]Br and deionized water were prepared by this laboratory.
1.2 Sample preparation
1.2.1 Fabrication of precursor solution
[BMIM]Cl and[BMIM]Br were synthesized following the literature procedures[30-31].
The preparation procedure is as follows:1 mmol Zn(CH3COO)2·2H2O and 1 mmol ZnCl2were dispersed in a mixed solution consisting of 30.0 mL deionized water and 2 mmol[BMIM]Br to form white turbid solution.Then 2 mmol alanine was added into the above solution,stirred until a clear solution was obtained.4 mmol NaOH was added into the clear solution under vigorous stirring.A white colloidal turbid liquid was finally obtained and transferred into a Teflon-lined stainless autoclave(volume of 50.0 mL).Then the autoclave was heated and maintained at 150℃for 15 h,the precursor solution was obtained after cooling down the system to room temperature.
1.2.2 Fabrication of the sample-1
1 mol·L-1NaOH solution was added dropwise into 25 mL of the above precursor solution until the precipitation was formed completely.After filtering, the solid precipitation was washed three times with deionized water and ethanol.Then,the sample-1 was obtained by drying to constant weight under vacuum at 50℃.By changing the zinc salt,surfactants and reaction temperature,other ZnO samples in different morphologies were obtained by the same method.
1.3 Photocatalytic studies
Methyl orange(MO)was used as the simulative pollutant to evaluate the photocatalytic activity of the as-prepared samples by standard curve method.In a typical experiment,3 mmol sample-1 was dispersed uniformly in 100 mL MO solution with 12 mg·L-1by ultrasonication.The above mixture was irradiated under UV lamp(3×8 W).3 mL suspension was taken out from the reaction system every 10 min,and immediately centrifuged.The absorbance of centrifugal solution was measured by a UV-Vis spectrophotometer at λmax=465 nm.
In addition,the influence of ZnO dosage on photocatalytic activity was studied.A certain amount of sample-1 was uniformly dispersed in 100 mL MO solution with 12 mg·L-1by ultrasonication,which was irradiated under UV lamp(3×8 W).After 80 min,the dagradation rate of MO was measured by a UV-Vis spectrophotometer at λmax=465 nm.
1.4 Characterization
Thephasestructureofthesamplewas characterized using an X-ray diffractometer(Philips-PW3040/60)with Cu Kα radiation(λ=0.154 18 nm, 10°·min-1,U=40 kV,I=40 mA,Ni filter)in the range of 2θ=10°~80°.The infrared spectrum was recorded on the Nicolet Nexus 670 Fourier transform infrared spectrometer(FTIR)using KBr pellets(scan range of 400~4 000 cm-1).The morphologies and microstructures were observed by a scanning electron microscopy (SEM,Hitachi S-4800,U=5.0 kV).The UV-Vis absorption of MO solution was measured with an ultraviolet -visible spectrophotometer(Shmadzu UV-2501PC).A photoluminescence(PL)spectrum of the sample-1 was recorded in an FL-920 fluorescence spectrophotometer in wavelength of 325 nm.The organic carbon of MO solution was measured by Total Organic Carbon Analyzer(ELEMENTAR Liqui TOCⅡ).
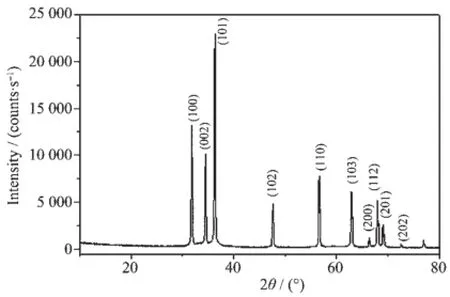
Fig.1 XRD pattern of the sample-1
2Results and discussion
2.1 X-ray diffraction
The XRD pattern of sample-1 is presented in Fig.1 .The strong and sharp diffraction peaks demonstrate that sample-1 has good crystallization.In addition,all peaks are in agreement with the characteristicpeaks of hexagonal ZnO(PDF-2 No 36-1451),and correspond to(100),(002),(101),(102),(110)and (103)crystal face.No diffraction peaks of impurities are observed,which illustrates that the as-prepared sample-1 is highly pure.
2.2 FTIR spectra
Fig.2 shows the FTIR spectra of sample-1.The bands at 435 and 534 cm-1are attributed to Zn-O axial stretching vibration[32].The band at 3 432 cm-1is assigned to-OH stretching vibration,which may be caused by the absorbed H2O on the surface of sample-1.The band at 1 099 cm-1is assigned to C-O stretching vibration.In addition,the weak bands at 1637 and 1 384 cm-1correspond to anti-symmetric and symmetric stretching vibration of COO-,suggesting that the CH3COO-groups are absorbed on the ZnO grain surface and there may be some coordination bonds between the Zn2+and CH3COO-.
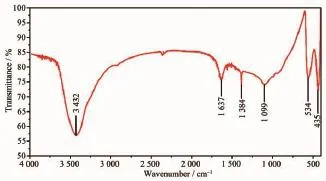
Fig.2 FTIR spectrum of the sample-1
2.3 Morphology
The SEM images of sample-1 are shown in Fig.3 . The ZnO particles possess a hierarchical structure with etching decorative pattern,indicating a high surfacearea.Thehigh-resolutionimage(Fig.3 b) clearlyshowsthatthewell-beddedsurfaceof hierarchical ZnO is constructed by nanoparticles of 50~100 nm,and has agglomeration to some extent. Some of these nanoparticles keep spherical morphology, while other shows the irregular morphology due to serious reunion.
2.3.1 Influenceofsurfactantonthesamplemorphology
Surfactant can regulate and control the nucleation and assembling of ZnO nanoparticles[33].In addition, due to the changes in spatial structure,solubility and functional groups of surfactants,the special regulating andcontrollingeffectisdifferent.Therefore,to investigate the effect of surfactants(IL and sodium citrate),the kind and dosage of the surfactant are considered.The experimental parameters and sample morphologies are given in Table1 (in section 1.2.2) and Fig.4 ,saparately.
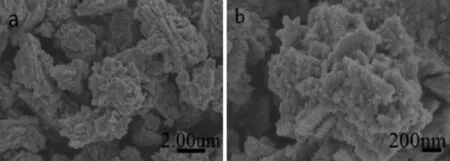
Fig.3 SEM images of the sample-1
The morphological differences are evident.For example,sample-2 presents the irregular spherical aggregates formed by nanospheres with 100~150 nm, and the nanospheres are actually assembled by the smaller spherical nanoparticles with 10~20 nm.While the irregular nanospheres convert to the triangulated petals,when the[BMIM]Cl is replaced by 1 mmol sodium citrate.So the flower-like ZnO(sample-3)is well revealed in Fig.4 b,which is constituted by a pistil and some petals with rugged surface due to the adhesion ZnO nanoparticles.Here one can reasonably speculate that the pistils and petals are assembled by ZnOnanoparticlesaccordingtothespecific orientation.Moreover,combining[BMIM]Clwith sodiumcitrateforsample-4,thepreparedZnOpresents multi-foot structure(Fig.4 c).In fact,the ZnO nanoparticles adhere on the surface of every foot,and the cone-like foot end is similar to the strawberry.At last,the ZnO nanospheres(sample-5)with the size of 100~200 nm(Fig.4 d)are obtained without any IL and sodium citrate,their surface is unsmooth due to being covered by the smaller particles.Compared Fig.4 a and 4d,their size and morphology are similar,but the dispersity of the latter(Fig.4 d)is better.
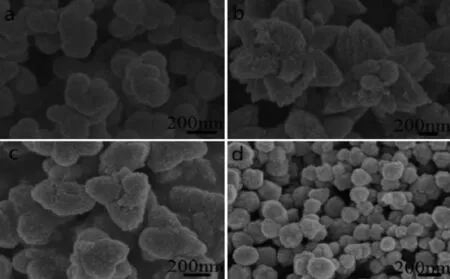
Fig.4 Effect of surfactants on ZnO sample morphology
The above results indicate that the surfactant plays a critical role in the formation process of hierarchical ZnO.No matter what the molar ratio of ILs to sodium citrate is,the minimum basic unit of all obtainedsamplesisnanoparticles.However,the fundamentaldifferenceamongaboveas-obtained samples attributes to the interaction forces among nanoparticles,leadingtodifferentorientationsof nanoparticles in the process of self-assembly,which illustrates that IL and sodium citrate have some influence on self-assembly kinetics of nanoparticles, or have the specific effect as template,structure orientedagentorbothontheassemblingof nanoparticles.The hierarchical structure of sample-1 hasthebiggestsizeandthemostcomplicated morphology.By contrast,sample-5 prepared without any surfactant is the smallest in the size,and it presents simple spherical structure,which is the most common morphology caused by nanoparticles reunion. In addition,comparing sample-3 and sample-4,the arrangeorientationofnanoparticlesdisappears completely,because of the replacement of[BMIM]Br by[BMIM]Cl.Thus,it can be concluded that the auxiliary effect of[BMIM]Cl on nanoparticle assembly is poorer than that of[BMIM]Br.It may because that the Cl-has a larger electronegativity and a smaller ionic radius compared with that of Br-,i.e.,Cl-has a weaker ability of donating electrons.However,the introductionofsodiumcitrateishelpfulfor nanoparticles to arrange orderly(Fig.4 b),and the effect of sodium citrate as the template agent(or structure oriented agent)is much better compared with that of[BMIM]Br.It is because that CH3COO-is more easily to combine with Zn2+as confirmed by FTIR.Moreover,when[BMIM]Cl is substituted for [BMIM]Br,thetemplateeffectofsodiumcitrate becomes weakened drastically,which also proves that [BMIM]Cl cannot work as the template.In addition, Cl-with a big electronegativity also weakens the template effect of sodium citrate.To sum up,the template effect of sodium citrate is the best,while that of the[BMIM]Cl is the worst among three surfactants in terms of constructing hierarchical ZnO.
2.3.2 Influence of zinc source on the sample morphology
Only the zinc source is changed in the following experiments.The precursor solutions with different mixed zinc salts are designed as shown in Table1 .
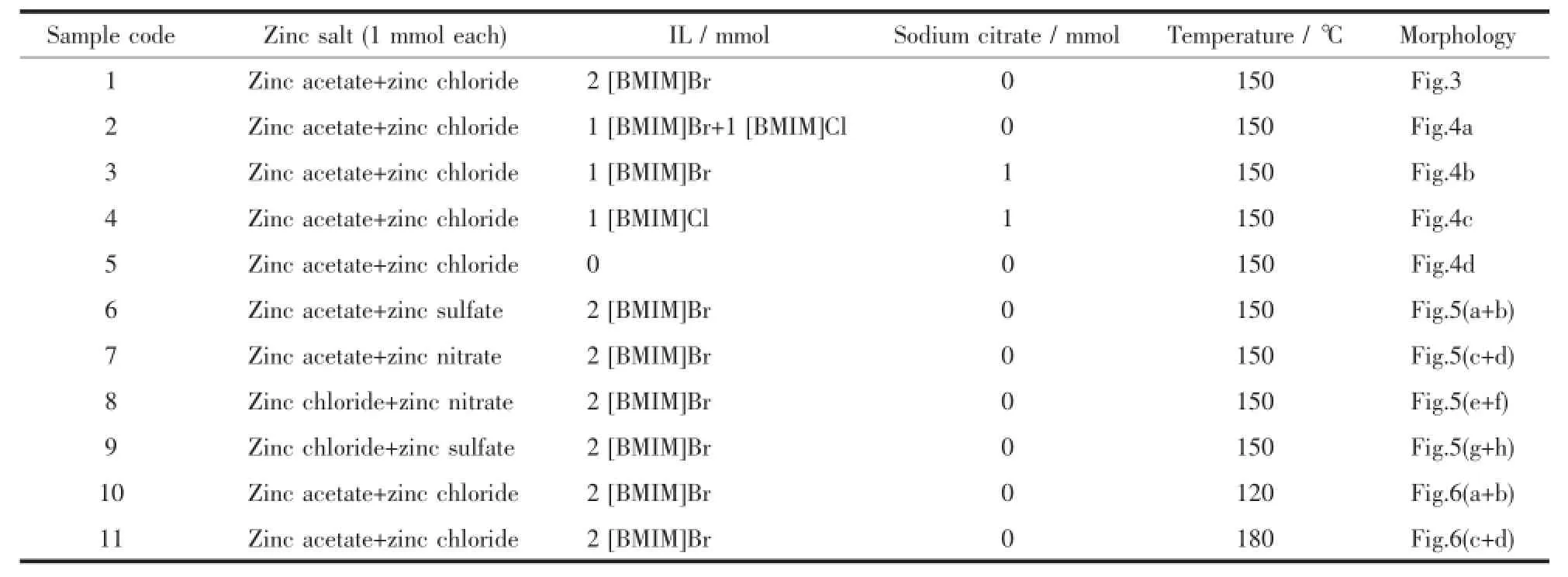
Table1 Experimental parameters for different sample
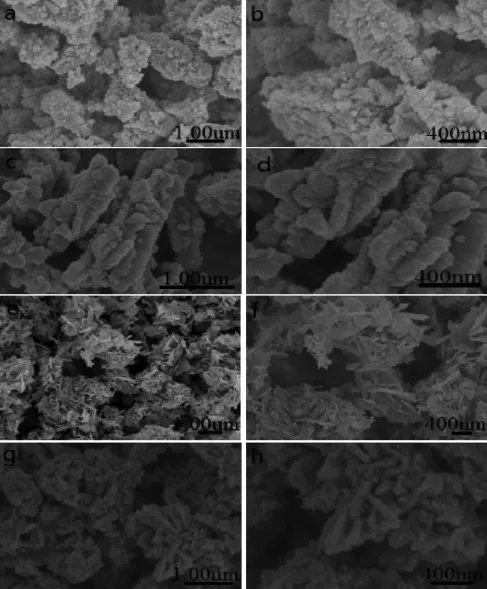
Fig.5 SEM images of ZnO samples prepared with different zinc sources
The ZnO morphologies and structures prepared by different zinc sources are shown in Fig.5 .The ZnO morphologies and structures are closely related to thezinc source.When the mixture of zinc acetate and zinc sulfate is used as the zinc source,sample-6 formedbyquasi-sphericalnanoparticlespresents inhomogenous size and irregular morphology(Fig.5 a). It can be seen from the high-resolution image(Fig.5 b) that the hierarchical structure of sample-6 is constituted by nanoparticles,and the cusp emissions are outward in the scattering form.Nevertheless,the obtained hierarchical structure of sample-7 is the agglomerate by multiple chains(Fig.5 c and 5d),whose surface is unsmooth due tothenanoparticlesassembly.In addition,the flake-like morphology of sample-8 can be found in Fig.5 e and 5f,when the mixed zinc salts are zinc chloride and zinc nitrate.The high-resolution image(Fig.5 f)ofsample-8showsthedisorder hierarchical structure with wheatear-like or flowervine morphology,some of which possess complicated structure arranged closely by the nanoflakes,while others have orderly structure arranged loosely by nanoflakes.The SEM images of sample-9 prepared by zinc chloride and zinc sulfate(Fig.5 g and 5h)show some flower patterns attached by some short chains. The surface of flower patterns is rough and the shape of flower patterns is different.The high-resolution image(Fig.5 h)shows that the hierarchical structure of sample-9issimilartocarvedpetal,andmany nanoparticles adhere well on the surface of sample-9.
It can be concluded that the samples prepared by altering zinc source are different in morphology. Although the samples are hierarchical structure assembled by nanoparticles,their assembling processes are not the same,which is mainly decided by the anion in zinc salts.Throughout Fig.5 ,only sample-8 is not assembled by nanoparticles,i.e.,Cl-and NO3-can directlyaffectthegrowthofZnOparticles.In addition,it can be found by comparing Fig.5 d with 5h that the two samples have well-organized hierarchical structures.It may be because that one crystal face of ZnO seed has a weaker absorption capacity to Cl-or NO3-,leading to the seed growth along this crystal face to form the regular grain,i.e.,Cl-makes grain grow into the chain-like structure,while NO3-makes grain grow into the flake-like structure,which may be related to anionic spatial structure and electronegativity.Interestingly,the morphology of sample-9 shown in Fig.5 g is very similar to that of sample-1(Fig.3 ), indicating that the effect of SO42-and CH3COO-as the template is similar.Maybe it is because that the two anions all have larger volume and possess the threedimensional structure,so that ZnO seed has weaker absorption on them.In addition,their larger volume may be propitious to assist the construction of the well-organized hierarchical structure.
2.3.3 Influence of temperature on the sample morphology
Tostudytheeffectoftemperatureonthe morphologyoffinalsample,experimentsare respectively carried out at 120 and 180℃under invariability of other conditions.
When the reaction temperature of the precursor solution is 120℃,the morphologies of the obtained sample-10 are shown in Fig.6 a and 6b.It can be observed from Fig.6 a that the sample-10 is thin flakelike agglomerates with irregular shape and rough surface.As shown in the high-resolution(Fig.6 b), theseagglomerateshavethedisorderedstructure constituted by nanoflakes with irregular shape.Some of nanoflakes are wide and short,on the contrary, others are narrow and long.On the whole,these nanoflakes are similar to the fallen leaves stacking in a mess.The nanoflake-like morphology disappears generally when the reaction temperature increases to 180℃,while the as-prepared sample-11 appears to bethemixedstructurecomposingtheirregular nanorods and nanoparticles aggregates(Fig.6 c and 6d),and the nanoparticles of 60~100 nm attach to the nanorod surface or fill in the interspace among the nanorods.It can also be found from Fig.6 d that the nanorodsstackirregularlyandtheirsurfaceis unsmooth.
From the above results,it can be learned that the structure unit of sample-10 presents the flake-like morphology at 120℃.While sample-11 has two structure units including the nanorods and nanoparticles at 180℃.Compared with sample-1(prepared at 150℃),the samples prepared at 120 and 180℃haveno obvious hierarchical structure.So,the temperature plays an important role in the formation process of hierarchicalZnO.Thepossiblereasonsofthis morphological difference are as follows.Firstly,the temperaturehasagreatinfluenceonthegrain nucleation and growth rate.The nucleation rate of grain is faster than its growth rate if the temperature is too low,so the obtained grain is usually irregular under this case.On the contrast,the growth rate of grainisfasterthanitsnucleationrateifthe temperatureishighenough,whichisalso disadvantageous to form the regular grains.When the growth rate of grain is faster than the diffusion rate of ions,the nonuniform distribution of concentration has anenormouseffectontheregularityofgrains. Secondly,hierarchical structure is usually assembled by small structure unit,while the assembly process needs the driving force.Hence,temperature can provide the driving force for grain assembly.But a too high temperature can dramatically enhance the Brown movement of particles,which would hinder the selfassembly of grains.On the contrary,a too low temperature can not provide enough driving force for grains assembly,which often leads to the incomplete assembly and forms disorder structure.In addition, the nano-flake is one dimensional,while the nano-rod is three dimensional in structure.So,with the increase of space dimension,the driving force needed for selfassemblyisbecominggreaterandgreater.In conclusion,sample-10preparedunderlow temperature(120℃)presents a flake-like morphology due to lack of driving force(Fig.6 a and 6b).However, owing to the enhanced Brown effect,some parts of ZnO grains form rod-like structure(Fig.6 c and 6d)at high temperature(180℃).
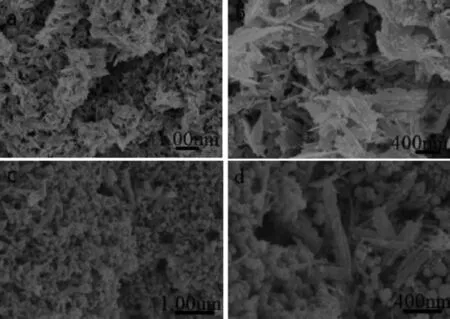
Fig.6 SEM images of ZnO samples prepared under different reaction temperature
2.4 Optical properties
Fig.7 a presents the UV-Vis spectrum of the aspreparedsample-1.Comparedwithcharacteristic absorption of common ZnO(373 nm),the main absorption peak at 368 nm in Fig.7 a shows some blue shift.It may be due to the nano-effect of nanoparticles on the surface of hierarchical ZnO,or the interaction between Zn2+and CH3COO-absorbed by the ZnO grains making the 3d valence electrons of Zn2+transfer easily to the π*orbital of CH3COO-.Fig.7 b shows the PL spectrum of sample-1 with the excitation wavelengthof 325 nm at room temperature.The PL spectrum possesses two emission bands,i.e.one is the narrow UV emission at about 320 nm corresponding to the electronic radiative transition from conduction band to valence band,and the other is a strong and broad emissionbandlocatedinthenearUVregion. Furthermore,the strong double bands in 375~450 nm are the blue emission of ZnO in the visible region, one of which corresponds to the red shift and the other is the blue shift relative to central transition frequency,it may be due to the emission of excitonic recombination or the ZnO crystal defect caused by the excess OH-in the process of adding NaOH dropwise. The weak green emission at 490 nm may be caused by the electronic transition from the low energy level to valence band.Compared with the conventional ZnO nanocrystals,allemissionpeaksexhibitthe phenomenon of blue shift to some extent.It may be becausethattheparticlesconstructingthe hierarchical structure belong to nano size,leading to that the emission peak position moves to the short wavelength,whichprovidesguaranteeforthe improvement of photocatalytic activity.
According to literature[34],the photoluminescence property of ZnO nanoparticle associates with the oxgen vacancies and defects on ZnO surface.In the processofphotoluminescence,superficialoxgen vacanciesanddefectscanmakephotogenerated electron to form the free exciton or bound exciton, leadingtoemittingfluorescence.Themorethe superficial oxgen vacancies and the more defects,the highter the intensity of fluorescence will be.The representativeZnOownsthestrongandbroad emission band in the range of 375~550 nm as shown in Fig.7 b,suggesting that hierarchical ZnO possesses more superficial oxgen vacancies and defects.However, the superficial oxgen vacancies and defects can make a large contribution to photocatalytic activity,because they can capture photogenerated electron to improve the efficiency of photoinduced charge separation.
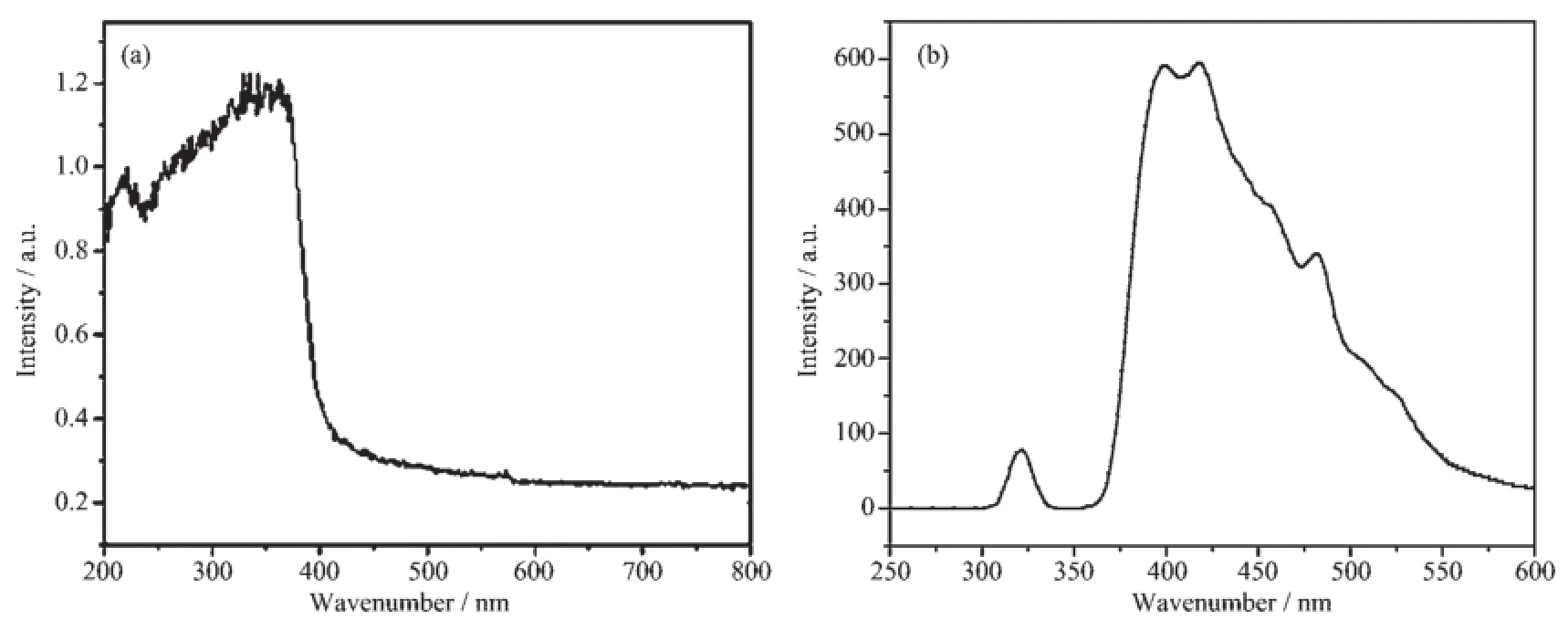
Fig.7 (a)UV-Vis diffuse reflectance spectrum and(b)PL spectrum of the sample-1
2.5 Evaluation of photocatalytic activity
By experiments,the standard curve equation of MO solution is A=0.074 2c+0.042 8(R2=0.999 8,A/ (mg·L-1)is the solution absorbance,c/(mol·L-1)is the solution concentration).The relative absorbance(A/A0) of solution is considered as 1,of which MO concentration is about 12.9 mg·L-1.In order to reach the adsorption-desorption equilibrium between the catalyst and MO,the mixed solutions including ZnO and MO have been stirred in the dark for 1 h.And C/C0values corresponding to 0 min in Fig.8 are all slightly less than 1,indicating that the three different ZnO samples have a weak absorption on MO.In the photocatalytic experiments,three kinds of as-prepared ZnO samples used as the catalyst are sample-1,sample-2,and sample-10 separately.
Fig.8 shows the photocatalytic activity of three different morphological ZnO samples on MO solution (12.9 mg·L-1)under UV irradiation.The C/C0values of all samples decrease with increasing time(Fig.8 ). Obviously,thedegradationrateofallsamples declines gradually,and compared with sample-2 andsample-10,the degradation rate of sample-1 is the fastest.Especially,when the irradiation time is up to 100 min,only the C/C0value of sample-1 reduces to below 0.05,proving that the MO has been degraded almost completely.By contrast,the C/C0value of sample-1 is the minimum under the same irradiation time among three samples,and the photocatalytic activity of sample is the best in the whole process of photocatalyticdegradation.Inaddition,thetotal organic carbon(w)of MO soultion degraded under sample-1 is shown in Table2 and the relational expression is
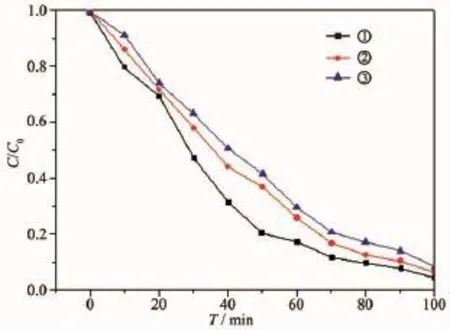
Fig.8 Photocatalytic activity of three different ZnO samples

Table2 Total content of organic carbon of MO soultion

Fig.9 (a)Photocatalytic activity under different the sample-1 dosages and(b)Reusability of the sample-1 evaluated for five cycles
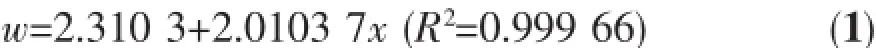
The w displays a dramatic variation as seen from Table2 ,incidating the effective photocatalyitc activity of sample-1.Besides,the decrement of carbon(η= 82%)is lower than the degradation rate(95%)of MO, which suggests that there are many intermediate products in the photocatalytic degradation.
It may be the hierarchical structure of sample-1 that improves the photocatalytic activity of ZnO,due to sample-2 and sample-10 without obvious hierarchical structure.So,the photocatalytic activity is closely associated with the microstructure and morphology of the catalyst.
The influence of ZnO dosage on photocatalytic activity is shown in Fig.9 a.The degradation rate of MO rises generally as the increase of ZnO dosage,but the change in degradation rate is becoming slow.It may be because that when the ZnO dosage is too high,it can lead to the light scattering effect,reducing the light absorption rate of solution[35].In addition,the inhomogenousdispersityofZnOhasalsosome influence on the photocatalytic activity.
To evaluate the reusability of sample-1,recycled experiments about the photocatalytic dagradation of MO have been performed.The photocatalytic activity of sample-1 is decreased to some extent after each recycle,while it still keeps above 90%after five recycles as shown in Fig.9 b,suggesting that sample-1 presents excellent photocatalytic stability.
3Conclusions
The hierarchical ZnO assembled by nanoparticles was prepared via a secondary deposition method by using the precursor solution including two zinc salts, IL and alanine as the raw materials,and the sodium hydroxideasprecipitant.Theas-preparedZnO samplesarehexagonalasevidencedbyXRD characterization.The kind of anion and temperature plays a vital role in constructing the morphology of hierarchical structure.The combination of anion has a great influence on the sample morphology,in which Cl-andhave the directing effect on the seed growth,but theand CH3COO-can play the role of the template in auxiliary constructing the hierarchical structure.In addition,IL and sodium citrate working as surfactant provide an assisted function for oriented growth of ZnO grain.Moreover,sample-1 presents a strong and broad absorption,and possesses a good emission performance in the UV and near UV region as seen from the UV-Vis and PL spectrum.The photocatalytic results indicate that the photocatalytic activity of three different morphological ZnO samples is different,among which sample-1 is the best.The photocatalytic activity of ZnO is closely related to the microstructure and morphology of ZnO.
[1]WANG Xin(汪信),LU Lu-De(陆路德).Chinese J.Inorg. Chem.(无机化学学报),2000,16(2):213-217
[2]Ren Y,Ma Z,Bruce P G.Chem.Soc.Rev.,2012,41(14): 4909-4927
[3]Lee K R,Lee J H,Yoo H I.J.Eur.Ceram.Soc.,2014,34 (10):2363-2370
[4]ZHENG Zhen-Miao(郑贞苗),TANG Xin-Cun(唐新村), WANG Yang(汪洋),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(4):731-738
[5]Yuan C Z,Wu H B,Xie Y,et al.Angew.Chem.Int.Ed., 2014,53(6):1488-1504
[6]XU De-Kang(徐德康),LIU Chu-Feng(刘楚枫),YAN Jia-Wei (阎佳薇),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31(4):689-695
[7]WANG Xin-Juan(王新娟),XIAO Yang(肖洋),XU Fei(徐斐), et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(8): 1821-1826
[8]Sharma R K,Ghose R.Ceram.Int.,2015,41(1):967-975
[9]Nevosad A,Hofstatter M,Supancic P,et al.J.Eur.Ceram. Soc.,2014,34(8):1963-1970
[10]Qiu Z W,Yang X P,Han J,et al.J.Am.Ceram.Soc.,2014,97 (7):2177-2184
[11]Ma S S,Li P,Lu C P,et al.J.Hazard.Mater.,2011,192(2): 730-740
[12]Cho S,Kim S,Jung D W,et al.Nanoscale,2011,3(9):3841-3848
[13]Deng S Z,Fan H M,Wang M,et al.ACS Nano,2010,4(1): 495-505
[14]Li H F,Huang Y H,Zhang Y,et al.Cryst.Growth Des., 2009,9(4):1863-1868
[15]Shang T M,Sun J H,Zhou Q F,et al.Cryst.Res.Technol., 2007,42(10):1002-1006
[16]Sangkhaprom N,Supaphol P,Pavarajarm V.Ceram.Int., 2010,36(1):357-363
[17]Gao X F,Jiang L.Nature,2004,432(7013):36-36
[18]Lao JY,WenJG,RenZF.NanoLett.,2002,2(11):1287-1291 [19]Yang Y H,Wang B,Yang G W.Cryst.Growth Des.,2007,7 (7):1242-1245
[20]Liu H,Li M,Wei Y,et al.Mater.Lett.,2014,137:300-303
[21]Huang Q,Cun T,Zuo W,et al.Appl.Surf.Sci.,2015,332: 581-590
[22]Shi R,Song X,Li J,et al.Mater.Chem.Phys.,2015,156:61 -68
[23]Chang G J,Lin S Y,Wu J J.Nanoscale,2014,6(3):1329-1334
[24]Kokotov M,Bar-Nachum S,Edri E,et al.J.Am.Chem.Soc., 2009,132(1):309-314
[25]Mclaren A,Valdes-Solis T,Li G,et al.J.Am.Chem.Soc., 2009,131(35):12540-12541
[26]Xu S,Wang Z L.Nano Res.,2011,4(11):1013-1098
[27]Zhou X,Xie Z X,Jiang Z Y,et al.Chem.Commun.,2005, 44:5572-5574
[28]Wang L,Chang L X,Wei L Q,et al.J.Mater.Chem.,2011, 21(39):15732-15740
[29]SHEN Jun-Hai(沈俊海),LI Jia-Jia(李佳佳),LI Liang-Chao (李良超),et al.Chem.J.Chinese Universites(高等学校化学学报),2014,35(6):1135-1141
[30]Huddleston J G,Willauer H D.Chem.Commun.,1998,16: 1765-1766
[31]Brindaban C R,Subhash B.Org.Lett.,2005,7(14):3049-3052
[32]Fernandes D M,Silva R,Hechenleitner A A,et al.Mater. Chem.Phys.,2009,115(1):110-115
[33]Xing R M,Zhu J H,Liu Q W,et al.Chem.Res.,2012,23 (5):57-60
[34]JING Li-Qiang(井立强),YUAN Fu-Long(袁福龙),HOU Hai-Ou(侯海鸥),et al.Sci.China Ser.B:Chem.(中国科学B辑:化学),2004,34(4):310-314
[35]HOU Chun-Yan(侯春燕).Thesis for the Master of Dalian Maritime University(大连海事大学硕士论文).2006.
Hierarchical ZnO:Architecture,Morphological Control and Photocatalytic Activity
QUAN Wei-LeiZHANG Jin-MinSHEN Jun-HaiLI Liang-Chao*LI Jia-Jia
(College of Chemistry and Life Sciences,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
Under the green and mild conditions,the hierarchical ZnO was fabricated by the secondary deposition with mixed two zinc salts(zinc source),ionic liquid(surfactant).The composition,structure,morphology, photoluminescence properties and photocatalytic activity of samples were characterized by XRD,IR,SEM,UVVis and PL,respectively.The influential factors on sample morphology,such as surfactant,the kind of anion and temperature,were discussed.The results indicate that surfactant has a significant regulation on the morphologies of as-prepared ZnO samples.Furthermore,the anion and temperature also play a critical role in the crystal structure and morphology of ZnO.In addition,all of as-prepared hierarchical ZnO show an excellent photocatalytic activity on methyl orange under UV lamp,where the sample-1 is slightly better than others.
ZnO;hierarchical structure;morphological control;photocatalysis
O614.24+1
A
1001-4861(2015)08-1626-11
10.11862/CJIC.2015.212
2015-04-21。收修改稿日期:2015-06-29。
国家自然科学基金(No.21071125)和浙江省大学生科技创新计划(No.2014R404056)资助项目。
*通讯联系人。E-mail:sky52@zjnu.cn;会员登记号:S06N6780M1401。

