一个具有四方锥和三角双锥构型的线性混合桥连三核铜配合物
吴迪 余柱 高大志 王欣 沈旋 朱敦如*,,2
(1南京工业大学化工学院,材料化学工程国家重点实验室,南京210009)
(2南京大学配位化学国家重点实验室,南京210093)
一个具有四方锥和三角双锥构型的线性混合桥连三核铜配合物
吴迪1余柱1高大志1王欣1沈旋1朱敦如*,1,2
(1南京工业大学化工学院,材料化学工程国家重点实验室,南京210009)
(2南京大学配位化学国家重点实验室,南京210093)
以反式-3-[N,N-二(2-吡啶甲基)氨甲基]-2-羟基-5-甲基苯甲醛肟(H2L)为配体,合成了一个新颖的线性三核铜(Ⅱ)配合物[Cu3L2(py)](ClO4)2·2THF(1)(py=吡啶,THF=四氢呋喃),对其进行了红外、紫外、热重和单晶结构表征。配合物1属于单斜晶系,空间群为C2/c,a=2.969 0(7)nm,b=1.302 8(3)nm,c=2.057 4(9)nm,β=132.435(2)°,V=5.873(3)nm3,Z=4,R1=0.047 9。单晶结构表明,这是一个混合桥(-N-O-和μ2-O-)连的线性五配位三核铜(Ⅱ)配合物:每个铜均由3个氮原子和2个氧原子配位,中间的铜为扭曲的三角双锥构型,而两侧的铜为扭曲的四方锥构型。变温磁化率显示1的铜离子间存在中等的反铁磁性(J=-33.3(5)cm-1)。
三核铜;五配位;晶体结构;混合桥连;反铁磁性
Trinuclear Cu(Ⅱ)complexes have received increasing attentions as models for the active sites of multicopper oxidases such as ascorbate oxidases,laccase and ceruloplasmin[1-2].A relatively large number of trinuclear Cu(Ⅱ)complexes have been reported over the past three decades[3-22].Generally,the trinuclearCu(Ⅱ)complexes can be classified as triangular[3-7]and linear arrangements[8-22].Up to now,linear trinuclear Cu(Ⅱ)complexes with respective coordination numbers of 4,5,4[9,14];4,5,5[20];5,4,5[19,21-22];5,5,5[8-13,15-17];5, 6,5[18,21];5,5,6[10]and 6,6,6[10],have been found. However,most of trinuclear Cu(Ⅱ)complexes with respective coordination numbers of 5,5,5 show the distorted square pyramidal configuration.Examples of five -coordinated trinuclear Cu(Ⅱ)complexes containing both the distorted square pyramidal and trigonal bipyramidal configuration are very limited[8].Herein we present the synthesis and crystal structure of a rare linear pentacoordinate trinuclear Cu(Ⅱ)complex:[Cu3L2(py)] (ClO4)2·2THF(1)(H2L=(E)-3-[N,N-di(2-pyridylmethyl) aminomethyl]-2-hydroxyl-5-methylbenzaldehyde oxime, py=pyridine,THF=tetrahydrofuran),showing the simultaneous presence of the distorted square pyramidal and the trigonal bipyramidal geometries.Moreover,the central trigonal bipyramidal Cu(Ⅱ)and the terminal square pyramidal Cu(Ⅱ)ions are linked by-N-O-/μ2-O-mixed-bridging,which is also uncommon within trinuclear Cu(Ⅱ)complexes[11,17,22].The spectral characterization,thermal stability and magnetic property of 1 are also reported.
1Experimental
1.1 Materials and measurements
All chemicals used were of analytical grade.Solvents were purified by conventional methods.The ligand H2L was prepared according to the literature method[23]and its structure is shown in Scheme 1.Elemental analyses(C,H,N)were carried out with a Thermo Finnigan Flash 1112A elemental analyzer.IR spectrum was recorded on a Nicolet Avatar 380 FT-IR instrument with KBr pellets in the range of 4 000~400 cm-1.UV-Vis spectra were recorded on a Perkin-Elmer Lambda 35 spectrometer at room temperature in methanol solution.Thermogravimetric analysis(TGA) was performed on a NETZSCH STA 449F3 thermal analyzer instrument under flowing N2with a heating rate of 10℃·min-1.The temperature dependence of the magnetic susceptibility for 1 was measured on a Quantum Design MPMS-7 SQUID magnetometer in the range of 1.8~300 K under 2 000 Oe of external field.Diamagnetic correction was made with Pascal′s constants.
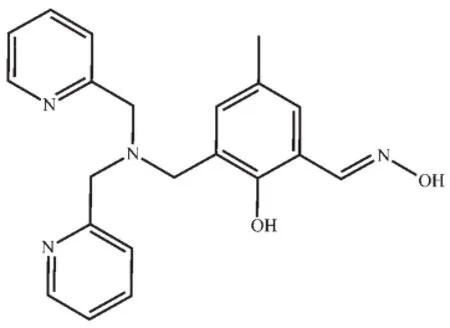
Scheme 1Structure of H2L
1.2 Synthesis of 1
A solution of Cu(OAc)2·H2O(0.186 3 mmol)in distilled water(0.8 mL)was added dropwise to a solution of H2L(0.124 2 mmol)in EtOH(1 mL).The mixture was stirred at room temperature for ten minutes, then the pyridine(5 μL)was added.Half an hour later,a solution of NaClO4·H2O(0.124 2 mmol)in distilled water(0.2 mL)was added and the dark green precipitate was immediately formed.After stirred for 8 h,the precipitate was separated by filtration,then washed with THF and dried under vacuum to give 55.4 mg(75%)of the complex.Diffusion from THF to the solution of the complex in pyridine afforded dark green single crystals of 1 suitable for X-ray crystallographic analysis.Elemental Analyses Calcd.for C55H61Cl2Cu3N9O14(%):C 49.53,H 4.61,N 9.45;Found(%): C 49.20,H 4.54,N 9.80.IR data(KBr,cm-1):ν(C=N) 1 605(s);ν(C=C),1 465(m),1 442(s);ν(Ar-O)1 293 (m);ν(C-O)1 027(s);ν(Cl-O)1 085(vs),936(w), 627(s).
1.3 Crystal structure determination
The well-shaped single crystals of 1 were selected for X-ray diffraction study.The unit cell parameters and intensity data were collected at 296(2)K on a Bruker SMART APEXⅡCCD diffractometer using a graphite-monochromated Mo Kα(λ=0.071 073 nm) radiation.The structure was solved by direct methods and refined on F2by full-matrix least squares procedures using SHELXTL software[24].All non-hydrogen atoms were anisotropically refined.Atoms O4,O5 andO6 of ClO4-anion were found to be highly disordered with occupancy of 0.50.All H atoms were located from a difference map and refined isotropically.Crystalographic data are listed in Table1 .Selected bond distances and angles are given in Table2 .
CCDC:916132.
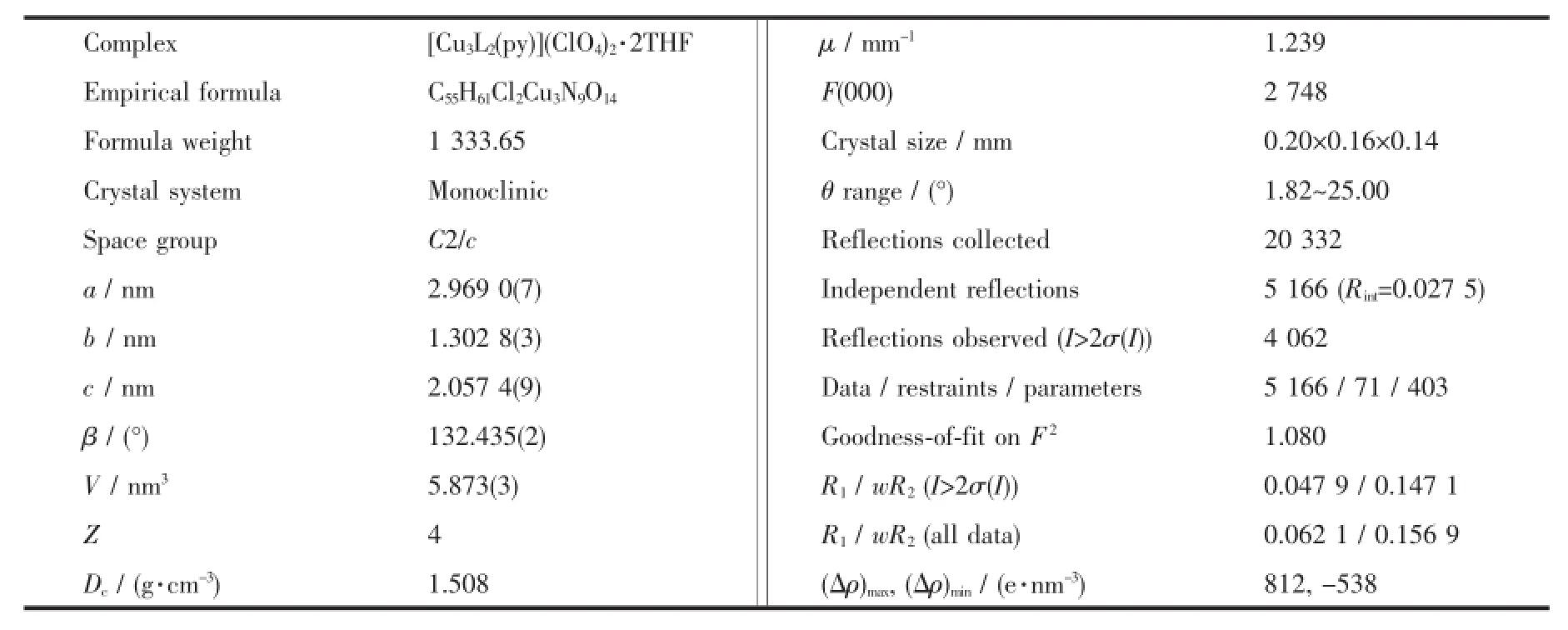
Table1 Crystal data and structure refinement for 1

Table2 Selected bond distances(nm)and bond angles(°)for 1
2Results and discussion
2.1 Crystal structure
Single crystal X-ray structure analysis shows that 1 crystallizes in the monoclinic space group C2/c.The asymmetric unit consists of three Cu(Ⅱ)cations,two L2-ligands,one pyridine,two perchlorate anions and two THF molecules.The structure of 1 with the atomic labeling system is shown in Fig.1 a.The central Cu1 cation is situated on a two-fold axis which also goes through N5 and C24 atoms of pyridine.The Cu1 cation is coordinated by three nitrogen atoms(N4,N4iand N5)in the equatorial plane and two phenolic oxygen atoms(O1 and O1i)in the axial positions to form a distorted trigonal bipyramidal configuration with the geometric parameter τ=0.623[25](Fig.1 b),whereas the peripheral Cu2 cation exhibits a distorted square pyramidal arrangement(τ=0.23)where three nitrogen atoms(N1,N2 and N3)and one oxime group O2iatom form the basal plane and the phenolic O1 atom is in the apical position(Fig.1 c).To the best of our knowledge,the simultaneous presence of a square pyramidal arrangement and a trigonal bipyramidal configuration within pentacoordinate trinuclear Cu(Ⅱ)complexes is very rare[8].In addition,Cu1 and Cu2 atoms are double bridged by the phenolic μ2-O1-and oxime group (-O2i-N4i-),resulting in a linear trinuclear Cu(Ⅱ)complex(∠Cu2…Cu1…Cu2i=161.64°).The square-pyra-midal Cu2 displaces 0.022 8 nm out of the basal plane consisting of N1,N2,N3 and O2i.The bond lengths of Cu-N are similar to those observed in the related trinuclear Cu(Ⅱ)complexes[8-13]though the Cu2 -N2 and Cu2-N3 distances in 1 are nearly equal.Interestingly,the Cu2-O1 bond length is 0.027 2 nm longer than the Cu2-O2ione,while the Cu1-O1 bond distance is 0.022 4 nm shorter than Cu2-O1 one. Moreover,the Cu1-N5 distance is 0.033 3 nm longer than Cu1-N4 one(Table2 ).The distance of Cu1 and Cu2 atoms is 0.340 9 nm,which is larger than the sum of van der Waals radii of two copper atoms(0.28 nm),revealing no metallphilic Cu…Cu interaction in 1.
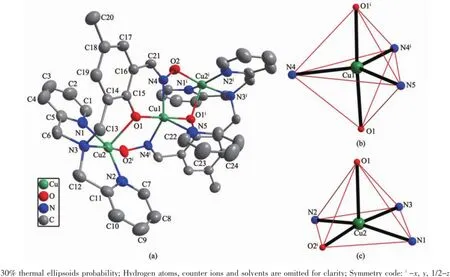
Fig.1 (a)Projection of the structure of 1 with the atomic labeling system;(b)Distorted trigonal bipyramidal configuration of Cu1;(c)Distorted square pyramidal arrangement of Cu2
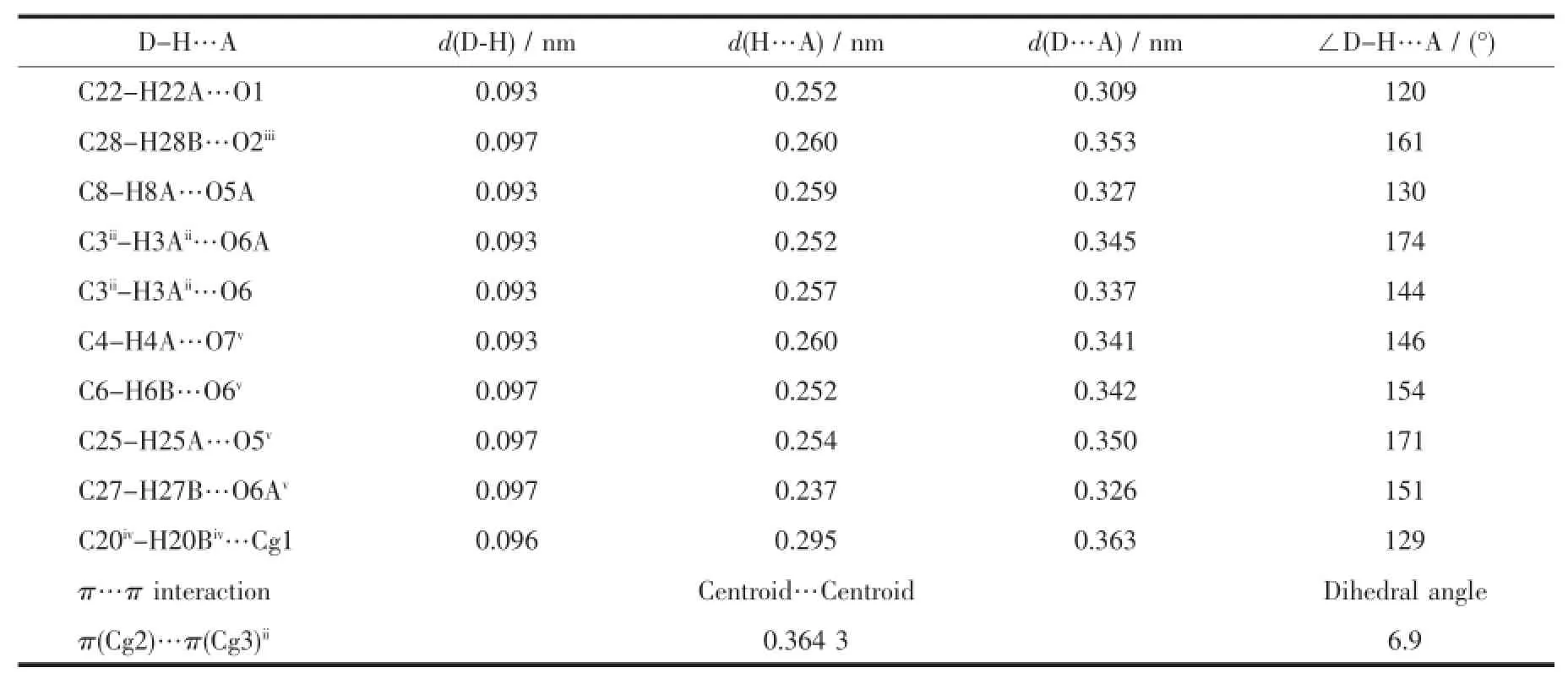
Table3 Hydrogen bonding and π-π stacking interactions in 1
There are a lot of C-H…O hydrogen bond interactions in the structure of 1(Table3 ),which is significantly associated with the closer crystal packing. Mainly,these hydrogen bond interactions can be clas-sified as three kinds:(1)one intramolecular C22-H22A…O1 hydrogen bond involving pyridine and phenolic oxygen;(2)one intermolecular C28-H28B…O2iiihydrogen bond involving THF and oxime group′s oxygen;(3)seven types of intermolecular C-H…O hydrogen bond involving ClO4-.In addition,there are one kind of intermolecular edge-to-face C-H…π interaction involving-CH3and phenolic ring(C20iv-H20Biv…Cg1,Cg1 is the centroid of the phenolic ring) and one kind of offset face-to-face π…π interaction between two pyridyl rings with the Cg2…Cg3iidistance of 0.364 3 nm and a dihedral angle of 6.9°(Cg2 and Cg3 is the centroid of pyridyl with N1 and pyridyl with N2,respectively).These interactions connect six trinuclear Cu(Ⅱ)molecules to form a 2D layer with THF solvents and ClO4-anions filled in the channel of a macrocycle(Fig.2 ).
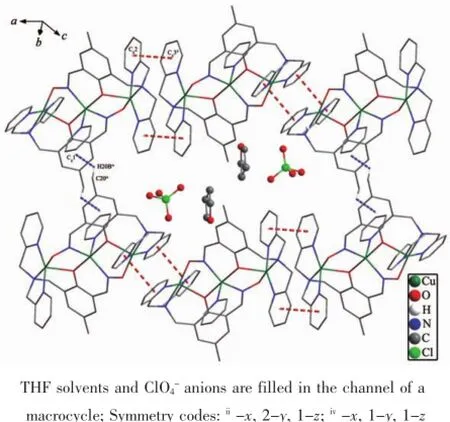
Fig.2 2D layer structure of 1 formed by face-to-face π…π stacking and edge-to-face C-H…Cg1 interactions
2.2 Spectral characterization
In the IR spectrum of 1,a strong band at 1 605 cm-1can be assigned to the C=N stretching vibration of the coordinated pyridyl ring.A strong band at 1 027 cm-1is attributed to the C-O stretching vibration of THF.The three bands at 1 085(vs),936(w)and 627 (s)cm-1,are attributable to the IR-allowed ν mode, IR-forbidden ν mode and the non-degenerate ClO3symmetrical bending vibrations of the ClO4-anion, respectively[26-28].These features are in agreement with the results of X-ray analysis.
The UV-Vis spectrum of 1 in CH3OH solution is shown in Fig.3 ,an intense band at 205 nm is attributed to π-π*transition of benzene ring(206 nm observed in the free H2L ligand).Two shoulder bands at 234 and 256 nm are attributed to n-π*transition of phenolic group in contrast to the single band at 262 nm for the free H2L ligand.A band due to π-π*transition of oxime group[29]is found at 291 nm,a blue shift compared with the band at 319 nm for the free H2L ligand,showing the conjugation between phenolic ring and oxime group decreases in 1 due to the coordination of the L2-ligand.A band at 340 nm can be attributed to LMCT[23].
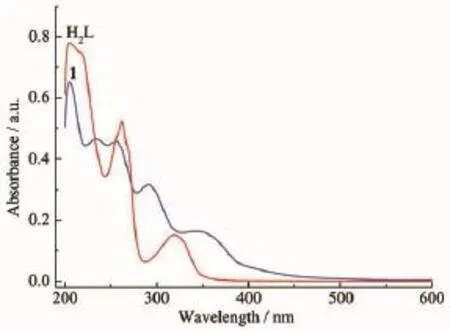
Fig.3 UV spectra of 1 and H2L ligand in CH3OH
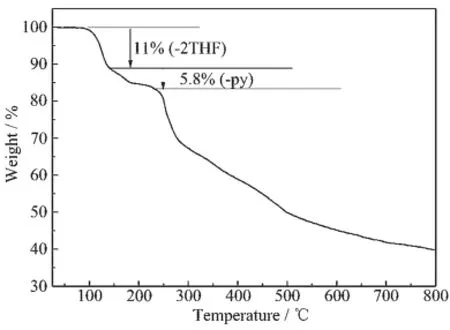
Fig.4 TGA curve of 1
2.3 TG analysis
TGA of 1(Fig.4 )was carried out in the range of 25~800℃under nitrogen atmosphere.The first weight loss of 11%from 25 to 138℃is attributed to the loss of two THF molecules(Calcd.10.8%).The next weight loss of 5.8%from 138 to 225℃correspondstothelossofthecoordinatedpyridine molecule(Calcd.6.7%).After that,1 began to decom-pose.
2.4 Magnetic property
As shown in Fig.5 ,the observed χMT value of 1 at 300 K is 1.25 cm3·K·mol-1,which is slightly larger than expected for three isolated Cu(Ⅱ)S=1/2 ions (1.125 cm3·K·mol-1for g=2.0).When further cooling, the χMT value decreases to reach 0.45 cm3·K·mol-1at 30 K that remains constant down to 5 K and decreases again below this temperature.This behavior shows the presence of predominant antiferromagnetic interactions between the Cu(Ⅱ)ions.In fact,the presence of a plateau with a value of about 0.4 cm3·K·mol-1is expected for an antiferromagnetically coupled linear trinuclear Cu(Ⅱ)complex where the central Cu(Ⅱ)ion is coupled with the two terminal ones,but there is no coupling between the terminal ones.It corresponds to the spin ground state of S=1/2 for the trinuclear complex.According to these data and the structure of 1, we can fit the magnetic properties to a simple symmetrical linear trimer Cu(Ⅱ)model[11]:
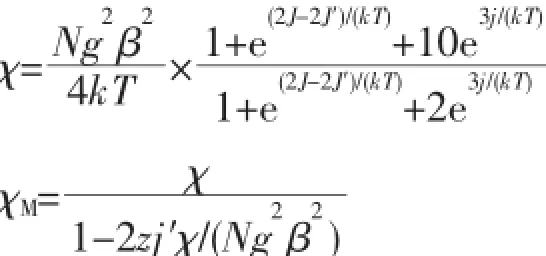
J is based on the spin Hamiltonianwith S=1/2,N,k,T and β have the common meanings.The best fitting results from 1.8~300 K gave:g=2.127(2), J=-33.3(5)cm-1a ndzj′=-0.66(1)cm-1withR=The negative J value indicates the medium antiferromagnetic coupling between Cu(Ⅱ)ions in 1[30].In addition,the present J value is also compared with those observed in the related linear trinuclear Cu(Ⅱ)complexes[11,18]. The negative value of zj′indicates that the face-to-face π…π stacking of pyridine rings of the ligands mediates the very weak antiferromagnetic interaction between molecules and corresponds to the decrease of χMT below 5 K.
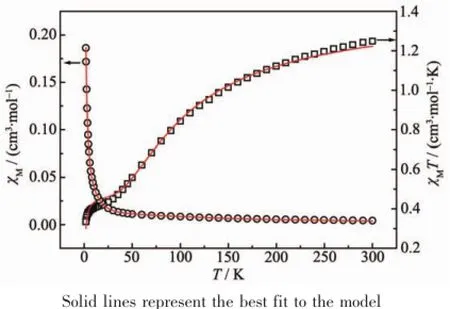
Fig.5 Thermal variation of the χM(○)and χMT(□)for 1
3Conclusions
A novel pentacoordinate trinuclear Cu(Ⅱ)complex [Cu3L2(py)](ClO4)2·2THF with(E)-3-[N,N-di(2-pyridylmethyl)aminomethyl]-2-hydroxyl-5-methylbenzaldehyde oxime(H2L)has been synthesized and characterized by IR,UV-Vis,TGA and single crystal X-ray analysis. The central trigonal bipyramidal Cu(Ⅱ)and two terminal square pyramidal Cu(Ⅱ)ions are linked by-N-O-/ μ2-O-mixed-bridging to from a linear trinuclear Cu(Ⅱ)arrangement.A medium antiferromagnetic coupling between adjacent Cu(Ⅱ)ions dominates the magnetic property of this complex.
[1]Rivera-Carrillo M,Chakraborty I,Mezei G,et al.Inorg.Chem., 2008,47:7644-7650
[2]Yang L,Powell D R,Klein E L,et al.Inorg.Chem.,2007, 46:6831-6833
[3]Ávila-Torres Y,López-Sandoval H,Mijangos E,et al.Polyhedron,2013,51:298-306
[4]Nicola C D,Garau F,Gazzano M,et al.Cryst.Growth Des., 2012,12:2890-2901
[5]Ferrer S,Lloret F,Pardo E,et al.Inorg.Chem.,2012,51:985 -1001
[6]Nicola C D,Forlin E,Garau F,et al.J.Organomet.Chem., 2012,714:74-80
[7]Qin T,Gong J,Ma J,et al.Chem.Commun.,2014,50:15886-15889
[8]Hasenknopf B,Lehn J M,Baum G,et al.Proc.Natl.Acad. Sci.,1996,93:1397-1340
[9]Zhao L,Thompson L K,Xu Z,et al.J.Chem.Soc.,Dalton Trans.,2001:1706-1710
[10]Seppälä P,Colacio E,Mota A J,et al.Dalton Trans.,2012, 41:2648-2658
[11]Biswas A,Drew M G B,Gómez-García C J,et al.Inorg. Chem.,2010,49:8155-8163
[12]Kannan S,Pillai M R A,Droege P A,et al.Inorg.Chim. Acta,1997,254:397-400
[13]Dey S,Sarkar S,Mukherjee T,et al.Inorg.Chim.Acta,2011, 376:129-135
[14]Singh J,Hundal G,Corbella M,et al.Polyhedron,2007,26: 3893-3903
[15]Nie F M,Dong Z Y,Lu F,et al.J.Coord.Chem.,2010,63: 4259-4270
[16]Chen X,Zhan S,Hu C,et al.J.Chem.Soc.Dalton Trans., 1997:245-250
[17]Gehring S,Fleischhauer P,Paulus H,et al.Inorg.Chem., 1993,32:54-60
[18]Jiang J,Chu Z,Huang W.Inorg.Chim.Acta,2009,362: 2933-2936
[19]Chen L F,Cao X Y,Li Z J,et al.Inorg.Chem.Commun., 2008,11:961-964
[20]Luo W,Meng X G,Xiang J F,et al.Inorg.Chim.Acta,2008, 361:2667-2676
[21]Botana L,Ruiz J,Seco J M,et al.Dalton Trans.,2011,40: 12462-12471
[22]Zhu Q,Tian C,Shen C,et al.CrystEngComm,2013,15:2120-2126
[23]Silva N M L,Pinheriro C B,Chacon E P,et al.J.Braz. Chem.Soc.,2011,22:660-668
[24]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[25]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc.Dalton Trans.,1984:1349-1356
[26]Lu W,Zhu D R,Xu Y,et al.Struct.Chem.,2010,21:237-244
[27]CHEN Lang(陈浪),CHEN Hui-Min(程慧敏),JIANG Jing-Jing(江静静),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28:381-385
[28]GAO Da-Zhi(高大志),WANG Ruo-Xu(王若徐),YE Fan(叶帆),et al.Chinese J.Inorg.Chem.(无机化学学报),2013, 29:2438-2444
[29]Joel T,Georgina A Á,Elia P B,et al.Spectrochim.Acta A, 2007,66:879-883
[30]Wang X Z,Zhu D R,Xu Y,et al.Cryst.Growth Des.,2010, 10:887-894
A Mixed-Bridged Linear Trinuclear Copper(Ⅱ)Complex Containing Both Square Pyramidal and Trigonal Bipyramidal Configuration
WU Di1YU Zhu1GAO Da-Zhi1WANG Xin1SHEN Xuan1ZHU Dun-Ru*,1,2
(1College of Chemical Engineering,State Key Laboratory of Materials-oriented Chemical Engineering,
Nanjing Tech University,Nanjing 210009,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
A novel trinuclear copper(Ⅱ)complex,[Cu3L2(py)](ClO4)2·2THF(1)(H2L=(E)-3-[N,N-di(2-pyridylmethyl) aminomethyl]-2-hydroxyl-5-methylbenzaldehyde oxime,py=pyridine,THF=tetrahydrofuran),has been successfully synthesized and characterized.1 crystallizes in monoclinic system with space group C2/c,a=2.969 0(7)nm,b= 1.302 8(3)nm,c=2.057 4(9)nm,β=132.435(2)°,V=5.873(3)nm3,Z=4 with final R1=0.047 9.X-ray crystallography analysis reveals that 1 is a mixed-bridged(-N-O-/μ2-O-)linear tricopper(Ⅱ)complex with all the copper cations being five-coordinated by three N and two O atoms:the central Cu(Ⅱ)in a distorted trigonal bipyramidal configuration,whereas the peripheral Cu(Ⅱ)in a distorted square pyramidal arrangement.Magnetic susceptibility measurement indicates that there is a medium antiferromagnetic exchange coupling between the Cu(Ⅱ)ions with a J value of-33.3(5)cm-1.CCDC:916132.
trinuclear copper;pentacoordinate;crystal structure;mixed-bridged;anti-ferromagnetism
O614.121
A
1001-4861(2015)08-1619-07
10.11862/CJIC.2015.220
2015-04-19。收修改稿日期:2015-06-02。
国家自然科学基金资助项目(No.21171093,21476115),南京大学配位化学国家重点实验室开放课题基金资助项目。
*通讯联系人。E-mail:zhudr@njtech.edu.cn;会员登记号:S060015982P。

