基于多齿希夫碱配体的四核和十核锰簇合物的合成、结构和磁性
杨晓迅 冷际东 刘俊良 贾建华 童明良
(中山大学化学与化学工程学院,生物无机与合成化学教育部重点实验室,广州510275)
基于多齿希夫碱配体的四核和十核锰簇合物的合成、结构和磁性
杨晓迅冷际东刘俊良贾建华*童明良
(中山大学化学与化学工程学院,生物无机与合成化学教育部重点实验室,广州510275)
合成了一种具有{NO4}给电子组成的多齿水杨醛希夫碱配体,3,5-二-叔丁基水杨醛-三(羟甲基)氨基甲烷(H4L),并利用元素分析、红外光谱以及核磁共振氢谱表征其结构。Mn(ClO4)2或MnCl2·4H2O分别与该配体在溶液中反应生成了一个四核锰簇合物[MnIII4(HL)2(H2L)2(MeCN)4](ClO4)2·2MeCN(1)和一个十核锰簇合物[MnIII6MnII4(bz)10(L)4(H2O)2]·10MeCN(2)。X-射线衍射分析表明化合物1的晶体结构空间群为三斜P1,而化合物2为正交Aba2。2~300 K温度区间的磁性测量数据表明化合物2中存在反铁磁相互作用。
希夫碱;锰簇合物;反铁磁相互作用
In 1993,Mn12Ac as the first single-molecule magnet(SMM)that could retain magnetization in the absence of magnetic field was discovered[1-2].This unexpected result has led to a great expansion in the investigation of the synthesis and magnetic property for polymetallic molecular species,especially those with large spin ground-state S and axial zero-field splittingparameterDvalues,whicharewidely accepted as the most important features as SMMs determination[3].True to its name,the SMM is a molecule that behaves as an individual nanomagnet. Becauseoftheirsmallsizeandprecisecharacterization,SMMs straddle the classical and quantummechanicalworlds,displayingmany interesting features and having important technological applicationsinfieldsofinformationstorageand quantumcomputation,etc[4].Complexesinvolving manganese ions have held a very important seat in SMMs family,up to now,particularly the Mn12and Mn6clusters[5-6].Generally speaking,the spin value can be successfully maximized when ferromagneticcoupling systems are generating[7].One important direction to reach this goal is developing the new synthetic procedure for polynuclear clusters,among whichthedesignofkeyligandsisextremely promising.In this regard,hydroxyl-rich ligands as well as acid ligands[8-14],such as tripodal alcohols,have been widely used for the development of polynuclear molecular species.
Recent years,multidentate hydroxyl-rich Schiffbase ligands have attracted much attention due to theirsignificantlypotentialapplicationsinsite selection,catalysis,biochemistryand magnetochemistry.Theyhavebeenidentifiedas highlyeffectiveligandsfortheconstructionof polymetallic SMMs because of the similar coordination superiorities as hydroxyl-rich ligands[15-21].For example, thetridentateSchiff-baseligand,5-bromo-2-salicylideneamino-1-propanol(H25-Br-sap),has been used to construct dinuclear and tetranuclear SMMs previously[16,18].
Inspired by these precedents,we recently began to investigate a multidentate salicylaldehyde Schiffbaseligand3,5-di-tert-butylsalicylaldehydetrihydroxymethylaminomethane(H4L)with{NO4}donor set,which may provide an effective direction for molecular magnets design.Fortunately,two novel manganese clusters[MnIII4(HL)2(H2L)2(MeCN)4](ClO4)2· 2MeCN(1)and[MnIII6MnII4(bz)10(L)4(H2O)2]·10MeCN (2),were obtained from reactions of Mn(Ⅱ)salts with H4L(Scheme 1).Herein,we reported their synthesis, structures and magnetic properties.
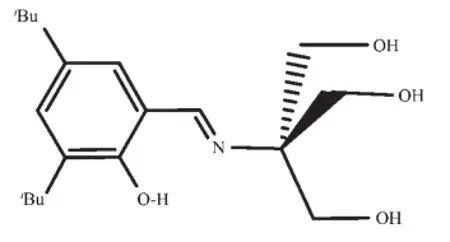
Scheme 1Schiff-base ligand H4L
1 Experimental Section
1.1Physical measurements
Elementalanalysiswasperformedonan Elementar Vario-EL CHNS elemental analyzer.IR spectra were recorded from KBr pellets in the range 4 000~400 cm-1on a Bruker-EQUINOX 55 spectrometer.1H NMR spectra were recorded on a Mercury-Plus 300 nuclear magnetic resonance analyzer using TMSasinternalstandard.Variable-temperature magnetic susceptibility and magnetization measurements were performed with a Quantum Design MPMSXL 7 SQUID.Dried powder samples were embedded in petroleum jelly to prevent torquing.Direct current (dc)susceptibility data were collected at zero dc field and were corrected with Pascal constants.
1.2Synthesis
All materials were commercially available and used as received without further purification.All manipulationswereperformedunderambient laboratory conditions.
H4L.Toamethanolsolution(100mL), trihydroxymethylaminomethane(1.79 g,15 mmol)was addedwithmagneticstirring.Then,3,5-di-tertbutylsalicylaldehyde(3.52 g,15 mmol)was added to the above solution slowly at 80℃under reflux.The resulting solution turned yellow,and was continued to reflux for further 1 h.After removing the solvent by reducedpressuredistillation,yellowpowderwith obvious scent was obtained(98.7%yield).IR data(KBr,cm-1):3 473s,3 356s,2 962m,2 360m,1 617s, 1 477w,1 365m,1 203s,1 049s,875m,850m,831s, 727m,649m,572m.1H NMR data:δ 8.36(1H);7.36 (1H);7.26(1H);4.90(1H);3.84(6H);1.92(3H);1.32 (18H).Anal.Calcd.for C19H31NO4(%):C,67.63;H, 9.26;N,4.15;Found(%):C,67.65;H,9.27;N,4.17.
[MnIII4(HL)2(H2L)2(MeCN)4](ClO4)2·2MeCN(1).To a solutionofMn(ClO4)2(0.543 g,1.5 mmol)in acetonitrile(15 mL),mixture solution of H4L(0.169 g, 0.5 mmol)and ethanolamine(0.092 g,1.5 mmol)in acetonitrile(10mL)wasaddeddropwisewith magnetic stirring.A black solution was obtained and continued to stir for 7 h.After filtration,the filtrate was allowed to evaporate at room temperature.Ten days later,only a few red block crystals suitable for X-ray analysis were obtained along with massive black microcrystals(less than 2%yield).
[MnIII6MnII4(bz)10(L)4(H2O)2]·10MeCN(2).To a solution of MnCl2·4H2O(0.059 g,0.3 mmol)in methanol(15 mL),a solution of H4L(0.034 g,0.1 mmol)in acetonitrile(15 mL)was added dropwise with magnetic stirring.The resulting solution turned black.About half an hour,solid sodium benzoate (0.086 g,0.6 mmol)was added,then the mixture was stirred at room temperature for 7 h and filtered.The filtrate with light reddish brown color was evaporated at room temperature.Three days later,red block crystals suitable for X-ray analysis were collected.(ca. 40%yield based on H4L).IR data(KBr,cm-1): 3 498m,3 396m,3 361w,2 989m,2 773m,2 032s, 1 778w,1 623s,1 473m,1 390w,1 203m,1 045w, 972w,835m,732m,655w,567m.Anal.Calcd.for C166H192N14O38Mn10(%):C,56.31;H,5.47;N,5.54; Found(%):C,56.28;H,5.48;N,5.56.
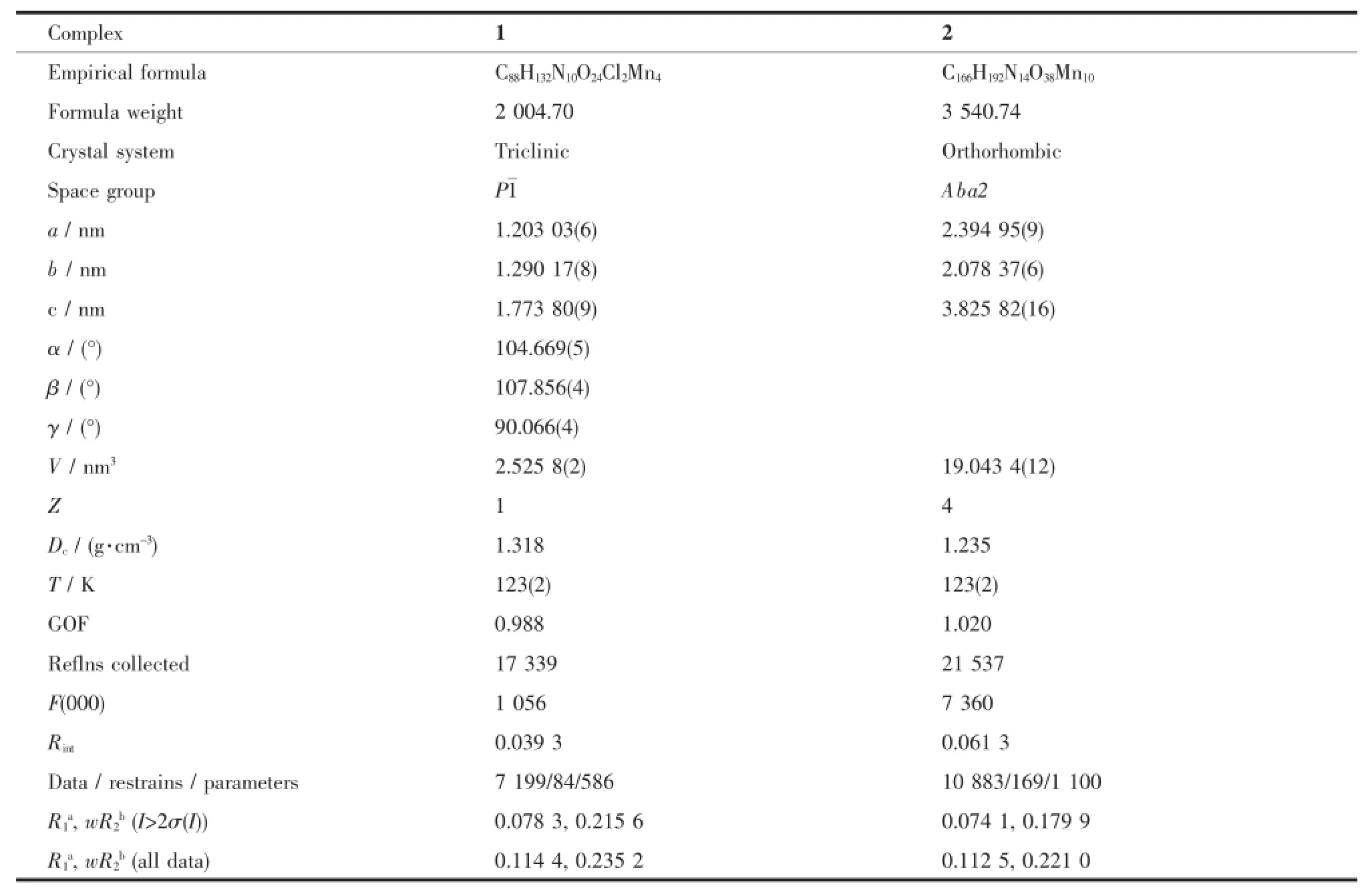
Table 1Crystallographic Data and Structural Refinements for 1 and 2
1.3X-ray crystallography
Diffractionintensitiesof 1 and 2 were collected on a Bruker Smart Apex CCD diffractometer using graphitemonochromated Cu Kα hradiation(λ=0.154 178 nm). Absorptioncorrectionswereprocessedthrough multiscan program SADABS[22].The structures weresolved with direct method and refined with a full-matrix least-squares technique with the SHELXTL program package[23].The hydrogen atoms were placed in idealised positions and refined using a riding model.
Datacollectionparametersandstructure refinement details are listed in Table 1.
CCDC:1403158,1;1403159,2.
2 Results and discussion
2.1Synthesis
The condensation reaction of trihydroxymethylaminomethanewith3,5-di-tert-butylsalicylaldehydein methanol solution under reflux gave the target product H4L in 98.7%yield(Scheme 2).
Solution reaction of Mn(ClO4)2with H4L in air can afford dark solution after seven hours.Evaporation of the resulting mixture at room temperature finally yielded crystals 1 in less than 2%yield.Crystals 2 were obtained in 40%yield from solution reaction of MnCl2with H4L and sodium benzoate.Given that the starting manganese sources were exclusively MnIIions, it is apparent that MnIIIions generated by oxidation of MnIIions,a common process in manganese cluster chemistry[24].As an immediate color change of solution to black was observed during the reagents mixing for both preparation process of 1 and 2,it is proposed that oxidation step occurs in the process of initial reactionratherthancrystallizationstep.Toour surprise,alcohol hydroxyls of H4L in 1 are partly deprotonated in the presence of base,whereas for 2 alcohol hydroxyls are completely deprotonated without any base.Hence,the extent of deprotonation of the ligand exerts great influence on the buildings of metal clusters,especially high nuclearity metal clusters. Generally speaking,the more thoroughly the ligands are deprotonated,the higher nuclearity species would be obtained.In order to figure out the conditions for deprotonation of the ligand and the impact of metal salts,auxiliaryligandsandsolventsonthe constructionsofcomplexeswithsuchSchiff-base ligand,many attempts have been done upon reaction systemof2.ReplacementofMnCl2byother manganese salts such as Mn(OAc)2,Mn(NO3)2and Mn (ClO4)2in the same reaction condition only yielded amorphouspowder.Inaddition,reactionsunder various solvent conditions were in vain.Due to the excellent property of N3-unit in magnetism transmission,moreover,NaN3was incorporated into the reaction system,unfortunately there is no any crystalobtainedexceptsomepowder.Sofar, combinationofMnCl2,sodiumbenzoate,mixed solvents of methanol and acetonitrile,seems to be the best choice for reaction system to generate 2.
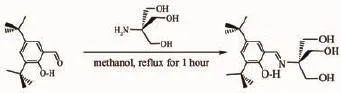
Scheme 2Synthetic route of H4L
2.2Structure description
Single-crystal X-ray structural analysis reveals that the structure of 1 consists of four MnIIIions(Fig. 1),crystallizing in triclinic space group P1.Four alcoholhydroxylsinligandH4Larepartly deprotonated while completely deprotonated in 2.All four MnIIIions in 1 are six-coordinated with nearoctahedral geometry.Four coordination sites of each MnIIIion in equatorial plane are occupied by phenoxyl oxygen atom,amine nitrogen atom,and two hydroxyl oxygen atoms from L4-.The other two axial sites are occupied by two nitrogen atoms from two acetonitrile molecules for Mn1 and two hydroxyl oxygen atoms from two L4-for Mn2,respectively(Fig.1).
The bond lengths of Mn1-O/N and Mn2-O/N are respectively in the range of 0.184 9(2)~0.195 5(3)nm, 0.185 3(3)~0.203 2(2)nm in the equatorial plane and 0.225 7(4)~0.234 6(4)nm,0.213 6(2)~0.215 4(2)nmin the axis,which indicates coordination geometry of MnIIIions are elongated octahedron,displaying a Jahn-Teller distortion.Meanwhile the mean bond length of Mn1-N(0.229 9(7)nm,Jahn-Telle axis)is longer than that of Mn2-O(0.2144(5)nm,Jahn-Telle axis). Similarly,the N3-Mn1-N4 angle of Jahn-Teller axis in Mn1(173.2(3)°)is found a little bit larger than that of O2-Mn2-O7A in Mn2(159.19(15)°).These comparisons indicate the octahedral configuration of Mn1 is more elongated than that of Mn2.
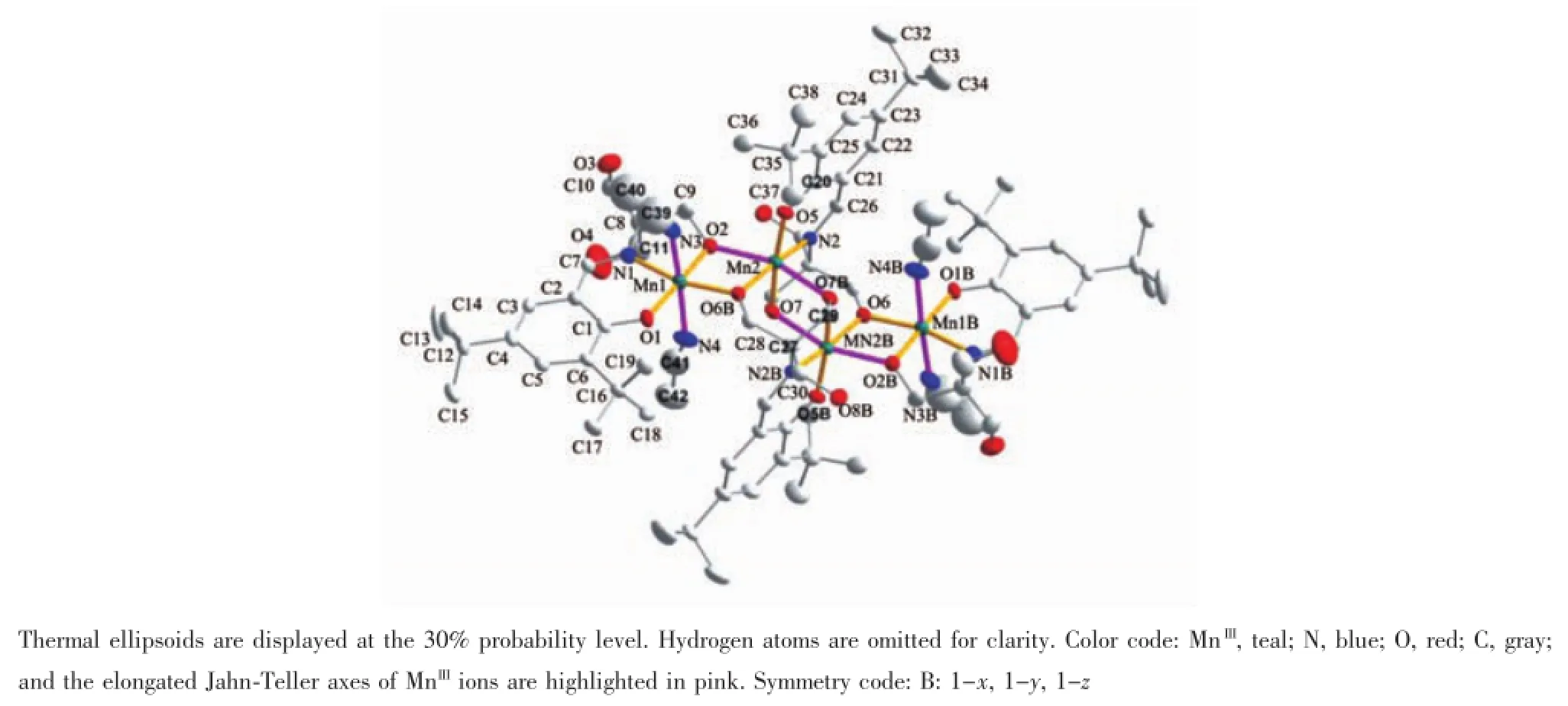
Fig.1 ORTEP diagram of 1
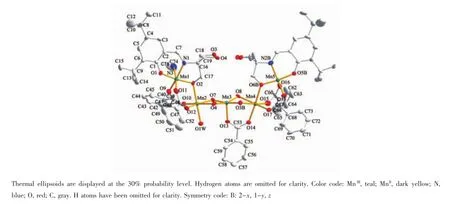
Fig.2 ORTEP diagram of the linear pentanuclear part of 2
Complex 2 crystallizing in orthorhombic space groupAba2consistsoftwolinearpentanuclear MnII2MnIII3parts(Fig.2),which are further connected by oxygen atoms from L4-and benzoate ions,forming a very beautiful cross-like MnII4MnIII6core(Fig.3).Three manganeseions(Mn1,Mn3,Mn5)ofeach pentanuclear MnII2MnIII3unit are found to be trivalent, the rest two manganese ions(Mn2,Mn4)are MnIIions. MnIIIand MnIIions are arranged alternately.All MnIIIions(Mn1,Mn3,Mn5,and their symmetry-related counterparts)are five-coordinated with square-pyramid as coordination polyhedron as shown in Fig.4(MnNO4for Mn1 and Mn5,MnO5for Mn3).Four coordination sites in square are occupied by phenoxyl oxygen atom, amine nitrogen atom(or replaced by hydroxyl oxygen atom),hydroxyl oxygen atom,and oxygen atom of benzoate.The apical site is occupied by benzoate oxygen atom.The bond lengths of MnIII-O/N are in the range of 0.184 9(8)~0.198 8(9)nm in the square and 0.210 9(7)~0.213 9(9)nm at the apex.In contrast to MnIIIions,each MnIIion(Mn2,Mn4,and their symmetry-related counterparts)is surrounded by six oxygen atoms in octahedral geometry(MnO6,Fig.4), which are respectively from hydroxyl groups of the L4-, benzoate,and watermolecule.Coordination bond lengths of MnII-O are in the range of 0.211 0(8)~0.221 8(7)nm and 0.211 8(9)~0.221 2(8)nm for Mn2 and Mn4,respectively.
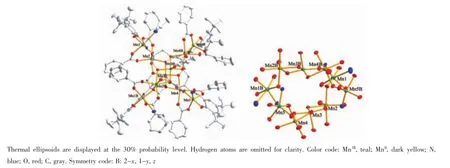
Fig.3 ORTEP diagram of the independent molecule(left)and the{MnII4MnIII6}core(right)of 2
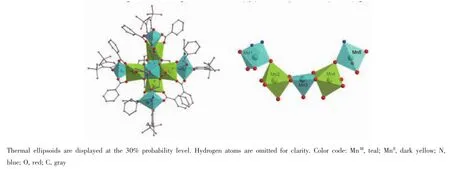
Fig.4 Polyhedral representation of molecule(left)and the linear pentanuclear part(right)of 2
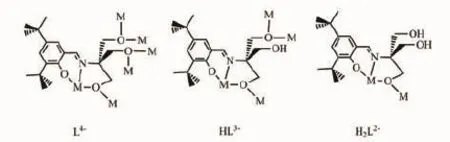
Scheme 3 Coordination modes of H4L in 1 and 2
Onlyweakintermolecularinteractionsare observed in both 1 and 2 involving that from benzene rings of ligands H4L.The H4L ligand displays the different coordination modes in 1 and 2(Scheme 3), however,both act as chelating and bridging ligands.
2.3Magnetic property study
Variable-temperature dc magnetic susceptibility data for 2 were collected in the temperature range 2~300 K,under an applied field of 1 000 Oe.The data plotted as χMT vs T in the Fig.5 are found to obey Curie-Weiss law χ(T)=C/(T-θ)in the temperature range 60~300 K,giving the Curie constant C=42.17 cm3· mol-1·K and Curie-Weiss temperature θ=-93.08 K. Such large negative θ value indicates antiferromagnetic exchange interactions among manganese ions,and the highCvalueemphasizesthesignificantorbital contribution to the susceptibility.The room-temperature value of χMT is 32.14 cm3·mol-1·K,smaller than the excepted spin-only value of 35.50 cm3·mol-1·K with SMn(Ⅱ)=5/2,SMn(Ⅱ)=2 and g=2 for a noninteracting MnII4MnIII6unit.Upon cooling,the χMT values gradually decrease to 5.16 cm3·mol-1·K at 2 K indicating an S>0groundstate.Fig.6showstheisothermal magnetization in the field of 0~7 T at 2 K.The data alsorevealthepresenceofthepredominant antiferromagnetic interactions in 2,which is consistent with the result obtained from magnetic susceptibility data.
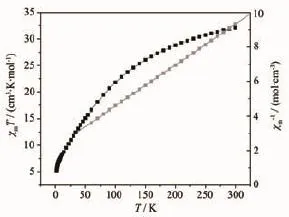
Fig.5 Plot of χMT versus T and plot χM-1versus T at an applied field of 1 000 Oe for 2
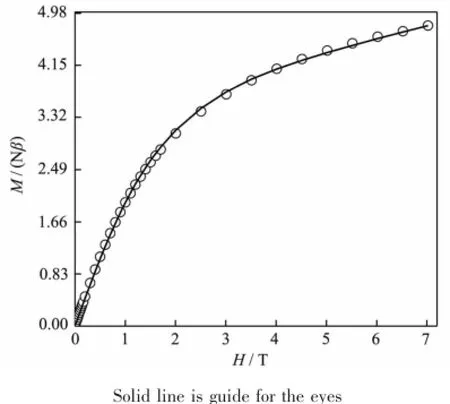
Fig.6 Field dependence of magnetization at T=2.0 K
3 Conclusions
In this work,two novel manganese clusters with tetranuclear MnIII4core and decanuclear cross-like MnII4MnIII6core surrounded by the new multidentate salicylaldehyde Schiff-base ligands H4L with{NO4} donorset.Magneticpropertystudiesrevealthat antiferromagnetic exchange interactions occur in 2, which may provide useful insights into magnetic exchangeinteractionsinpolynuclearmanganese complexes.Moreover,the extent of deprotonation of H4L ligands and the choice of proper auxiliary ligands are the key points in constructing high-nuclearity clusters with Schiff-base ligands.
References:
[1]Sessoli R,Tsai H L,Schake A R,et al.J.Am.Chem.Soc., 1993,115:1804-1816
[2]Sessoli R,Gatteschi D,Caneschi A,et al.Nature,1993,365: 141-143
[3]Aromí G,Brechin E K.Struct.Bond.,2006,122:1-67
[4]Friedman J R,Sarachik M P.Annu.Rev.Condens.Matter Phys.,2010,1:109-128
[5]Gatteschi D,Sessoli R.Angew.Chem.Int.Ed.,2003,42: 268-297
[6]Christou G,Gatteschi D,Hendrickson D N,et al.MRS Bull., 2000,25:66-71
[7]Ako A M,Hewitt I J,Mereacre V,et al.Angew.Chem.Int. Ed.,2006,45:4926-4929
[8]Manoli M,Prescimone A,Bagai R,et al.Inorg.Chem.,2007, 46:6968-6979
[9]Moushi E E,Lampropoulos C,Wernsdorfer W,et al.Inorg. Chem.,2007,46:3795-3797
[10]Murugesu M,Raftery J,Wernsdorfer W,et al.Inorg.Chem.,2004,43:4203-4209
[11]Rumberger E M,Shah S J,Beedle C C,et al.Inorg.Chem., 2005,44:2742-2752
[12]YAO Hong-Chang(要红昌),WANG Ning(王宁),LI Miao-Miao(李淼淼),et al.J.Zhengzhou Univ.:Nat.Sci.Ed. (郑州大学学报:理学版),2008,40:88-94
[13]WANG Peng-Fei(汪鹏飞),WANG Xin(汪新),WU Guang-Hui(伍光辉),et al.Chinese J.Inorg.Chem.(无机化学学报),2011,27:1881-1886
[14]ZHOU Ai-Ju(周爱菊),LIANG Jing-Jing(梁晶晶),ZHENG Yan-Zhen(郑彦臻),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28:2425-2430
[15]Fontecha J B,Goetz S,McKee V.Dalton Trans.,2005:923 -929
[16]Oshio H,Hoshino N,Ito T,et al.J.Am.Chem.Soc.,2004, 126:8805-8812
[17]Oshio H,Hoshino N,Ito T,et al.Angew.Chem.Int.Ed., 2003,42:223-225
[18]Oshio H,Nihei M,Yoshida A,et al.Chem.Eur.J.,2005, 11:843-848
[19]Oshio H,Nakano M.Chem.Eur.J.,2005,11:5178-5185
[20]Bagai R,Abboud K A,Christou G.Dalton Trans.,2006: 3306-3312
[21]Hoshiko J A,Wang G,Ziller J W,et al.Dalton Trans., 2008:5712-5714
[22]Sheldrick G M.SADABS,University of Göttingen,Germany, 2002.
[23]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[24]Hewitt I J,Tang J K,Madhu N T,et al.Chem.Commun., 2006:2650-2652
Synthesis,Structures and Magnetic Properties of Two Tetranuclear and Decanuclear Manganese Clusters Bearing the Multidentate Schiff-Base Ligands
YANG Xiao-XunLENG Ji-DongLIU Jun-LiangJIA Jian-Hua*TONG Ming-Liang
(MOE Key Laboratory of Bioinorganic and Synthetic Chemistry,School of Chemistry and Chemical Engineering, Sun Yat-Sen University,Guangzhou 510275,China)
A multidentate salicylaldehyde Schiff-base ligand with{NO4}donor set,3,5-di-tert-butylsalicylaldehydetrihydroxymethylaminomethane(H4L),has been synthesized for the first time and characterized by elemental analysis,IR and1H NMR.Solution reactions of Mn(ClO4)2or MnCl2·4H2O with H4L in air generated a tetranuclear complex[MnIII4(HL)2(H2L)2(MeCN)4](ClO4)2·2MeCN(1)and a decanuclear complex[MnIII6MnII4(bz)10(L)4(H2O)2]· 10MeCN(2),respectively.X-ray studies reveal that complex 1 crystallizes in triclinic space group P1,while complex 2 crystallizes in orthorhombic space group Aba2.Magnetism data in the temperature range 2~300 K have been carried out,indicating the presence of the antiferromagnetic interaction within 2.
Schiff-base;manganese cluster;antiferromagnetic interaction
O614.71+1
A
1001-4861(2015)09-1831-08
10.11862/CJIC.2015.260
2015-05-29。收修改稿日期:2015-07-13。
国家重点基础研究发展规划(“973计划”,No.2014CB845602,2012CB821704),国家自然科学基金(No.21301197,91122032,21371183,91422302),广东省自然科学基金(S2013020013002),以及中央高校基本科研业务费专项资金(14lgpy10)资助项目。
*通讯联系人。E-mail:jiajh3@mail.sysu.edu.cn

