Long-term nitritation performance of ammonium-rich land fill leachate☆
Hongwei Sun *,Xintao LÜ,Yongzhen Peng ,Shuying Wang ,Juan Ma ,
1 School of Environmental and Municipal Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China
2 Key Laboratory of Beijing Water Quality Science and Water Environment Recovery Engineering,Beijing University of Technology,Beijing 100124,China
Keywords:Land fill leachate Nitritation Low temperature Free ammonia AOB and NOB
ABSTRACT This study presents a biological system combined up flow anaerobic sludge bed(UASB)with sequencing batch reactor(SBR)to treat ammonium-rich land fill leachate.The start-up and operation of the nitritation at low temperatures were investigated.The synergetic interaction of free ammonia(FA)inhibition on nitriteoxidizing bacteria(NOB)and process control was used to achieve nitritation in the SBR.It is demonstrated that nitritation was successfully started up in the SBR at low temperatures(14.0 °C-18.2 °C)by using FA inhibition coupled with process control,and then was maintained for 482 days at normal/low temperature.Although ammonia-oxidizing bacteria(AOB)and NOB co-existed within bacterial clusters in the SBR sludge,AOB were confirmed to be dominant nitrifying population species by scanning electron microscopic(SEM)observation and fluorescence in situ hybridization(FISH)analysis.This confirmation not only emphasized that cultivating the appropriate bacteria is essential for achieving stable nitritation performance,but it also revealed that NOB activity was strongly inhibited by FA rather than being eliminated altogether from the system.
1.Introduction
This leachate is characterized by small amounts of biodegradable organics;high concentrations of ammonia,chemical oxygen demand(COD),and suspended solids(SS);low ratios of B/C and C/N[1,2].If not treated safely,land fill leachate can be a major source of environmental pollution.
To treat land fill leachate in a more economic and sustainable way,researchers[3-5]have recently introduced a method for removing nitrogen via the nitrite pathway because this pathway has distinct advantages over the nitrate pathway[6,7].In order to establish the nitrite pathway,stable nitritation(ammonia being oxidized to nitrite)must be achieved in nitrification.
It has been widely reported that a high concentration of free ammonia(FA)can promote nitrite accumulation by selectively inhibiting the activity of nitrite oxidizing bacteria(NOB)but not ammonia oxidizing bacteria(AOB)[8].Therefore,FA inhibition is an effective way to remove nitrogen from leachate via the nitrite pathway.
Moreover,process control has been found effective for achieving nitritation in SBRs treating domestic wastewater[9-11].Previous literatures[12-14]have also reported that the nitrifying bacterial population can be optimized through long-term application of process control,which implicates AOB,rather than NOB,as the dominant nitrifying bacteria involved in nitritation.
Temperature is typically an important parameter for achieving nitritation.High temperatures will cause AOB to outcompete NOB because they grow faster than NOB at temperatures higher than 25°C,leading to nitrite accumulation[15].For this reason,nitritation is easily achieved at high temperatures.Interestingly,several studies[13,14,16]have also shown that nitritation can be achieved and maintained at low temperatures.For example,Yang et al.[13]treated municipal wastewater in a pilot-scale sequencing batch reactor(SBR)and Qiao et al.[16]treated high-ammonium wastewater in a swim-bed reactor;both studies reported that nitritation was initiated at normal temperatures(above 20°C)and maintained at low temperatures(blow 20°C).To the best of our knowledge,however,only Gu et al.[14]successfully started up nitritation at low temperatures(11 °C-16 °C)using a pilot-plant SBR to treat municipal wastewater.
In this research,we established a biological system coupled up flow anaerobic sludge bed(UASB)and sequencing batch reactor(SBR)to treat ammonium-rich land fill leachate.Based on the achievement of simultaneous removal of organics and nitrogen from leachate,we expect to develop a novel method for starting up and maintaining nitritation at low temperatures.Moreover,microbial spatial distribution and the morphology of nitrifying bacteria population in the sludge flocs were analyzed.
2.Materials and Methods
2.1.Experimental lab-scale reactor
Fig.1 shows the two-component lab-scale system,which includes an UASB and a SBR.The working volumes of UASB and SBR were 3 L and 9 L,respectively.The SBR contained three sidewall ports in which sensors for dissolved oxygen(DO),pH,and oxidation-reduction potential(ORP)were inserted.An equalization tank was designed to adjust the conflict between continuous effluent in the UASB and intermittent influent in the SBR.
The temperature of the mixed liquor in the UASB was maintained at 30°C±2°C using a heating water jacket and a temperature control system.The SBR was operated at room temperature(9.0 °C-32.1 °C).DO was supplied by an air compressor through a porous diffuser installed at the bottom of the SBR.Complete mixing was provided by a mechanical stirrer rotating at a speed of 40 r·min-1.
2.2.Operational procedures
A wastewater mixture,consisting of the raw land fill leachate and SBR-nitrified supernatant(SNS),was continuously pumped into the UASB using peristaltic pumps.The SBR was fed with the UASB effluent.Each cycle of the SBR consisted of 2-min feeding,aerobic reaction,30-min settling,15-min SNS recycling anoxic reaction,30-min settling,10-min decanting,and idling period.The duration of the aerobic and anoxic reactions were controlled by monitoring characteristic points on DO,pH,and ORP profiles.
The UASB-SBR system was operated for 623 days.The experiment was divided into three periods according to the nitrogen removal pathway in the SBR.In Period I(0-115 days),nitrogen removal was performed via the nitrate pathway.In Period II(116-141 days),nitrogen removal was initiated via the nitrite pathway at low temperatures.In Period III(142-623 days),the nitrite pathway was maintained at normal and low temperatures.
Table 1 summarizes the detailed operational conditions of the UASB-SBR system during the whole experimental period.After inoculation,the UASB was fed with a mixture of the raw leachate and the returned SNS at suitable ratios.For the first 89 days of operation,raw leachate was diluted by tap water to acclimate the inoculated sludge to leachate.The designed dilution ratios were 5,3,2,and 1.5.Subsequently,raw leachate was not diluted.During the first 89 days,the influent COD of the UASB gradually increased due to the decrease of the dilution ratios.The influent biodegradable COD was removed by denitrification and methanogenesis in the UASB.
The low effluent COD of the UASB benefited the rapid nitrification of the ammonia in the SBR.
2.3.Land fill leachate
The land fill leachate was taken from the Liulitun municipal land fill site in Beijing,China.The leachate samples were collected monthly,transported to the laboratory,and then stored at 4°C.Characteristics of the leachate are described in Table 2.
2.4.Inoculums
The UASB was inoculated with anaerobic granulated sludge taken from a methanogenic reactor of a wastewater treatment plant of Harbin brewery(located in Heilongjiang province,China).The SBR was seeded with aerobic activated sludge taken from a Municipal Wastewater Treatment Plant(located in Beijing,China),where the oxidation ditch process was employed.The concentrations of mixed liquor suspended solids(MLSS)in the SBR were 1340-4400 mg·L-1.
2.5.Analyses
COD,BOD5,NH4+-N,NO3--N,NO2--N,MLSS,TS,and alkalinity were examined according to the Standard Methods[17].TN was analyzed using a multi N/C 3000 analyzer(Analytik Jena AG,Germany).DO,pH,and ORP were continuously detected using pH/oxi-340 analyzer(WTW Company,Germany).
2.6.Microbiology analysis
Fluorescence in situ hybridization(FISH)was performed as specified by Amann[18].Specific oligonucleotide probes used in this experiment were EUBmix for detecting all bacteria;NSO1225 specific for ammonia-oxidizing β-proteobacteria;NIT3 specific for Nitrobacter;and Ntspa662 specific for Nitrospira.FISH images were captured using an OLYMPUS-BX52 fluorescence microscope(Japan).The quantitative analysis of FISH images was performed using Leica QW in quantitative microscopy software,where the relative abundance of the target bacteria was determined in triplicate as the mean percentage of all bacteria.
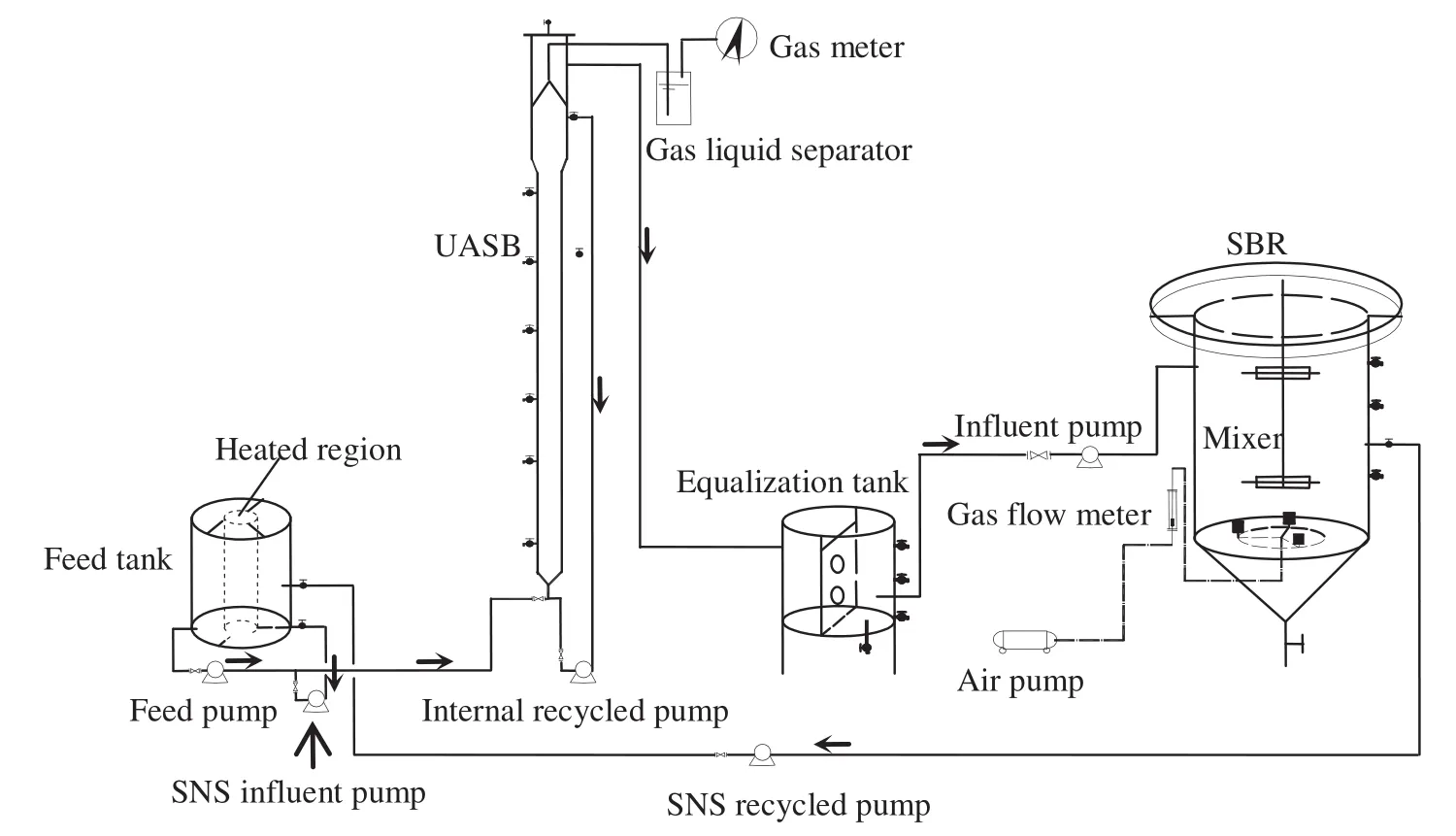
Fig.1.Schematic diagram of the UASB-SBR biological system.

Table 1 Operational conditions of the UASB-SBR system during the whole experimental period.

Table 2 Characteristics of the land fill leachate used in this study.Values are in mg·L-1,except for pH.
Scanning electron microscopy(SEM)was used to observe the morphological characteristics of the dominant nitrifying bacteria in the SBR.SEM was performed as follows: first,sludge samples were fixed with 2.5%glutaraldehyde in a 0.1 mol·L-1phosphate buffer for 1.5 h at 4°C.Second,the samples were washed and dehydrated in a graded series of ethanol solution(50%,70%,80%,90%,and 100%).Third,the dewatered samples were dried with a critical-point drier and sputter coated with a 1500-nm goldpalladium mixture.Finally,the coated samples were detected using SEM(HIT ACHIS-4300;Hitachi;Tokyo,Japan)and SEM images were captured digitally.
2.7.Calculations
The FA(mg·L-1)concentration was calculated as a function of pH,temperature,and total ammonium as nitrogen(TAN);the calculation was modified from Anthonisen et al.[19]:

The nitrite accumulation ratio(NAR,%)was calculated using Eq.(2):

3.Results and Discussion
3.1.Organic and nitrogen removal performance
The UASB-SBR system was operated for 20 months with 623 cycles during the whole operation period.Fig.2 shows the removal performance of organics and ammonium in the system.As shown in Fig.2a,the raw COD of the system was 1237-13000 mg·L-1,while effluent COD was 100-650 mg·L-1,which led to COD removal efficiency of 76.8%-98.9%.On the other hand,as illustrated in Fig.2b,the raw NH+4-N of the system was 710-2256 mg·L-1,when effluent NH4+-N was 3.8 and 18.1 mg·L-1,which resulted in the NH4+-N removal efficiency of 89.0%-99.9%.Although the influent concentrations of organics and ammonium fluctuated in the wide ranges,stable removal performances of organics and ammonium were achieved in this biological system.

Fig.2.Removal of organics and ammonium in the UASB-SBR system during the experimental period:(a)organic removal;(b)ammonium removal.
3.2.Achieving nitrogen removal via the nitrite pathway in the SBR
3.2.1.Period I:Establishing the nitrate pathway at normal and low temperatures
During Period I,the SBR was operated for 115 days by applying process control based on DO,pH,and ORP signals in order to switch between aerobic and anoxic periods.At the end of nitrification in the SBR,the average levels of nitrate,nitrite,and the NAR were 47.1 mg·L-1,1.2 mg·L-1,and 2.9%,respectively(Fig.3a),indicating that complete nitrification occurred in the SBR,even though the operational temperature decreased from 23.6 °C to 10.3 °C(Fig.3c).While the average FA concentration at the beginning of nitrification was about 3.0 mg·L-1(Fig.3b),this value was much higher than those reported by Anthonisen et al.[19],who suggest that FA inhibition on Nitrobacter could be initiated at 0.1-1.0 mg·L-1.However,as shown in Fig.4a,no nitrite accumulation was observed,indicating this FA level did not obviously inhibit the activity of Nitrobacte r.Though,in another study[20],it was suggested that FA would only have a small inhibitory effect on the respiratory capability of NOB at the level we used:In their study,Vadivelu et al.[20]found that an increase in FA concentration from 0 to 4.0 mg·L-1caused the respiration rate of NOB to only drop by 12%in the absence of inorganic carbon.

Fig.3.Evolution of the nitrite pathway in the SBR during the experimental period:(a)concentrations of nitrate and nitrite,and NAR at the end of the aerobic period;(b)FA concentration at the beginning of the aerobic period;(c)operational temperature.
3.2.2.Period II:Initiating the Nitrite Pathway at Low Temperatures
Process control was maintained in the SBR during Period II.As Fig.3a also shows,the nitrate concentration at the end of nitrification gradually decreased from61.2 to 5.2 mg·L-1;however,the nitrite concentration increased stepwise from 1.1 to 55.0 mg·L-1,suggesting that nitrite started to accumulate as the experiment progressed.During this period,the temperature in the SBR was maintained at 14.0 °C-18.2 °C and the NAR rapidly reached 91.3%on day 141(Fig.3c).It is clear that at these low temperatures,up to 90%nitrite pathway was successfully established.

Fig.4.Typical profiles of nitrogen,organic,DO,pH,ORP,and FA levels during nitritation and denitritation of an SBR cycle.
Comparing Period II with Period I,the average FA concentration increased from 3.0 mg·L-1in Period I to 16.2 mg·L-1in Period II(resulting from the higher influent ammonia concentration and pH,data not shown).During Period II,the FA concentration ranged between 10.8 and 33.5 mg·L-1,with an average value of 16.2 mg·L-1.The nitrite accumulation that occurred in Period II suggests that the activity of NOB was strongly inhibited by FA at this level range.Nevertheless,the ammonium oxidation reaction still proceeded.This further suggests that AOB can tolerate higher levels of FA than are tolerable by NOB.
The inhibitory effects of FA on AOB and NOB activity have been widely reported in previous literatures,and varying inhibitory threshold levels have been discussed.For example,Anthonisen et al.[19] first found that FA started inhibiting AOB and NOB at 10-150 mg·L-1and 0.1-1.0 mg·L-1,respectively.However,Van Hulle et al.[21]reported that AOB cultures were not inhibited by FA at concentration of 70-300 mg·L-1in SHARON(single reactor system for high activity ammonium removal over nitrite)reactors.Furthermore,Vadivelu et al.[22]demonstrated that an FA concentration of 16.0 mg·L-1did not inhibit either the anabolic or catabolic processes of an enriched AOB culture.Meanwhile,Vadivelu et al.[20]also found that an enriched NOB culture likely ceased growing at an FA concentration of 6.0-9.0 mg·L-1.Therefore,the results obtained in our study,together with the findings from previous studies,clearly show that the concentration of FA during Period II strongly inhibited NOB activity,but did not inhibit AOB activity.
3.2.3.Period III:Maintaining the nitrite pathway at normal and low temperatures
During Period III,nitrite was the primary product of nitrification in the SBR,which accumulated to about 108.2 mg·L-1at the end of nitrification.The nitrate concentration remained low,at an average of 5.3 mg·L-1.The average NAR was steady at approximately 94.6%for 482 days until the end of Period III,when the operational temperature in the SBR varied widely(9.0 °C-32.1 °C).Moreover,the average initial FA concentration during this period still maintained at 24.1 mg·L-1and decreased slightly between days 519 and 623 as the temperature decreased(Fig.3b and c).This means that operational conditions of the SBR worked to continually suppress NOB activity rather than eliminating NOB from the activated sludge system.
Because NOB has a higher activity than AOB at temperatures below 25°C,it has proven that it is difficult to achieve and stabilize nitrite accumulation below this temperature.In this study,however,during the low-temperature conditions of Period II(14.0 °C-18.2 °C),we observed nitrite accumulation and the NAR was 91.3%.Especially,on days when the operational temperature dropped below 15°C during Period III(days 262,287,289-308,315-377,and 565-623),the SBR consistently maintained a high level of nitrite accumulation(about 95.5%).
Despite the existence of temperature-related limitations,we found that the nitrite pathway functioned at lower temperatures in this study.To our knowledge,this study is the first to show that the nitrite pathway can be established and maintained in a low-temperature SBR treating leachate by using synergetic interaction of FA inhibition and process control.While,it is acknowledged that FA was an effective long-term inhibitor for NOB activity,the way in which process control induced nitrite accumulation must be further discussed below.
3.3.The mechanism for achieving nitritation at low temperatures
The level of FA relied on the ammonium concentration,pH,and the temperature.As such,Fig.4b gives plots of the FA levels calculated during the nitritation process,which clearly show that the FA gradually decreased from 18.8 mg·L-1at the beginning of nitritation to 0.21 mg·L-1at the end of nitritation because both the ammonium concentration and the pH decreased throughout the SBR cycle.This decreasing FA level implies that the inhibitory effect that FA placed on NOB activity gradually weakened as nitritation proceeded.
If the aeration is continued after ammonia oxidation,the extremely low level of FA would lose its inhibition on NOB activity,resulting in the conversion of nitrite to nitrate by NOB.In other words,excess aeration(aeration is still on after ammonia oxidization finishes)could promote the conversion of nitritation to nitrification,which has also been found by Gao et al.[23]in an SBR treating municipal wastewater.On the contrary,if the aeration is stopped as soon as nitritation is completed,the NOB activity would be continuously inhibited by FA in the nitritation,which ensures that nitrite is no longer available for NOB.However,the end-point of nitritation can be reliably detected by characteristic points on the DO,pH,and ORP curves.Thus,process control is available to prevent the occurrence of excess aeration.In this study,we were able to prolong favorable FA inhibition using process control to achieve nitritation in the SBR.
In order to further explain the results on stable nitritation obtained in the SBR at low temperature,the nitrifying population in the SBR sludge was detected using SEM and FISH techniques.On day 345,a sludge sample was taken from the SBR for examination by SEM and FISH.
Fig.5 shows that SEM result of microbial population in the SBR sludge system.SEM images revealed abundance of spherical cells(0.65 × 0.45-1.06 × 0.63 μm)and rod-shaped cells(0.88-5.65 μm)in the sludge flocs.For more detailed localization,as shown in Fig.6,FISH observation showed that AOB percentage was calculated to be 4.6%of all bacteria,whereas NOB fraction was less than 0.3%of all bacteria,and no Nitrospira was detected.This result explains the essential reason for successful nitrite accumulation in the SBR because AOB were dominant in the nitrifying bacterial clusters.
4.Conclusions

Fig.5.SEM result of microbial population:(a)spherical cells;(b)rod-shaped cells.

Fig.6.FISH results for AOB and NOB:(a)NSO1225 target for AOB;(b)NIT3 target for Nitrobacter,Ntspa 662 target for Nitrospira.(Red color signifies AOB in Fig.6a,NOB was notdetected in Fig.6b,green color signifies all bacteria in Fig.6a and b.)
This study demonstrated that the FA inhibition on NOB activity coupled with process control was effective for starting up nitritation at low temperatures of 14.0 °C-18.2 °C in the SBR treating ammoniumrich land fill leachate.The end-point of ammonium oxidation was clearly and reliably detected using characteristic points the DO,pH,and ORP curves.Ceasing aeration at the end of ammonium oxidation allowed FA to continue to inhibit NOB activity.Based on the observation of SEM and FISH,AOB were confirmed to be the dominant bacteria,which contributed to stable nitritation performance obtained in this study.
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
