N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
K.Krishnaveni,K.Sampath ,J.Ravichandran ,*,C.Jayabalakrishnan
1 Research Department of Chemistry,Sri Ramakrishna Mission Vidyalaya College of Arts and Science,Coimbatore 641020,Tamilnadu,India
2 Department of Chemistry,Kumaraguru College of Technology,Coimbatore 641049,Tamilnadu,India
Keywords:Mild steel EIS Mass loss SEM Acid corrosion
ABSTRACT The corrosion inhibition of mild steel in 1 mol·L-1 hydrochloric acid by N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide(MNBHC)was studied using weight loss and electrochemical studies.Results obtained indicate that the inhibitor is effective in hydrochloric acid medium and the efficiency decreases with increase in temperature.Added halide additives improve the efficiency of the inhibitor.The AC impedance studies reveal that the process of inhibition is through charge transfer.Polarization studies indicate the mixed nature of the inhibitor.From the thermodynamic,spectraland surface analyses the nature of adsorption has been found out.The adsorption of the inhibitor on mild steel follows the Langmuir isotherm.
1.Introduction
Corrosion inhibition of metals is an important process in metal finishing industries.The treatment of inhibitor increases the life period of metal utensils.Several efforts have been made to control or reduce corrosion and out of numerous methods,the use of inhibitors is one of the most practical methods for the prevention of corrosion particularly in acidic media[1-3].A number of organic and inorganic compounds are known to act as corrosion inhibitors for metals in various environments.Compounds containing nitrogen,sulfur and oxygen atoms adsorb on the metal surface and block the active sites and thereby decrease the corrosion rate.The efficiency of these compounds depends upon electron density present around the hetero atoms,number of adsorption active centers in the molecule,their charge density,molecular size,mode of adsorption and formation of metallic complexes[4-6].Compounds with π-bonds generally exhibit good inhibitive properties due to interaction of π-electrons with metal surface.Schiff bases are one of the widely used corrosion inhibitors due to their ability to form protective layer on the metal surface.The presence of>C═N-group in the molecules of schiff bases makes these compounds effective corrosion inhibitors.Many corrosion protection works have been studied with the use of synthetic compounds especially organic compounds[7-9].In the present study an attempt has been made to study the adsorption and inhibition effect N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide(MNBHC)as a new inhibitor for the corrosion of mild steel in 1 mol·L-1HCl using mass loss and electrochemical methods.
2.Materials and Methods
2.1.Materials preparation
The mild steel(MS)specimen of dimension 1 cm×5 cm×0.2 cm with an area of 12.3 cm2was used for the mass loss study and the specimen with an exposed area of 0.95 cm2was used for electrochemical study.The surface of the specimens was mechanically polished with different grades(600,800 and 1000)of emery papers and then degreased with acetone and stored in a desicator.The composition(by mass)of MS is:C 0.14,Mn 0.57,Al 0.05,Cr 0.03,Si 0.02,Cu 0.01 and balance is Fe.The inhibitor MNBHC was prepared according to the reported procedure of Sampath et al.[10].The product so obtained was taken for the investigation of corrosion inhibition study.Fig.1 shows the molecular structure of the examined compound MNBHC.
2.2.Mass loss method
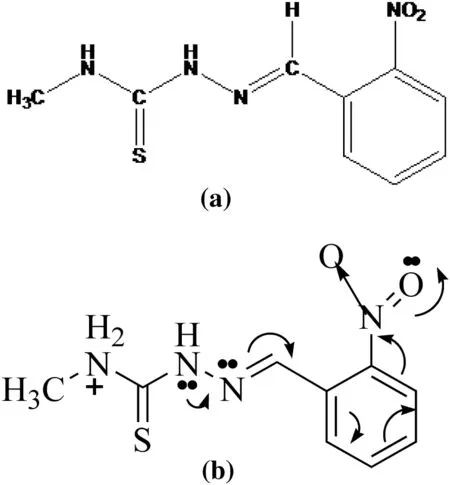
Fig.1.(a)Chemical structure of studied schiff base N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide.(b)Direction of electron movements in the chain of studied schiff base N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide.
The pre-cleaned and pre-weighed MS specimens in triplicate were suspended in 100 ml test solution with and without inhibitor at different concentrations(4-32 mol·L-1)of MNBHC in 1 mol·L-1HCl for a period of 1 to 48 h.After that,the specimens were taken out,washed with distilled water,dried,and weighed.From the mass loss data,percentage inhibition efficiency(IE,%)was calculated and the optimum concentration of the inhibitor was established.To study the influence of the concentration of the acid on the effectiveness of the inhibitor,mass loss experiments were carried out in 1-3 mol·L-1HCl.The inhibitor concentration was kept constant at 32 mol·L-1in the test solution.The synergistic influence of halide ions was studied by adding the halide additives(1 mol·L-1of KCl,KBr and KI)to MNBHC.All these studies were carried out at(303±1)K.The influence of temperature on the corrosion behavior of MS in the presence of MNBHC was studied in the range of 303-333 K.The inhibiting power was calculated using the following equation.

where,WBand WIare the mass loss of the MS specimens in the absence and in the presence of inhibitor respectively.The corrosion rate(CR,mm·a-1)was calculated employing the equation,

where,W is the mass loss of the specimen(mg),ρ is the density of the specimen(g·cm-3),A is the area of specimen(cm2),and t is the exposure time(h).
2.3.Electrochemical method
Electrochemical studies were carried out using Electrochemical Analyzer of HCH Instruments(Model 608D).The experiments were carried out in a three electrode cell assembly with a platinum wire meshelectrode and a saturated calomel electrode used as auxiliary and reference electrodes respectively.MS specimen was used as the working electrode.AC impendence studies were conducted in the frequency range of 10,000-1 Hz at the rest potential using 0.02 V sine wave as the excitation signal.Rctand Cdlvalues were obtained from the Nyquist plots.The IE(%)was calculated from the following equation,
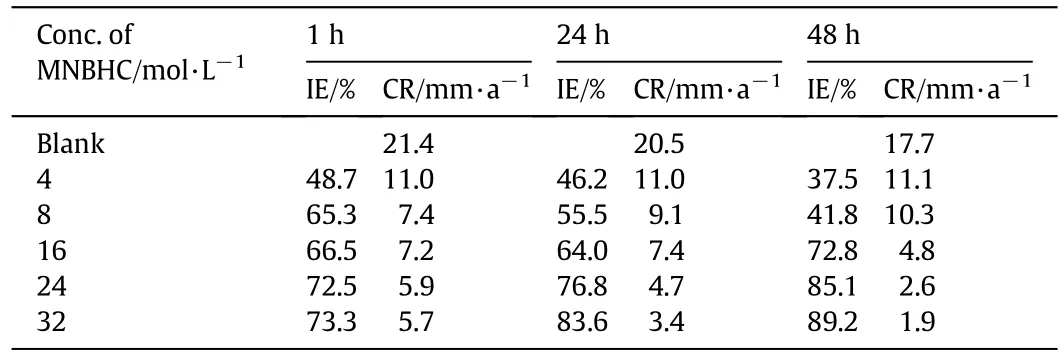
Table 1 Effect of concentration of MNBHC and immersion time on the corrosion inhibition of MS in 1 mol·L-1 HCl at room temperature
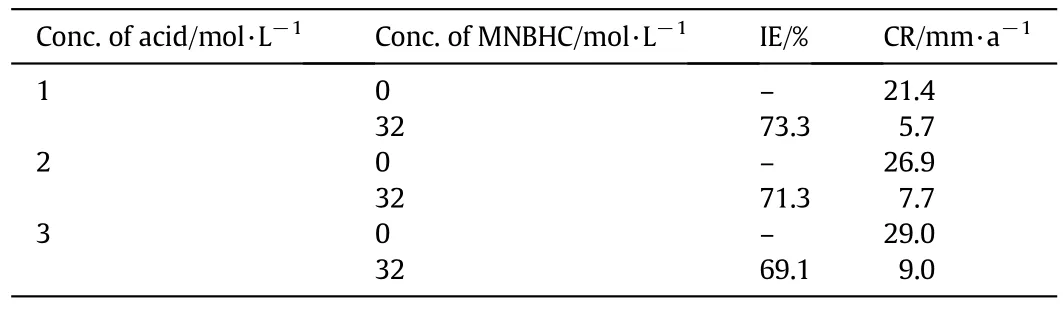
Table 2 Effect of concentration of HCl on the corrosion inhibition of MS with 32 mol·L-1 MNBHC at room temperature


2.4.Surface analysis
Surface morphology of the MS for fresh,inhibited and uninhibited specimens were also examined using scanning electron microscope(SEM)of JOEL model(JSM 6390).Fourier transform infra-red(FT-IR)spectra were recorded using Perkin Elmer Spectrophotometer to study the characteristic adsorption of the functional groups present in the inhibitor on to the metal surface.FT-IR was recorded for the inhibitor MNBHC and the material obtained by scrapping the specimens after the immersion experiment.
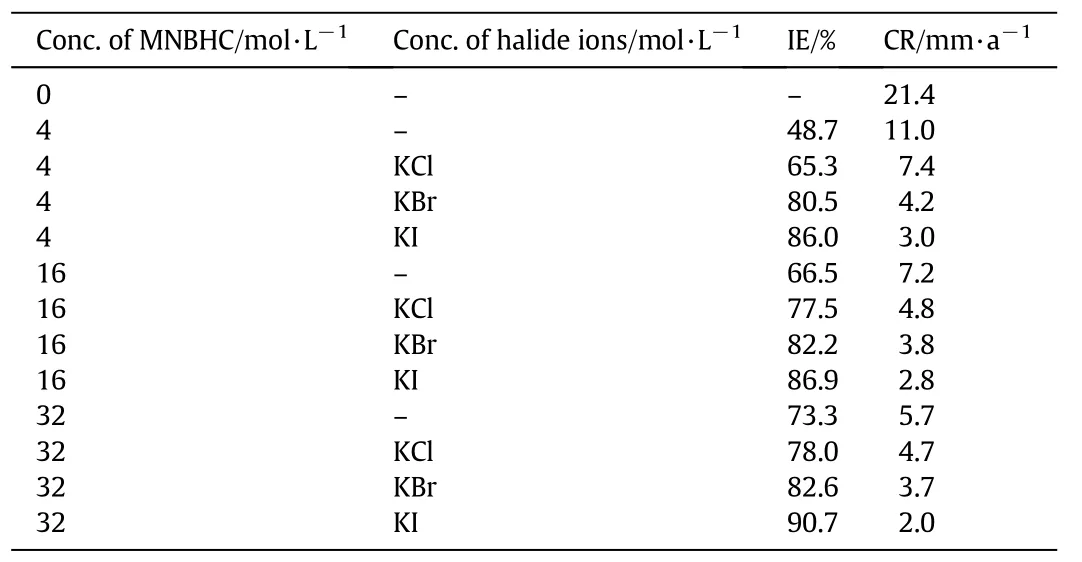
Table 3 Effect of addition of halide additives(1 mol·L-1)on the corrosion inhibition of MS in 1 mol·L-1 HCl with different concentrations of MNBHC
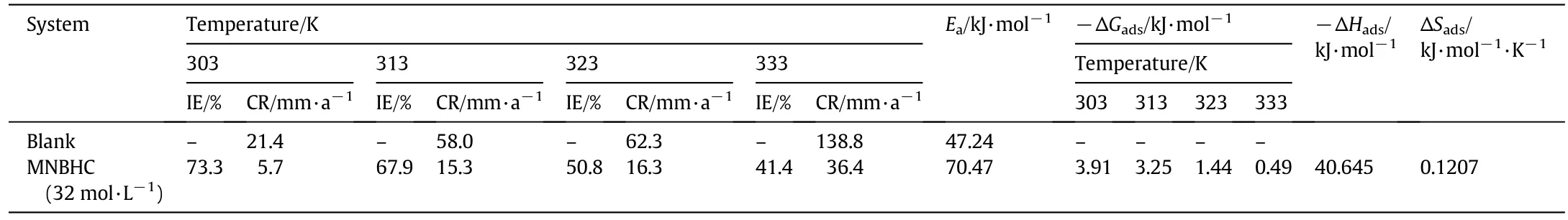
Table 4 Effect of temperature on the corrosion inhibition of MS in 1 mol·L-1 HCl with and without MNBHC
3.Results and Discussion
3.1.Mass loss method
The results obtained for the corrosion inhibition of MS in 1 mol·L-1HCl with MNBHC by mass loss studies are given in Table 1,and it is clear from the table that the IE increases with increasing the concentration of MNBHC.The maximum IE at1 h immersion time for MNBHC was noticed at an inhibitor concentration of 32 mol·L-1.Further addition of MNBHC did not produce significant improvement in the performance of the inhibitor.Also,the IE increases with increase in immersion time from 1 to 48 h.The increase in IE or decrease in corrosion rate reveals the adsorption of active functional groups present in the MNBHC on MS.The increase in inhibition efficiency at higher immersion time is due to the higher adsorption of the inhibitor molecules on the metal surface on standing.The adsorption of the inhibitor molecules on the metal surface could be due to any of the following reasons:
(1)The inhibitor molecules can directly get adsorbed on the metal surface via.the lone pair of electrons and π electrons of the inhibitor molecules.
(2)The inhibitor molecules can get protonated in the acid medium and the positively charged protonated molecules can get adsorbed on the metal surface via the chloride ions which are strongly adsorbed on the positively charged metal surface.
An observation of the structure of the inhibitor molecule[Fig.1(a)]clearly shows that the-NO2being electron withdrawing in nature attracts the bonding electrons towards itself.Hence,the direction of movement of the electrons in the chain is towards the phenyl ring.As a result,due to the+I effect of the methyl group the electron density on the methyl nitrogen is relatively more when compared to the other nitrogens and sulfur atom and hence gets protonated.This is pictorially represented in Fig.1(b).
Based on the above facts it is possible to suggest that the inhibitor molecules can get adsorbed on to the metal surface by following means.
(1)The inhibitor molecules can get directly adsorbed on to the positively charged metal surface through theπ electrons or the electron rich nitro group attached to the aromatic ring.
(2)The adsorption of the inhibitor molecule can also be through the interaction between the protonated inhibitor molecule and the chloride ions which are already adsorbed on the positively charged metal surface.
The data from Table 2 shows that with the increase in concentration of the acid the inhibition efficiency decreases.This could be due the fact that at higher concentrations due to the increase in aggressiveness of the acid,the corrosion rate increases and hence,the inhibition efficiency decreases.It is also possible that at higher concentrations,the surface is saturated with anions of the acids allowing less space for the inhibitor molecules to get adsorbed through the electron rich centers of the inhibitor molecule.
Table 3 indicates the results due to the addition of halide ions to different concentrations of MNBHC.It is clear from the table that the addition of halide ions improves the IE of the MNBHC to considerable extent.The order of increase has been found to be Cl¯< Br¯< I¯.Many of the earlier authors have reported an improvement in the IE of inhibitor in the presence of halide ions and they have noticed a similar trend in the performance of the halide ions following the order Cl¯< Br¯< I¯[11-13].They have attributed the higher synergistic effect of I¯in comparison to Cl¯and Br¯to its larger size.It has also been reported that the halide ions act synergistically with inhibitor molecules by acting as a bridge between the positively charged metal surface and positively charged inhibitor molecules[14].

Fig.2.(a)Arrhenius plot for MS corrosion in 1 mol·L-1 HClin the absence and presence of MNBHC.(b)Langmuir adsorption isotherm for the corrosion of MS in the presence of MNBHC.(c)Dependence of ΔG ads on temperature for mild steel in 1 mol·L-1 HCl containing MNBHC.

Fig.3.a)Nyquist plots for MS corrosion in 1 mol·L-1 HCl containing different concentrations of MNBHC.b)Anodic and cathodic polarization curves of MS in uninhibited and inhibited systems containing different concentrations of MNBHC.
3.2.Effect of temperature
The temperature can modify the interaction between metal surface and the acid in the presence of inhibitor.The result due to the influence of temperature on the performance of the inhibitor is given in Table 4.The energy of activation(Ea)for MS corrosion is calculated from the slope of the Arrhenius plot(lg CR vs.1/T plot)[Fig.2(a)]where slope is Ea/2.303 R,CR is the corrosion rate,R is the gas constant,T is the temperature in absolute scale.The values of Eafor free and inhibited acids obtained from the plots are also given in Table 4.Inspection of the above results indicates that an increase in temperature decreases the inhibition efficiency of the MNBHC,suggesting physisorption of the inhibitor on the metal surface.As reported by Amin et al.[15],the decrease in the IE at higher temperature might be due to the shift of the adsorption desorption equilibrium towards desorption at higher temperatures andalso due to the roughening of the metal surface at higher temperatures because of the increase in corrosion rate.
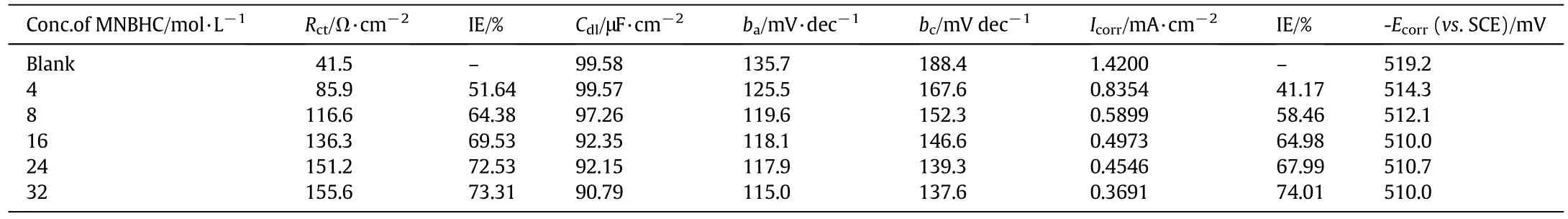
Table 5 Electrochemical parameters for the corrosion inhibition of MS in 1 mol·L-1 HCl with different concentrations of MNBHC at room temperature
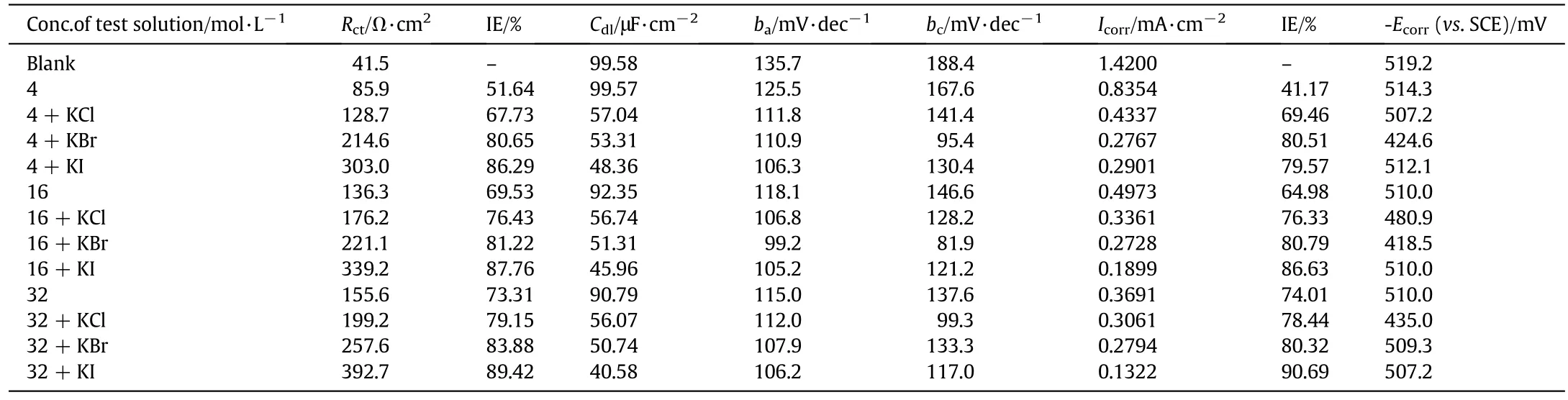
Table 6 Electrochemical parameters for the corrosion inhibition of MS in 1 mol·L-1 HCl at different concentrations of MNBHC with halide additives at room temperature
From the table,it is also clear that the Eavalue in the presence of inhibitor is higher than that in the absence of inhibitor.This behavior is an indication of physical adsorption of the constituents of the inhibitor on the metal surface[16].An increase in the activation energy of inhibited system is due to the adsorption of the inhibitor molecules on active sites.
3.3.Adsorption isotherm
Corrosion inhibition is a surface process with specific adsorption of inhibitor on the metal surface.Adsorption isotherms are very important in understanding the mechanism of inhibition of corrosion reaction.An attempt has been made to understand the nature of interaction between the inhibitor and metal surface in terms of adsorption isotherm.The surface coverage values obtained from weight loss studies were used to find out the suitable adsorption isotherm.Among various adsorption isotherms tested,the Langmuir isotherm gave the best fit in the temperature range of 303 to 333 K.The Langmuir isotherm is represented by the following equation.

where Kadsis adsorption equilibrium constant and is related to the free energy of adsorption.The Langmuirisotherm is obtained by plotting log[θ /(1- θ)]vs.lg C and is shown in Fig.2(b).The free energy of adsorption(ΔGads)at various concentrations of inhibitor at different temperatures was calculated using the following equation.

where,K= θ/Cinh(1-θ),θ is the surface coverage,Cinhis the concentration of inhibitor and the constant value of 55.5 represents the concentration of water in solution.Generally a value of ΔGadsless negative than-20 kJ·mol-1signifies physisorption and a value more negative than about-40 kJ·mol-1indicates chemisorption[17].Analysis of Table 4 shows that the values of ΔGadsare less negative than-20 kJ·mol-1indicating that the process of inhibition is through physisorption.Further,the negative values of ΔGadspointout the stability of the adsorbed layer and the spontaneity of adsorption.The ΔHadsand ΔSadsvalues were obtained from the ΔGadsvs.T plot(Fig.2(c)).The results are given in Table 4.Analysis of the results in Table 4 indicates that ΔHadsvalues are negative and ΔSadsvalues are positive suggesting that the reaction is feasible.Further,the ΔGads,ΔHads,and ΔSadsvalues indicate that the process of adsorption of MNBHC is exothermic in nature.

Fig.4.SEM images of(a)fresh MS,(b)MS in 1 mol·L-1 HCl,(c)MS in 1 mol·L-1 HCl with MNBHC.
3.4.Electrochemical method
Impedance studies were carried out by varying the concentration of inhibitor and the concentration of added halide ions.The Randles equivalent circuit used for impedance studies[18]is given in Fig.3(a),where Rsis solution resistance,Cdlis the double layer capacitance and Rctis the charge transfer resistance.The impedance data obtained from Nyquist plots for the above mentioned systems are given in Tables 5 and 6.The representative Nyquist plots for various concentrations of inhibitor MNBHC is given in Fig.3(b).The values of Rctincreased with increase in concentrations of the inhibitor.The impedance diagrams obtained are approximately semi-circle in nature and shape is varied upon increasing the concentration of inhibitor and added halide ions suggesting that the corrosion of MS is controlled by a charge transfer process[19].The values of Cdldecrease with the increase in the concentration of the organic compounds which undergo adsorption on metal surface.This is due to the increase in the thickness of the double layer and also the gradual replacement of water molecules by the organic molecules on the metal surface.
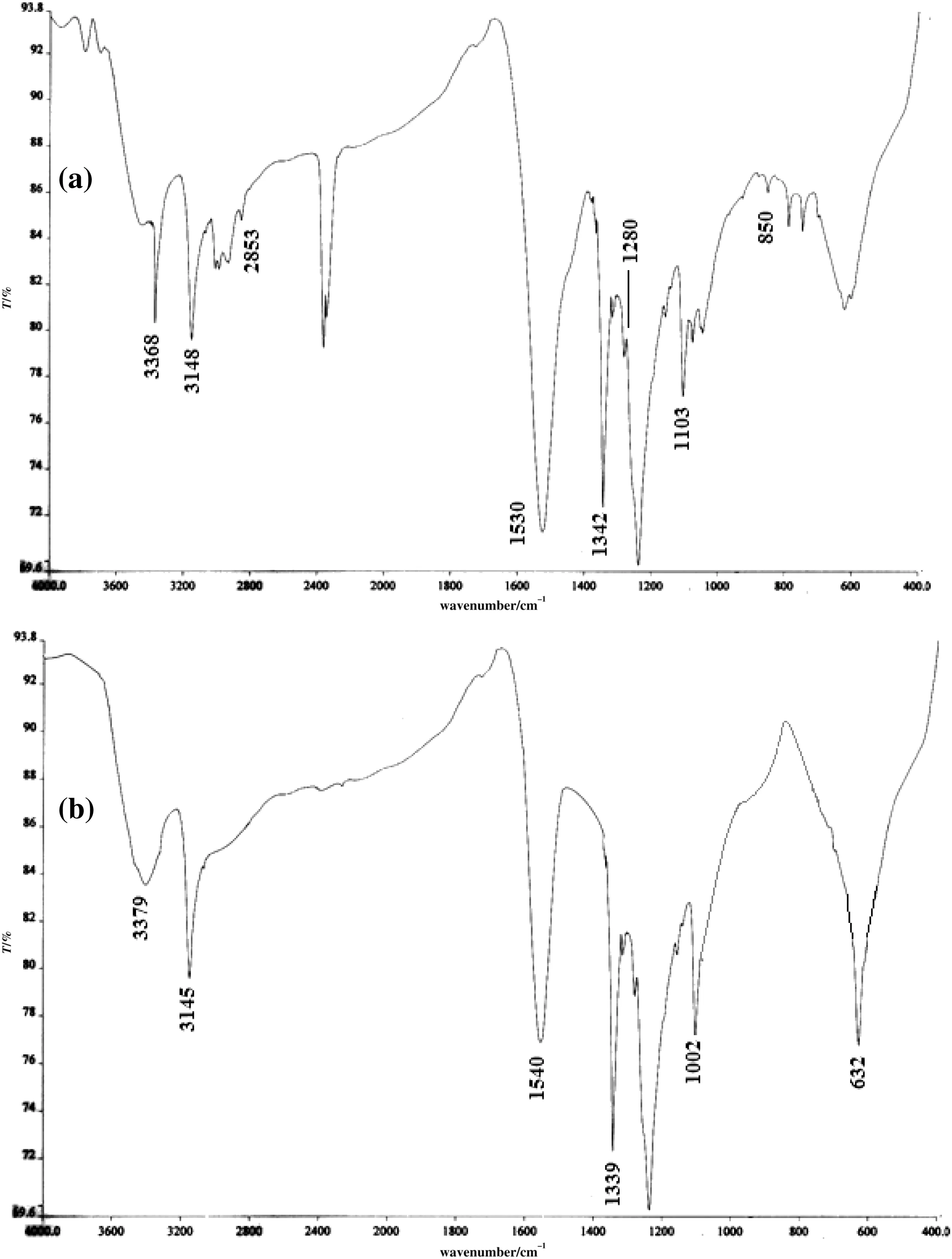
Fig.5.FT-IR spectra of(a)MNBHC and(b)scrapped material from MS after immersion.
Fig.3(c),depicts the representative Tafel plots of the corrosion inhibition studies on mild steel with varying the concentration of the inhibitor.The polarization data obtained are given in Tables 5 and 6.Results show that on increasing the concentration of inhibitor,the Icorrvalues decrease which is due to the increase in the surface coverage by the inhibitor.Also,addition of inhibitor and halide ions alters both baand bcvalues suggesting that the inhibitor reduces both anodic dissolution and cathodic hydrogen evolution and thereby indicating the mixed nature of the inhibitor[20].The corrosion potential(Ecorr)is found to be high in the absence of inhibitor for all the studied systems.Competitive adsorption mechanism directs the corrosion potential of blank solution to more negative values.Addition of inhibitor produces a positive shift(less negative)in the Ecorrvalues which is an indication of the interaction of the inhibitor molecules with metal surface.Analysis of the results also reveals that in inhibited system Ecorrvalues are shifted towards less negative values representing the nobility.Further,there has not been much change in the Ecorrvalues of the inhibited test solutions indicating the mixed nature of the inhibitor preventing both anodic and cathodic reactions.This is further confirmed by the variation of baand bcvalues of blank solution from that of the inhibited solution.
4.Surface Analysis
The SEM images of fresh,uninhibited and inhibited specimens are shown in Fig.4(a)-(c).Analysis of Fig.4 reveals that the MS surface is damaged more in the absence of inhibitor and less in the presence of inhibitor and the image shows good protective film formation by the adsorbed functional groups on the metal surface.This is further confirmed by FT-IR studies.Fig.5 shows the IR spectra of the inhibitor and the material obtained by scrapping the inhibited specimen.In Fig.5(a),N-H asymmetric and symmetric stretching is obtained at 3368 and 3148 cm-1respectively.The band at 2853 cm-1corresponds to C-H stretching.A strong band at 1530 cm-1and a weak band near 1280 cm-1are assigned for C-NH vibrations resulting from the coupling of N-Hin plane bending and C-Nstretching vibrations.The strong band at1342 cm-1is attributed to NO2symmetric stretching in aromatic nitro group and the band at1103 cm-1is assigned for N-Nstretching.The C═S stretching vibration is found at 850 cm-1[10,21,22].Fig.5(b)shows that the absorption frequencies corresponding to N-Hare shifted from 3368 to 3379 cm-1and 3148 to 3145 cm-1respectively.The peak at 1530 cm-1which is assigned for C═N is shifted from 1530 to 1540 cm-1.The band related to NO2symmetric stretching in aromatic nitro group at 1342 cm-1is shifted to1339 cm-1.The N-N stretching at 1103 cm-1is shifted to 1002 cm-1.The new band at 632 cm-1in the IR spectrum of the scrapped material is due to γ-Fe2O3[16].The shift in the absorption frequencies indicates that there is an interaction between the inhibitor molecules and the metal surface advocating the adsorption of MNBHC on metal surface.
5.Conclusions
(1)Increase in the concentration ofMNBHC increases the IE whereas increase in temperature decreases the IE.
(2)Thermodynamic data suggests that the process of inhibition occurs through physisorption and follow the Langmuir isotherm.
(3)The potentiodynamic polarization studies reveal that MNBHC acts as a mixed type inhibitor.
(4)It is thus,evident that the inhibitor MNBHC is effective in bringing down the corrosion of mild steel in 1 mol·L-1HCl.
Acknowledgments
The authors wish to acknowledge the PG and Research Department of Chemistry,Sri Ramakrishna Mission Vidyalaya College of Arts and Science,Coimbatore-20 for providing the facilities.
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- Facile synthesis ofporous Pd nano flowers with excellentcatalytic activity towards CO oxidation☆
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
