Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
Guo Lin,Qiang Zhuang,Qun Cui*,Haiyan Wang,Huqing Yao
College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
Keywords:Lithium slag FAU/LTA Co-crystalline zeolite Mother liquid Detergent builder
ABSTRACT Co-crystalline zeolite FAU/LTA-0 was synthesized by hydrothermal method from lithium slag.To make the most of excess silicon and alkalisources in motherliquid derived from FAU/LTA-0,zeolite FAU/LTA-1b was synthesized in the same method with the use of mother liquid by adding a certain amount of aluminum source.Influences of different adding ways of aluminum source and recycling dosages of mother liquid on synthesis of zeolites FAU/LTA with mother liquid were investigated.The phase,microstructure and thermostability of FAU/LTA-0 and FAU/LTA-1b were characterized by XRD,SEM and TG-DTA.The calcium and magnesium cation exchange capacities(CECs)of the zeolites were determined.The results have shown that co-crystalline zeolite can be synthesized with the use of mother liquid by adding aluminum source after 2 h of reaction.Compared with FAU/LTA-0,the crystal twinning structure of FAU/LTA-1b became weaker,the grain size was smaller,and the thermostability was better.With a lower dosage of mother liquid,the content of P-type impurity in product decreased significantly,and the content of LTA phase increased.The reuse rate of mother liquid can reach 48%.The CECs of FAU/LTA-1b-150 can reach 343 mg CaCO3·g-1 and 180 mg MgCO3·g-1,showing more excellent adsorption capacities than FAU/LTA-0 and commercial zeolite 4A.The full recycling use of mother liquid to synthesize zeolite FAU/LTA which can be applied for detergent not only improves resource utilization but also reduces production cost.
1.Introduction
Lithium slag is a solid by-productin the process of producing lithium carbonate from spodumene by sulfuric acid method.Currently,only a small amount of lithium slag is used as building materials such as cement and concrete[1],and most of them are dealt with land fill or open-air pile-up,with low utilization and added value.Lithium slag mainly contains silicon and aluminum,the ratio of which is generally between 2 and 3.Thus,the author's group has proposed processes to synthesize zeolite FAU(NaX or 13X)from lithium slag[2,3],opening up a new way for the efficient use of lithium slag.
Recently,zeolite 4A is widely used for phosphate-free builder[4,5],which has a calcium ion exchange capacity and an exchange rate better than those of sodium tripolyphosphate(STPP).The adsorption capacity of surfactant on 4A is five times on STPP,which contributes to improve detergency.However,the magnesium ion exchange capacity of 4A is low.Zeolite 13X has a higher magnesium ion exchange capacity than that of zeolite 4A,but a lower calcium ion exchange capacity.It is known that certain amounts of zeolite 13X have been added in some high-grade detergents in order to improve the removal ability of magnesium in water.On the other hand,the price of 13X is relatively high,limiting its use as a detergent builder.Zeolite FAU/LTA synthesized from lithium slag by the author's group has a good application prospect as a detergent builder[6].
Co-crystalline zeolite FAU/LTA synthesized from chemical reagents has been reported.It was first reported by Zatta et al.[7]that zeolite FAU/LTA had a high CEC and an exchange rate for both calcium and magnesium.Traa et al.[8]synthesized zeolite A/X with the proportion of A-type to X-type controlled by adjusting the ratio of Na to K in the synthesis system.FAU/LTA composite was synthesized by using TMA+as a template,by adjusting only the concentration of Na+ions in the initial solution[9].Zhan et al.[10]found that Cl-,F-and K+had a great influence on phase selection of FAU and LTA zeolites.Wang et al.[11]prepared zeolite Na-A/X with a low molar ratio of Si to Al in the pure sodium aluminosilicate system.Meanwhile,zeolite FAU/LTA was synthesized without template by Hu et al.,and the CECs of the zeolite for calcium and magnesium could reach 295 mg CaCO3·g-1and 150 mg MgCO3·g-1,respectively[12].The author's group[6]prepared zeolite FAU/LTA by alkali dissolution and hydrothermal reaction from lithium slag.However,the mother liquid that is generated from the process of synthesis contained a large amount of surplus silicon which has become the issue most in need of a solution.
It is a key and difficulty to comprehensive utilization of mother liquid in the process of zeolite synthesis from minerals.Engelhard company[13]studied recycle of motherliquid derived from synthesis of zeolite Y from kaolin,and SiO2and Na2O were reused which were high in the mother liquid.Hiraki et al.[14]prepared zeolite NaX by making the most of silicon sludge and aluminum dross with NaOH solution,and utilization ratio of Si and Na were 21.4%and 7.8%respectively after 3 cycles.Pengthamkeerati et al.[15]synthesized silicalite-1 by using fly ash as a silicon source.The use of silicon and seed crystal in mother liquid is conducive to synthesis of silicalite-1.Rani et al.[16]found that mother liquid had memory effect in the process of zeolite synthesis.Crystal under the effect of mother liquid which contained structure units of zeolite grew faster.Pan et al.[17]used silica gel powder as a silicon source to synthesize ZSM-5 with NaY zeolite as a seed.The addition of mother liquid made products prone to secondary nucleation with a lower co-crystallization,a smaller grain size and a better dispersity.
In the present paper,utilization of mother liquid derived from synthesis of co-crystalline zeolite FAU/LTA from lithium slag was studied.A certain amount of sodium metaaluminate was added to synthesize zeolite FAU/LTA according to the content of silica in the mother liquid.Effects of different adding ways of aluminum source and recycling dosages of mother liquid on adsorption capacity(calcium or magnesium cation exchange capacity)of zeolites FAU/LTA were investigated.The products were characterized by X-ray diffraction(XRD),scanning electron microscopy(SEM),thermogravimetric analysis(TGA)and differential thermal analysis(DTA).The study is aimed to realize efficient utilization of lithium slag,and to provide a steady and inexpensive detergent builder for detergent market.
2.Experimental
2.1.Materials and reagents
Lithium slag was obtained from General Lithium(Haimen)Co.,Ltd.The chemical composition of lithium slag was determined by ARL ADVANTXP X-ray fluorescence spectrometer(XRF)which is listed in Table 1.Commercial zeolite 4A was obtained from Nanjing Inorganic Chemical Plant of China.
Sodium hydroxide(NaOH,AR,provided by Xilong Chemical Co.,Ltd.),sodium metaaluminate(NaAlO2,AR,supplied by Aladdin Chemical Co.,Ltd.),calcium chloride(CaCl2,AR,provided by Xilong Chemical Co.,Ltd.)and magnesium chloride(MgCl2·6H2O,AR,provided by Xilong Chemical Co.,Ltd.)were used.Deionized water was made in author's laboratory.
2.2.Synthesis of zeolites FAU/LTA
2.2.1.Synthesis of zeolite FAU/LTA from lithium slag
The general procedures for synthesizing zeolite without mother liquid added are described below.Firstly,200 ml of NaOH solution was added to 50 g of lithium slag,followed by gentle agitation for 10 min and moderate temperature for 2 h.Then 250 ml of deionized water was added into the solution.After aging for 2 h,the resultingmixture kept heated at an appropriate temperature for 9 h.At last,the solid product was filtrated,washed with deionized water and dried in an oven at 110°C overnight.The sample was marked as FAU/LTA-0,and the mother liquid was in reserve.

Table 1 Chemical component of lithium slag
2.2.2.Synthesis of zeolite FAU/LTA with utilization of mother liquid
200 ml of mother liquid and a certain amount of NaOH solution were added to 50 g of lithium slag to satisfy base concentration required by zeolite synthesis.Then a certain amount of NaAlO2was added to keep molar ratio of Si to Al stay the same,followed by gentle agitation for 10 min and moderate temperature for 2 h.Then 250 ml of deionized water was added into the solution.After aging for 2 h,the resulting mixture kept heated at an appropriate temperature for 9 h.At last,the solid product was filtrated,washed with deionized water and dried in an oven at 110°C overnight.The solid sample was marked as FAU/LTA-1a,and the mother liquid was marked as L-FAU/LTA-1a.
In dreams, a strange city, denotes you will have a sorrowful occasion to change your abode64 or mode of living (Miller 146). Both the above meanings are shown within the tale, the daughter s change in statues and the happy ending.Return to place in story.
In above system,if NaAlO2was added after lithium slag was dissolved by mother liquid or NaOH solution for 2 h,the final product was then marked as FAU/LTA-1b or FAU/LTA-1b-200,and the corresponding mother liquid was marked as L-FAU/LTA-1b-200.Then,zeolite FAU/LTA-1b could also be prepared if dosage of mother liquid decreased to 100 ml(or 150 ml)along with 100 ml(or 50 ml)of water added.The solid sample was referred as FAU/LTA-1b-100(or FAU/LTA-1b-150),and the corresponding mother liquid was referred as L-FAU/LTA-1b-100(or L-FAU/LTA-1b-150).
2.3.Characterization of zeolite FAU/LTA
XRD measurement was performed on a SmartLab series X-ray diffractometer by Rigaku using Cu Kαradiation with a step size of 0.02°,a tube current of 40 mA and a tube voltage of 100 kV.The mass ratios of FAU to LTA in zeolites FAU/LTA were calculated by RIR-value method[6]as shown in Eq.(1)according to XRD patterns.The calculated RIRFAUand RIRLTAwere 1.11 and 0.483,respectively.

Morphology analysis was performed using a S4800 emission scanning electron microscope by Hitachi.Each sample was coated with 5 nm gold.TG-DTA analysis was performed with a WCT-1 simultaneous thermal analyzer by Beijing Optical Instrument Factory.
2.4.Content analysis of main components of mother liquid
Content of silicon in mother liquid was measured by silicomolybdic blue spectrophotometric method reduced by ammonium ferrous sulfate on the base of national standard of GB/T 6730.9-2006[18].Content of aluminum in mother liquid was measured by EDTA volumetric method according to national standard of YB/T 109.3-1997[19].Concentration of alkali in mother liquid was determined by acid-base titration.
2.5.Measurement of cation exchange capacity of zeolite FAU/LTA
Cation exchange capacity of zeolite FAU/LTA for calcium ion in aqueous solution was determined on the basis of national standard“4A zeolite for detergents”[20].Cation exchange capacity for magnesium ion was measured referring to the determination of CEC for calcium ion.
3.Results and Discussion
3.1.Contents of main components of mother liquid
Part of silicon was not involved in synthesis of FAU/LTA-0 resulting in a large amount of silicon left in mother liquid.The mother liquid can be used to synthesize zeolite FAU/LTA by adding aluminum source into the mother liquid to keep the molar ratios of Si to Al the same as that of lithium slag.
The main components of mother liquid which are derived from zeolites FAU/LTA synthesis are shown in Table 2.There existed large amounts of silicon which reached 14.1 kg·m-3in the mother liquid in the process for synthesizing FAU/LTA-0.The content of aluminum was smaller by contrast,only about0.03 kg·m-3.The concentration of alkali in mother liquid remained the same,which was 3.25 kmol·m-3.When 200 mlofmother liquid were added into synthesis system,itwas equivalent to 2.82 g of silicon to participate,which accounted for 17%of silicon in 50 g of lithium slag.Thus,only a small amount of aluminum source needs to be added to synthesis system,in order to maintain molar ratio of Si to Al constantly.Because the main component in the secondary mother liquid derived from FAU/LTA-1a and FAU/LTA-1b remains unchanged compared with that of FAU/LTA-0,the utilization of mother liquid can be recycled.About 310 ml of mother liquid could be collected from synthesis of FAU/LTA-0,so that the reuse rate of mother liquid reached 48%when 150 ml of mother liquid was added to reaction system.
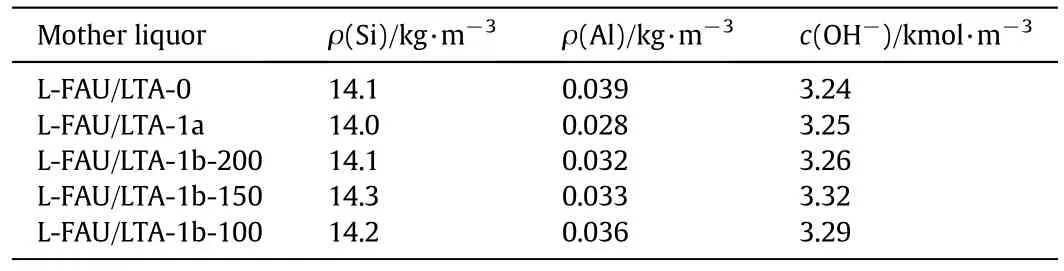
Table 2 Main components of mother liquid corresponding to different synthetic processes
3.2.Synthesis of zeolites FAU/LTA with utilization of mother liquid
3.2.1.Effect of adding ways of aluminum source on synthesis of zeolites FAU/LTA
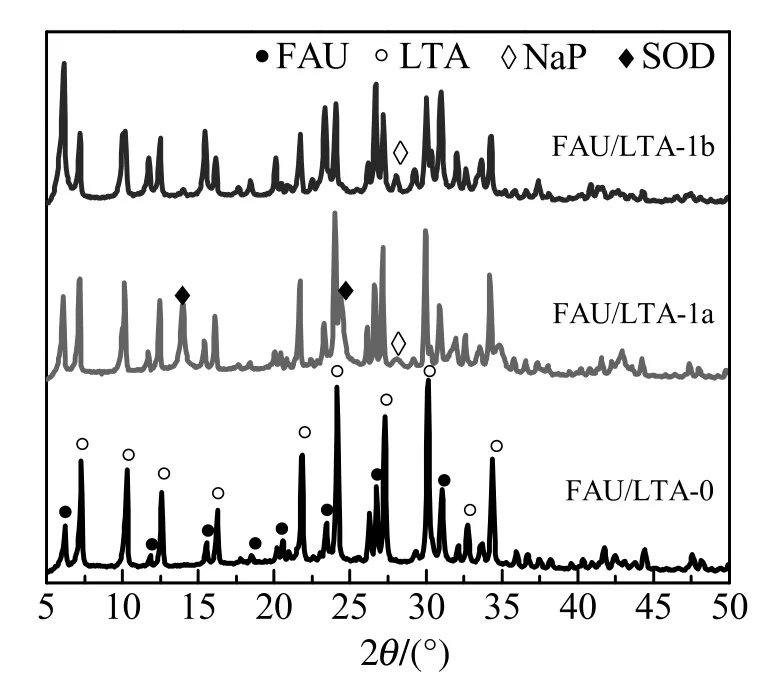
Fig.1.XRD patterns of zeolites FAU/LTA synthesized with different adding ways of aluminum.

Table 3 Contents of crystalline phases of zeolites FAU/LTA with different adding ways of aluminum
FAU/LTA-0 synthesized from lithium slag had typical diffraction peaks of FAU-type and LTA-type without any impurity peak.The content of LTA phase was as high as 82%,and FAU phase 18%.However,except for features of FAU-type and LTA-type in the zeolites with mother liquid involved in synthesis,there still had other crystal phases.For FAU/LTA-1a,it contained obvious amounts of SOD-type and little P-type.The content of LTA and FAU phase were 61%and 22%,respectively.For FAU/LTA-1b,it contained little P-type only.The content of LTA phase was down to 45%,and the content of FAU phase was up to 50%.This is due to the fact that the mother liquid derived from FAU/LTA-0 contains a lot of active silicon source.When active aluminum source is joined initially,it is easy to form the primary structure units of four-membered ring under influence of high alkali concentration,resulting in generation of dense phase of SOD-type.On the other hand,when aluminum source is added after reaction at 2 h,the influence of aluminum on mother liquid becomes weak extremely because aluminosilicate in lithium slag which has structure of leached spodumene has changed to be active silicon and aluminum sources.Furthermore,the primary structure units that may be existed in the mother liquid have more advantage to promote the formation of low-silica FAU-type.
3.2.2.Morphology of zeolites FAU/LTA with utilization of mother liquid
Fig.2 shows SEM images of zeolites FAU/LTA-0 and FAU/LTA-1b.Notably,FAU/LTA-0 mainly had a cubic shape which was a LTA-type structure.It contained a small amount of faujasite crystals which was a FAU-type structure.However,the particle size of the synthetic zeolite was not uniform,and the average size was about 2 μm.Moreover,the crystal twinning and agglomerate phenomena were serious.This is because the reaction of lithium slag with NaOH solution belongs to a heterogeneous solid-liquid reaction system.The crystal nucleation and growth of FAU/LTA-0 is limited by particle size of lithium slag which is large(up to 20 μm).It is easy to prevent active silicon and aluminum from transporting to crystal nucleus,so that competitive growth of nucleus leads to twins and aggregation.
Compared with FAU/LTA-0,the number of cubic crystals of FAU/LTA-1b synthesized with mother liquid reduced significantly,and a certain amount of octahedral crystals increased.The twin structure became weak and the particles had a good dispersity at the same time.What was more,the number of crystals with small size rose to some extent.These suggest that there is a certain amount of primary structural units of zeolite in mother liquid,providing a crystal nucleus for synthesis with mother liquid,so that it is prone to the secondary nucleation.Competition of crystal growth reduces,and product has a good dispersity with small particles.
3.2.3.Thermostability of zeolites FAU/LTA with utilization of mother liquid
TG-DTA Thermogravimetric(TG)curves of as-synthesized zeolites(Fig.3)shows that both FAU/LTA-0 and FAU/LTA-1b existed mass losses before 250°C which were mainly arisen from water desorption.Total mass loss of FAU/LTA-0 was about 20.27%,while total mass loss of FAU/LTA-1b was about 22.05%,higher than that of FAU/LTA-0.This is because FAU-type contains only 18%in FAU/LTA-0,and it occupies as high as 50%in FAU/LTA-1b.Obviously,the ability of FAU-type to water adsorption is stronger than LTA-type,leading to more distinct mass loss of FAU/LTA-1b.
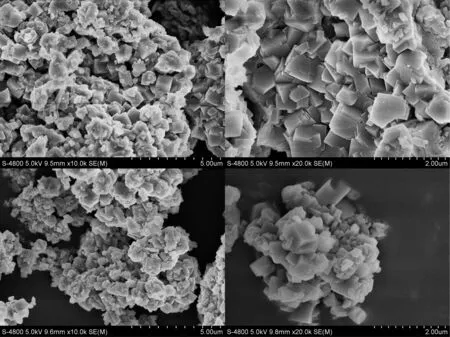
Fig.2.SEM morphology of zeolites FAU/LTA-0 and FAU/LTA-1b.

Fig.3.TG-DTA curves of zeolites FAU/LTA-0 and FAU/LTA-1b.
Correspondingly,differential thermal analysis(DTA)curves(Fig.3)show that both FAU/LTA-0 and FAU/LTA-1b had obvious endothermic peaks center at about 162°C which are attributed to removal of the adsorbed water in the holes of zeolites.There had no lattice damage when temperature continued to rise to 800°C,suggesting that both FAU/LTA-0 and FAU/LTA-1b synthesized with mother liquid have good thermostability.
3.3.Effect of dosages of mother liquid on synthesis of zeolites FAU/LTA
Different dosages of mother liquor have influence on nucleation and crystallization of zeolites because there are some of crystal nucleus and primary structure units.XRD patterns of FAU/LTA-1b-200,FAU/LTA-1b-150 and FAU/LTA-1b-100 are determined as shown in Fig.4,and phase contents of zeolites are listed in Table 4.
For FAU/LTA-1b-x(x=100,150,200),it could be found that with the decrease of volume of mother liquid,the content of P-type reduced from 4%to 1%,and the content of LTA increased.Supposedly,the more the dosage of mother liquid is added,the more the number of four membered rings which are the primary structure units of zeolite NaP exists,When mother liquid is added in low level,the four-membered rings can act as templates of zeolite LTA instead of NaP.So the content of LTA-type in zeolite FAU/LTA-1b-200 can reach about 67.3%.It is appropriate to synthesis zeolite FAU/LTA by using mother liquid with 100 or 150 ml per 50 g lithium slag,that is,the dosage ratio of mother liquid to lithium slag is 2 or 3 ml·g-1.

Fig.4.XRD patterns of zeolites FAU/LTA synthesized with different dosages of mother liquid.

Table 4 Contents of crystalline phase of zeolites FAU/LTA with different dosages of mother liquid
3.4.Cation exchange capacities of zeolites FAU/LTA with utilization of mother liquid
The CECs of FAU/LTA-0,FAU/LTA-1a,FAU/LTA-1b and commercial zeolite 4A for calcium and magnesium are shown in Table 5.The CEC of FAU/LTA-0 for calcium was 324 mg CaCO3·g-1,meeting the requirements of grade A of zeolite 4Afor detergent builder(310 mg CaCO3·g-1or higher).The CEC of FAU/LTA-0 for magnesium was 177 mg MgCO3·g-1,better than that of zeolite 4A.Compared with FAU/LTA-0,the CEC of FAU/LTA-1b for calcium fell slightly,dropping to 312 mg CaCO3·g-1because of less proportion of LTA-type and more proportion of NaP;the CEC for magnesium was 175 mg MgCO3·g-1,remaining unchanged.The CECs of FAU/LTA-1a for calcium and magnesium were 251 mg·(g CaCO3)-1and 140 mg·(g CaCO3)-1,respectively,which was due to the formation of SOD.Therefore,co-crystalline zeolites FAU/LTA with good performance can be successfully synthesized by adding aluminum source after 2 h of reaction.By this time,lithium slag is dissolved into active silicon and aluminum.

Table 5 CECs of zeolites FAU/LTA with different adding ways of aluminum compared with zeolite 4A
The CECs of FAU/LTA-1b-x(x=100,150,200)and commercial zeolite 4A for calcium and magnesium are shown in Table 6.Because of the decrease of P-type with decreasing dosage of mother liquid,the CEC for calcium of zeolite synthesized with 150 or 100 ml mother liquid improved.When the dosage of mother liquid reached 150 ml,the CECs for both calcium and magnesium came to a head.On the one hand,the less impurity phase of P-type promotes the CEC of FAU/LTA-1b-150 for calcium.On the other hand,the low-silica FAU-type which is still occupied for 45%in FAU/LTA-1b-150 with large pore size makes it easy to hydrated magnesium ions to enter,and thus leads to improve magnesium ion exchange capacity.
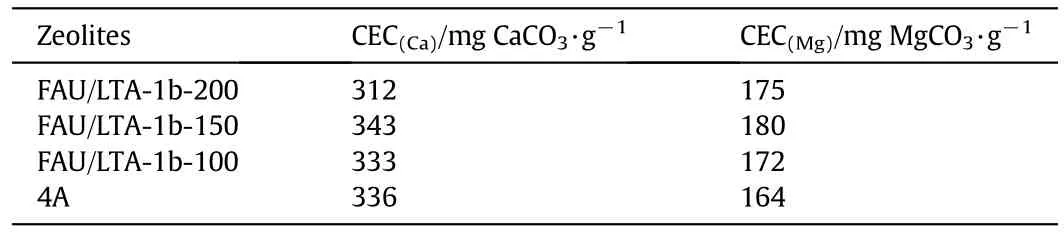
Table 6 CECs of zeolites FAU/LTAwith different dosages of mother liquid compared with zeolite 4A
4.Conclusions
(1)Co-crystalline zeolite FAU/LTA-1b can be synthesized successfully by adding aluminum source after 2 h of reaction with lithium slag as a start material.FAU/LTA-1b had typical diffraction peaks of FAU and LTA,which was similar to FAU/LTA-0 synthesized without mother liquid.The main components of mother liquid derived from FAU/LTA-0 and FAU/LTA-1b kept the same basically.So the motherliquid can be recycled,and the utilization rate of mother can reach 48%.
(2)The main crystal phase of FAU/LTA-0 was LTA-type with cubic structure,and particle size was about 2 μm with twin crystal.However,the mass fraction of LTA-type in FAU/LTA-1b was only 50%,and particle size became small with less crystal,which was beneficial to promote CECs for calcium and magnesium.Zeolite frameworks of both FAU/LTA-0 and FAU/LTA-1b were not destroyed until 800°C,manifesting good thermostability.
(3)With deceasing dosage of mother liquid,P-type in co-crystalline zeolite with utilization of mother liquid reduced obviously,and LTA type increased,so that CECs of co-crystalline zeolites for calcium and magnesium improved.The CECs ofFAU/LTA-1b-150 for calcium and magnesium can reach 343 mg CaCO3·g-1and 180 mg MgCO3·g-1respectively when the dosage ratio of mother liquid to lithium slag is 3 ml·g-1,which are higher than commercial zeolite 4A and satisfies the requirement of detergent builder.Co-crystalline zeolite for detergent builder synthesized with utilization of mother liquid and using lithium slag as a start material has a good application prospect.
Nomenclature
CEC(Ca)calcium cation exchange capacity,mg CaCO3·g-1
CEC(Mg)magnesium cation exchange capacity,mg MgCO3·g-1
c molar concentration,kmol·m-3
Iithe strongest peak intensity of XRD of phase i,cps
i crystalline phase of zeolite FAU/LTA
RIRiRIR value of phase i,1
T temperature,°C
Wimass fraction of phase i,%
ρ mass concentration,kg·m-3
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Simulation and analysis of multi-stage centrifugal fractional extraction process of 4-nitrobenzene glycine enantiomers☆
