Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
Yanjie Wang,Qing Liao,Yan Chen,Libin Zhuang,Haihui Wang*
School of Chemistry&Chemical Engineering,South China University of Technology,Guangzhou 510640,China
Keywords:Perovskite Membranes Permeation BaFeO3-δ Oxygen separation
ABSTRACT Cobalt-free oxides Gd x Ba1-x FeO3-δ(0.01≤x≤0.1)were achieved by a solid state reaction method.Itis found that Gd x Ba1-x FeO3-δ(0.025≤x≤0.1)exhibits the cubic perovskite structure.Among Gd x Ba1-x FeO3-δ(0.025≤x≤0.1),the Gd0.025Ba0.975FeO3-δ(GBF2.5)membrane shows the outstanding phase structure stability and the highest oxygen permeation,which can reach 1.44 ml·cm-2·min-1 at950 °C underair/He oxygen partial pressure gradient.The GBF2.5 membrane was successfully operated for more than 100 h at800°Cand the oxygen permeation flux through the membrane is 0.62 ml·cm-2·min-1.After 100 h oxygen permeation experiment at 800°C,X-ray diffraction(XRD)and energy dispersive X-ray spectrometer(EDXS)demonstrate that the GBF2.5 exhibits phase structure stability even at intermediate temperature.
1.Introduction
Perovskite-type(ABO3)oxides have attracted considerable attention due to their wide range of applications in catalysis,superconductors,gas sensors,and batteries[1-4].In addition to the above applications,oxygen transporting membranes(OTMs)on the basis of mixed electronic and ionic conductors are regarded as a new technology for oxygen production[5-10].Theoretically,the defect-free membrane has 100%oxygen selectivity and could potentially reduce the cost and energy of oxygen production[11-13].For industrial applications,the membrane is required possessing both high oxygen permeation and excellent stability under harsh conditions,such as reducing atmospheres.Since Teraoka et al.[14]reported that SrCo1-xFexO3-δshows high oxygen permeation,lots of cobalt-based perovskite-type membranes have been developed.However,the easy reduction of cobalt ions leads to the poor stability of cobalt-based perovskite-type membranes[15],especially under reducing atmospheres or at intermediate temperatures.Therefore,it is necessary to develop the cobalt-free perovskite-type membranes with high oxygen permeation and excellent stability.So far,many cobalt-free perovskite-type membranes have been reported,such as BaxSr1-xFey(Al,Cu,Zn)1-yO3-δ(0≤x≤1,0≤y≤1)[7,16-18],BaFe1-xCexO3-δ[19,20],LaxBa1-xFeO3-δ[21,22],BaFe1-yZryO3-δ[23]and SrFe0.7Al0.3O3-δ[24].Usually,the cobalt-free membranes are based on BaFeO3-δoxides as it possesses a high oxygen vacancy concentration[25,26].However,for BaFeO3-δ,the ionic radius of Ba2+is too large to stabilize the cubic perovskite structure,which results in a low oxygen permeation.An effective way to stabilize the cubic perovskite structure of BaFeO3-δis the partial substitution on the A or B sites,which is good for oxygen permeation and structure stability.
Recently,we have also reported a novel cobalt-free BaFeO3-δ-based material Gd0.33Ba0.67FeO3-δ[27].Particularly,the Gd0.33Ba0.67FeO3-δshows excellent phase structure stability under syngas production conditions.Here,we will further investigate the influence of the content of Gd in the GdxBa1-xFeO3-δon the crystal structure,structure stability and oxygen permeation.Then the membrane with high oxygen permeability and excellent structure stability will be studied in detail.
2.Experimental
All the GdxBa1-xFeO3-δ(0.01≤x≤0.1)powders were synthesized by a solid state reaction method.The calculated quantities of Gd2O3,BaCO3and Fe2O3(all reagents with A.R.purity)in their appropriate stoichiometric ratios were mixed in an agate mortar and ball-milled for 10 h in acetone.Then,the compounds were calcined at 1050°C for 10 h.The calcined powders were pressed into green membranes under a pressure of 20 MPa and then sintered at 1200°C for 10 h in air.All the membranes used for the oxygen permeation tests have relative density higher than 95%.
The crystal structures of the GdxBa1-xFeO3-δ(0.01≤x≤0.1)were characterized on X-ray diffraction(XRD,Bruker-D8 ADVANCE,Cu Kαradiation)in the 2θ range of 20°-80°with intervals of 0.02°.Lattice parameter refinement was performed by Rietveld method with an error of 0.0001 nm.Scanning electron microscopy(SEM,HITACHI S-3700N)with an energy dispersive X-ray spectrometer(EDXS)unit was used to analyze the microstructure and the elemental compositions of the membrane.
Oxygen temperature-programmed desorption(O2-TPD)was performed by a Micromeritics AutoChem 2920™.Before O2desorption,the GdxBa1-xFeO3-δ(0.025≤x≤0.1)material(about 1.0 g)was pretreated in flowing high purity O2(30 ml·min-1)at 850 °C for 2 h.Then,it was cooled to 50 °C at a speed of 10 °C·min-1,and O2-TPD was operated from50 °C to 900 °C ata speed of10 °C·min-1.The carrier gas was high purity He(30 ml·min-1).A thermal conductivity detector was used to detect the amount of desorbed oxygen.To further research the reversibility of oxygen adsorption and desorption,the sample was pretreated again under flowing O2at 850°C after O2-TPD,and then the next O2-TPD run was operated again.The sample was characterized by XRD after the last run.
To investigate the phase structure stability of these GdxBa1-xFeO3-δ(0.025≤x≤0.1)materials under a more reducing atmosphere,10%H2-Ar(30 ml·min-1)was used to treat these materials(about 0.8 g)at 900°C for 1 h.Then these materials were characterized by XRD.
The oxygen permeation tests were conducted on a homemade high temperature gas permeation system,as described elsewhere[28].Both sides of the sintered GdxBa1-xFeO3-δ(0.025≤x≤0.1)membranes were polished with 2000 mesh SiC paper.These polished membranes were sealed on a dense alumina tube with a commercialceramic sealant(Hubei,China).Temperature was controlled by a temperature controller(Model AI-708P,Xiamen,China)within±1 K of the set points through a type-K thermocouple.Dried air was fed to the feed side,while high-purity helium was used as the sweep gas on the permeate side of the membrane.All gas flows were controlled by gas mass flow controllers(MFC,model D08-4F/ZM,Beijing,China).The effluents were analyzed by an on-line gas chromatograph(GC,Agilent Technologies,7890A).Due to the minor gas leakage through imperfect sealing,N2was also detected in the effluents by the gas chromatograph.Fluxes of leaked N2and O2are related by:=× 0.79:0.21=4.02.The O2flux (JO2)was then calculated as follows:

where CO2and CN2are the concentration of O2and N2detected by GC,respectively.F is the flow rate of the effluents and S is the effective surface permeation areas.
3.Results and Discussion
3.1.Crystal structures
Fig.1 displays the room-temperature XRD patterns of GdxBa1-xFeO3-δ(0.01≤x≤0.1)samples sintered at 1200°C for 10 h.As shown in Fig.1,cubic perovskite structure could be effectively stabilized by partly substituting Ba2+(0.161 nm)with Gd3+(0.0938 nm)in the substitution range of x=0.025-0.1.However,for Gd0.01Ba0.99FeO3-δ(GBF1),tetragonal and triclinic phase can be detected,indicating that the smaller Gd content(x=0.01)fails to stabilize the cubic structure.Kida et al.[29]found that La0.025Ba0.975FeO3-δis a triclinic phase together with the cubic phase instead of the cubic structure.In contrast,Gd0.025Ba0.975FeO3-δ(GBF2.5)is a cubic perovskite phase.The reason is that the smaller ionic radius(La3+:0.103 nm,Gd3+:0.0938 nm)is more beneficial for stabilizing the perovskite phase.
To evaluate the impact of the incorporated Gd to the structure in detail,the united cell parameters were obtained by using the Rietveld method and the main peaks of GdxBa1-xFeO3-δ(0.025≤x≤0.1)were amplified.As shown in Fig.1(b),the peak shifts gradually to the higher angles with increasing the Gd amount,which agrees well with the calculated value in Table 1.The lattice parameter decreases from 0.40183 nm(x=0.025)to 0.40068 nm(x=0.1).These results further verify that the Gd is doped into the A-sites.

Table 1 Room-temperature unit cell parameters of Gd x Ba1-x FeO3-δ(0.025≤x≤0.1)

Fig.2.O2-TPD profiles of Gd x Ba1-x FeO3-δ(0.025≤x≤0.1)oxides.
The O2desorption properties ofoxide is not only associated with the oxygen permeation,but also an indirect evidence for the structure stability[30,31].Fig.2 shows the O2-TPD profiles of GdxBa1-xFeO3-δ(0.025≤x≤0.1).As shown in Fig.2,only one desorption peak is observed for all samples from 300 °C to 700 °C,which is attributed to the reduction of Fe4+to Fe3+as both of them exist in the samples[7].For the cobalt-based perovskite oxides,there are often two desorption peaks.Generally,the reason is that the desorption peak at the lower temperature zone is bound up with the reduction of Co4+to Co3+,and that at the higher temperature zone is bound up with the reduction of Co3+(0.063 nm)to Co2+(0.074 nm)[32],leading to a bigger expansion of the lattice.In contrast,there is no desorption peak at the higher temperature zone related to the reduction of Fe3+to Fe2+in GdxBa1-xFeO3-δ(0.025≤x≤0.1),which demonstrates that these materials are relatively stable.In addition,the amount of the O2desorption decreases with the increasing of the doping amount of Gd.Higher O2desorption indicates higher oxygen permeation as more oxygen vacancy forms[33].
For the oxygen permeation process,oxygen continuously transports from the high oxygen pressure side to the low oxygen pressure side.And the unit cell is constantly reduced and oxidized.Thus,the oxygen permeable materials should possess excellent phase reversibility.The multi-run O2-TPD is an effective way to examine the phase reversibility of perovskite oxides.GBF2.5 sample was chosen because it has the highest O2desorption amount.As can be seen in Fig.3,the four O2-TPD profiles of the GBF2.5 oxide possesses the same curve,revealing that it has excellent phase reversibility under the repeated O2adsorption and desorption.After O2-TPD,the sample was analyzed by XRD.As displayed in Fig.4,the GBF2.5 sample after O2-TPD test still keeps the perovskite structure,which demonstrates that the GBF2.5 shows outstanding phase structure reversibility.

Fig.3.Multirun O2-TPD profiles of the GBF2.5.

Fig.4.XRD patterns of the GBF2.5 samples,(a)fresh powder;(b)after O2-TPD test;(c)spent membrane after long term operation at 800°C.
It is important to evaluate the ability of reduction resistance when applying the membrane to partial oxidation of methane(POM)reaction.Fig.5 shows the XRD patterns of GdxBa1-xFeO3-δ(0.025≤x≤0.1)after reduction for 1 h at 900°C and GBF2.5 before reduction.As can be seen in Fig.5,these GdxBa1-xFeO3-δ(0.025≤x≤0.1)oxides mainly keep the perovskite structures and no Fe is detected for GBF2.5.

Fig.5.XRD patterns of the Gd x Ba1-x FeO3-δ(0.025≤x≤0.1)after reduction for 1 h at 900 °C and the GBF2.5 before reduction.(▼)Fe.
However,for GBF5,GBF7.5 and GBF10,ferric ion is partly deoxidized to Fe and the amount of Fe increases with the enhancing amount of Gddoping.This result demonstrates that the GBF2.5 has excellent phase structure stability under a more reducing atmosphere.Comparing XRD patterns of GBF2.5 before and after reduction,we could find that the XRD peaks of GBF2.5 after reduction shiftto lower angles,indicating that the lattice parameters of the cubic phase enlarge.The reason is that the radius of the Fe ion expands when the Fe ion is reduced from high valence state to low valence state.
3.2.Oxygen permeation measurements
Fig.6 shows the oxygen fluxes through the GdxBa1-xFeO3-δ(0.025≤x≤0.1)membranes.It can be seen in Fig.6(a)that the Arrhenius activation energy(Ea)decreases from 75.5 kJ·mol-1(GBF2.5)to 59.8 kJ·mol-1(GBF10).Generally,there are two controlling steps about the oxygen permeation process,which are surface exchange reaction and bulk diffusion process.Usually,the limiting step is the bulk diffusion process controlled under the higher temperature zone.Thus,the increasing doping Gd could reduce the activation energy of bulk diffusion.Fig.6(b)displays that the oxygen flux through the GdxBa1-xFeO3-δ(0.025≤x≤0.1)membrane increases with decreasing the doping amount of Gd.It is consistent with the O2-TPD result.And the GBF2.5 shows the highest oxygen permeation with an oxygen flux of 1.44 ml·cm-2·min-1at 950 °C under air/He oxygen partial pressure gradient.As mentioned above,the oxygen vacancy concentration and the lattice parameter increase with decreasing the Gd-doping,which contributes to the higher oxygen permeability.
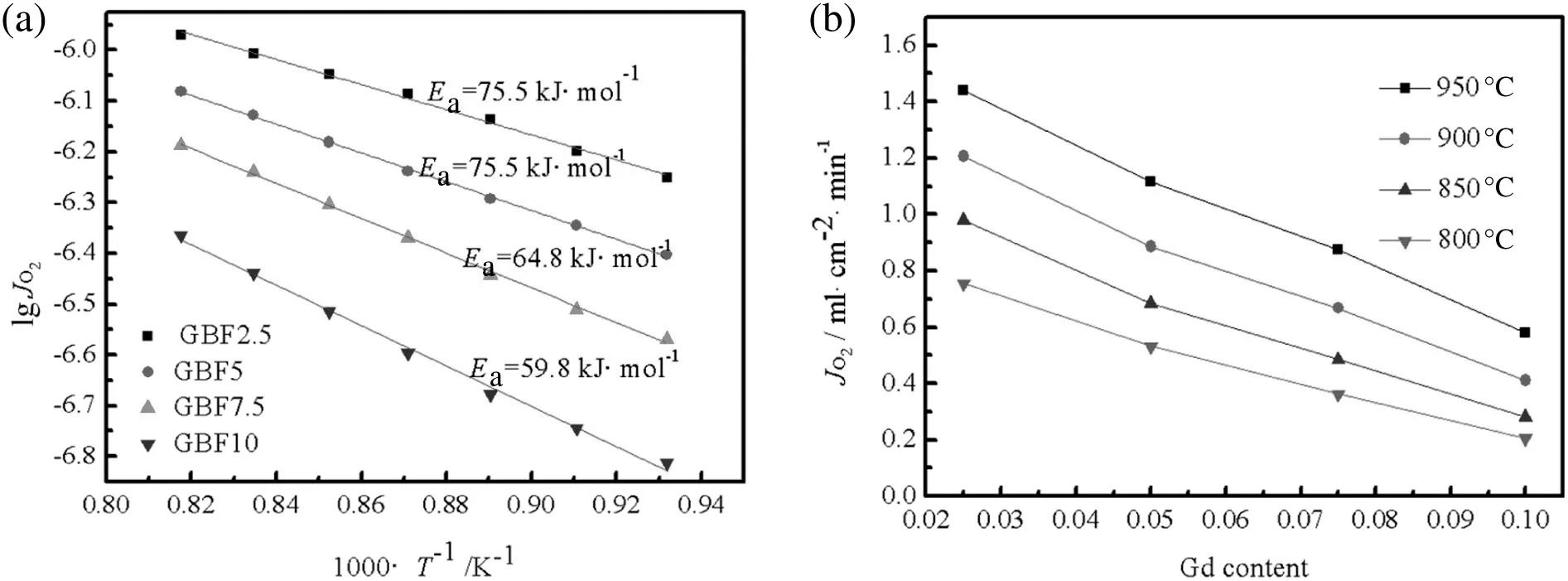
Fig.6.Oxygen permeation fluxes:(a)temperature dependence for Gd x Ba1-x FeO3-δ(0.025≤x≤0.1)membranes;(b)for membranes with different Gd amounts at different temperatures.L=0.5 mm,F air=150 ml·min-1,F He=60 ml·min-1.

Fig.7.Long term oxygen permeation test,oxygen permeation flux of GBF2.5 membranes at 800 °C and 950 °C as a function of time.L=0.5 mm,F air=150 ml·min-1,F He=60 ml·min-1.
Fig.7 shows that the membranes could be operated for more than 100 h at 950 °C and 800 °C,respectively.The oxygen permeation flux can reach 1.4 ml·cm-2·min-1and 0.62 ml·cm-2·min-1during the operation.After the long term operation test at 800°C,the microstructure of GBF2.5 membrane was characterized by XRD,SEM and EDXS.As can be seen in Fig.4,the XRD pattern of the GBF2.5 after the permeation test is the same to that of the fresh sample.Fig.8 depicts the SEM and EDXS results of the cross-section views of the GBF2.5 membrane after long term operation test.Obviously,elements disperse evenly and no element diffusion can be detected.Therefore,these results demonstrate that the GBF2.5 material is stable at 800°C.
4.Conclusions
In this study,the GdxBa1-xFeO3-δ(0.01≤x≤0.1)powders were synthesized by a solid state reaction method.It is found that the GdxBa1-xFeO3-δ(0.025≤x≤0.1)exhibits the cubic perovskite structure except for the GBF1.The lattice parameter decreases from 0.40183 nm(x=0.025)to 0.40068 nm(x=0.1)and the Gd is doped into the A-sites.The GBF2.5 sample shows the highest O2desorption amount and the most excellent phase stability under a more reducing atmosphere.The result of multirun O2-TPD demonstrates that the GBF2.5 possesses outstanding structure reversibility and stability.Besides,among GdxBa1-xFeO3-δ(0.025≤x≤0.1),the GBF2.5 membrane shows the highest oxygen permeation,which can reach 1.44 ml·cm-2·min-1at 950 °C under air/He oxygen partial pressure gradient.The oxygen permeation flux through the GBF2.5 membranes is 1.4 ml·cm-2·min-1and 0.62 ml·cm-2·min-1when the membranes were operated for more than 100 h at 950 °C and 800 °C,respectively.After 100 h oxygen permeation experiment at 800°C,XRD and EDXS demonstrate that the GBF2.5 exhibits phase structure stability even at intermediate temperature.To summarize,the GBF2.5 could potentially be used in oxygen separation at intermediate temperature.
Nomenclature
CN2the concentration of N2
CO2the concentration of O2
EaArrhenius activation energy,kJ·mol-1
F flux of gas,ml·min-1
JO2the permeation flux of oxygen,ml·cm-2·min-1
L membrane thickness,mm
S effective membrane area,cm2
T temperature,K
δ oxygen vacancy concentration

Fig.8.(a)SEM and(b,c)EDXS images of the cross-section views of the GBF2.5 membrane after long term operation at 800°C.
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
- Simulation and analysis of multi-stage centrifugal fractional extraction process of 4-nitrobenzene glycine enantiomers☆
