Removal of elemental mercury by modified bamboo carbon☆
Zengqiang Tan *,Guoping Niu Xiaowen Chen
1 Xi'an Thermal Power Research Institute Co.Ltd.,Xi'an 710032,China
2 Qinghai Huanghe Hydropower Development Co.,Ltd(Xining Branch),Xining 810007,China
ABSTRACT The mercury removal performance of modified bamboo charcoal(BC)was investigated with a bench-scale fixed bed reactor.A simple impregnation method was used to modify the BC with ZnCl2 and FeCl3 separately.BET and XPS were used to determine the pore structure and surface chemistry of the sorbents.The role of Fe3+in the removal of elemental mercury by modified sorbents was discussed.The experimental results suggest that the modified BCs have excellent adsorption potential for elemental mercury at a relatively higher temperature,140°C.The BET surface area and average pore size of modified sorbents do not show noticeable priority compared to unmodified BC.XPS spectra indicate that Fe atoms mainly exist in the form of Fe3+for the FeCl3-impregnated BC.Better performance of FeCl3-impregnated BC at different temperatures(20,140 and 180°C)suggests the enhancement of non-chloride functional groups(Fe3+).Inhibition effect of SOx and NOfor Hg0 removal by BC samples is present in the study.
1.Introduction
Coal- fired power plants have become primary anthropogenic emissions of mercury to atmosphere[1].There are three forms of mercury in the coal- fired flue gas:elemental mercury(Hg0),oxidized mercury(Hg2+)and particle bound mercury(HgP).Elemental mercury is of high concern due to its volatility,high toxicity,bioaccumulation in the environment,and neurological health impact[2].The United States Environmental Protection Agency has issued a national regulation for mercury emissions from the coal-burning power plants in 2011.
Many methods have been developed to control Hg0emissions,such as sorbent injection[3],photochemical oxidation[4,5],and pollution control devices[6].The activated carbon[7]is one of the most promising techniques for elemental mercury removal,which has been successfully applied for the control of mercury emissions from incinerators[8].Recently,many investigations have been focused on improving mercury adsorption ability of activated carbon,alternative low-costsorbents and other treatment techniques,including the introduction of various impregnants onto activated carbon surface such as chlorine,iodine,sulfur,bromine[9-12],zeolites[13],and calcium-based sorbents[14].Some cheaper materials have been used,with pine and oak woods,olive seed,and tire wastes[15]as the precursors for production of carbon sorbents.These trials have provided useful knowledge about alternative cheap sorbents and sorbent processing methods.
Due to energy shortage and serious environmental pollution with the use of fossil fuels,renewable energy has become a worldwide focus.Bamboo has an outstanding potential due to its fast growth.In China,there are about 40 genera,500 species of bamboo,with a forest area of more than 4 million ha,and the total species,growing stock and harvesting amount of bamboo are the most in the Asia-Pacific region[16].Bamboo and its residues can be transformed into bamboo charcoal(BC)in a high temperature and low oxygen atmosphere,which is a technology broadly used in China.BC is an environmentally friendly,low-cost and renewable bioresource.
In this study,a simple and reliable impregnation method is used to modify BC materials with ZnCl2and FeCl3,which are relatively cheap materials and easy to treat with water.The relation of chemical properties of sorbents to Hg0removal process,especially for the role of chlorine and Fe3+,is discussed in detail.
2.Experimental
2.1.Sample preparation
All BC materials used in this study were commercial products from Zhejiang,China.The BC materials were pulverized and sieved in a 40-80 Chinese mesh(0.45-0.20 mm),washed with deionized water,stirred at 80 °C for 3 h to remove dust,and dried at 110 °C in an oven for 24 h.The pretreated BC materials were impregnated with the prepared chemical reagent solution for 12 h.The ratio of solution to BC was 0.8 ml·g-1.The impregnated BC materials were dried at 90 °C in an oven and then cooled to room temperature for use.The chemical reagent solutions used in this study were as follows:ZnCl2solution with a molar concentration of 0.21 mol·L-1(BC1)and 0.42 mol·L-1(BC2),FeCl3solution with a molar concentration of 0.14 mol·L-1(BC3)and 0.28 mol·L-1(BC4).
2.2.Sample characterization
The Brunauer-Emmett-Teller(BET)surface area and pore size distribution were determined using a Micromeritics ASAP 2020 nitrogen adsorption apparatus.The surface binding and elemental speciation were analyzed by X-ray photoelectron spectroscopy(XPS)using VG Multilab 2000 X-ray photoelectron spectrometer,with the surface excitation at1253 eVby an Mg X-ray source.The survey and high-resolution spectra of Fe2p,Zn2p,Cl2pand O1swere collected,calibrated with the binding energy of C1sat 284.6 eV.For comparison,original BC was used in all analyses.
2.3.Experimental apparatus and methods
The schema of experiments is shown in Fig.1.The mercury permeation device(VICI Metronics,United States)was placed in a sealed U shaped quartz tube immersed in a temperature-controlled water bath.A regulated volume of nitrogen gas was introduced into the inlet of the U tube as carrier gas.Mercury concentration was 50 μg·m-3.Other components in flue gases including oxygen(O2,6 vol%),nitrogen(N2,balance),SO2(2800 mg·m-3),NO(130 mg·m-3)and carbon dioxide(CO2,12 vol%)were supplied from gas cylinders.Minor gases including SO2and NO were introduced into the main flow individually or in combination.These concentrations were monitored by a Kane KM940 gas analyzer(British manufacture).The simulated flue gas after being mixed fully(by gas mixer)passed through the blank reactor and then the RA-915M mercury analyzer(Lumex)was used to determine the baseline of Hg0concentration.The experimental error of the system was±6%error.The total flux of gas was 1.5 L·min-1and the weight of sorbents was 0.75 g in each test.

Fig.1.Schematic of the experimental setup.1.Reducing valve;2.mercury vapor generator;3.gas mixer;4. flue gas analyzer;5.adsorption bed reactor;6.mercury analyzer.
The performance of sorbent for elemental mercury removal is evaluated by the removal efficiency η

where Cinletand Coutletrepresent the elemental mercury concentration(μg·m-3)at inlet and outlet,respectively.
3.Results and Discussion
3.1.BET analysis of sorbents
A summary of the BET testis given in Table 1.The BET surface area of sorbents follows a descending order:BC1>BC>BC2>BC3>BC4.This is the same order for their total pore volumes,indicating a positive relation between the total pore volume and BET surface area.The average pore size of modified and unmodified BC sorbents does not show noticeable difference,close to micropore range(2 nm).
The effect of impregnant on the porous structure of sorbents depends on the concentration and species of impregnant.Table 1 shows that a relatively low concentration of ZnCl2(BC1)has a certain beneficial activation effect for BC,presenting higher BET surface area and total pore volume than the original BC.However,there is a negative effect with relatively high ZnCl2and FeCl3concentrations:noticeable decrease in surface areas and pore volumes,possibly resulted from the destruction of thin pore walls and blockage of pore entrances.
3.2.XPS analysis of sorbents
High-resolution scans for selected elements show specific chemistry of element and content of surface functional groups.The complicated envelope revealed by high-resolution XPS spectra(of C1sexcitation)indicates several carbon species atthe carbon surface.Surface information on synthesized samples is analyzed by XPS and XPS spectra over the spectral regions of Fe2p,Zn2p,Cl2pand C1sare shown in Fig.2.
The C1sspectra of BC2 and BC4 in Fig.2(a,b)show a characteristic peak value of(284.5±0.5)eV,which is close to that for graphite[(284.3-284.6)eV][17],suggesting that the C atoms in ZnCl2-impregnated and FeCl3-impregnated BCs basically maintain their states in graphite.However,the bordered peak width indicates that the C atoms are affected a little in their electronic states by intercalating atoms.The peak separation of C1sindicates the existence of another C1speak at 286.1 eV,which is the C1scharacteristic peak in C-Cl bond[17],proving the influence of Clatom on C atom.In other words,certain bonding action exists between Cl and C atoms.
The binding energy of Fe3+state is 711.5 eV[17].In iron 2p and zinc 2p region,the binding energy of iron in FeCl3-solution-impregnated BC is found to be 711.6 eV[Fig.2(c)],indicating that Fe atom mainly exists in the form of FeCl3.XPS spectra reveal a Zn2p3/2binding energy of 1022.9 eV[Fig.2(d)]in ZnCl2-doped BC samples,which corresponds to Zn(II)state,possibly for ZnCl2.
The information on the nature of Cl bonding is available from the analysis of Cl2pregion.Fig.2 contains detailed scans at the energies corresponding to the Cl2pspectral region.Multiple peaks corresponding to Cl2pbinding energies are observed,and the curve fitting the Cl2pregion of BC2 and BC4 shows that some of the chlorine is at(198.7±0.2)eV and another Cl2pcharacteristic peak is at(200.1±0.2)eV.The peak at binding energy of 198.8 eV is ionic chlorine(Cl-),suggesting that the compound intercalated into graphite should contain Cl-[17].The observed binding energy ofCl2pin FeCl3is 198.8 eV[17],while the binding energy of Cl2pin ZnCl2is 198.8 eV.The strong bonding between Cl and Fe,as indicated by the existence of sharp peak in Fig.2(c),shows that FeCl3in graphite layers maintains its binding energy for crystal state.The Cl2pcharacteristic peak at 200.1 eV is possibly contributed to polymerized Cl,that is C-C-Cl-,suggesting that chlorine of BC2 and BC4 is covalently bound to carbon[17].
3.3.The performance of sorbents for elemental mercury removal
3.3.1.Effect of reaction time
The efficiency-time curves of the sorbents at 140°C are given in Fig.3.BC3 and BC4 show better performance for elemental mercury removal,while BC1 and BC2 present considerable variation,with the maximum and final removal efficiency of 88%and 92%,respectively.With the elapse of reaction time,the Hg0removal efficiency of BCs remains constant(above 87%)in 5 h.It is important to note conspicuous removal efficiency(above 99.9%)of BC3 and BC4 in the long experimental time.Their mercury removal performance is similar.When the stability of mercury removal at reaction temperature of 140°C is taken into consideration,the result is satisfactory.

Table 1 BET analysis of sorbents
The mercury removal performance of ZnCl2-impregnated BC(BC1,BC2)and FeCl3-impregnated BC(BC3,BC4)are also compared.The C1 content of BC1 and BC3 is the same,and it is similar for BC2 and BC4.The experimental results reveal the important role of non-chloride functional groups Fe3+in the elemental mercury removal process.

Fig.2.XPS spectra of high-resolution scan of C1s,Fe2p,Zn2p,and Cl2p for sorbents.(a)BC2-C1s;(b)BC4-C1s;(c)BC4-Fe2p;(d)BC2-Zn2p;(e)BC2-Cl2p;(f)BC4-Cl2p.
3.3.2.Mercury removal performance of BC,BC1 and BC3
The flue gas temperature of the cold-side electrostatic precipitator is 120-160 °C,so we conduct Hg0removal experiments mainly at 140 °C.The performance of sorbent BC at ambient temperature(20°C)and 180°C are used for comparison.As can be seen from Fig.4,the BC is poor for removal of elemental mercury.Its Hg0uptake at adsorption temperatures of 100,140 and 180 °C is much less than that at 20 °C.This could ascribe to a drop of physical adsorptive capacity at higher temperatures,which is the characteristic of exothermal adsorption process[18].

Fig.3.Efficiency-time curves of sorbents at 140°C.

Fig.4.Efficiency-time curves of BC.
Fig.5 shows that the Hg0removal efficiency of sample BC3 maintains stable and above 98%at adsorption temperatures20 and 140°C.The solid mercury measurement instrument(Hydra II C)was used to test the total mercury content of the sorbents,with the result of 12953.14 ng·g-1.As the temperature increases to 180°C,the Hg0removal efficiency of sample BC3 is still above 90%.Sample BC1 shows relatively poor Hg0removal performance at these temperatures,which is nearly 90%at140°C,but reduces sharply,with 40%at 180°C.
From previous study[19],it is known that mercury reaction with chloride presents an exothermic behavior,so the dynamic adsorption capacity is inversely proportional to the operating temperature.However,in this study,BC3 shows excellent heat-resistant characteristic and its Hg0removal efficiency maintains higher than 99%at operating temperatures from 20 °C to 140 °C.Even at 180 °C,it is approximately 92%.This is important for possible application at high temperatures such as prior to the electrostatic precipitators.
Excellent performance of ZnCl2-impregnated and FeCl3-impregnated BCs at reaction temperatures from 20 °C to 180 °C suggests the enhancement of chemisorption.It can be assumed that Hg0reacts with impregnated Cl on the sorbent surface and the mechanism involves adsorbed Cl on the sorbent surface(such as adsorbed atomic and ionic chlorine).Adsorbed Hg0will react with adsorbed Cl(C-Cl(ad))to form mercurous chloride and final mercuric chloride on the carbon surface.The enhanced performance of elemental mercury removal by FeCl3-impregnated and ZnCl2-impregnated BCs can be explained as follows.
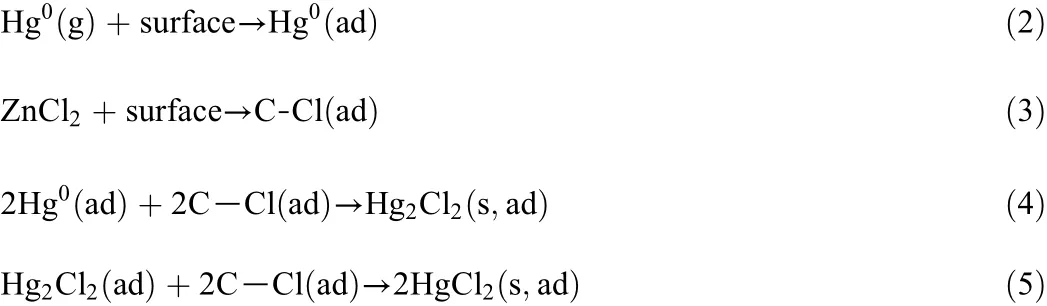
Better performance of FeCl3-impregnated BC at temperatures from 20 °C to 180 °C suggests the enhancement of non-chloride functional groups(Fe3+).

Fig.5.Efficiency-time curves of BC1 and BC3.(a)20 °C;(b)140 °C;(c)180 °C.
It is found that the oxidation of Fe3O4to γ-Fe2O3involves the addition of free oxygen at the surface of crystal and the diffusion of Fe3+through an oxygen framework to the crystal surface[20].The enhanced performance of elemental mercury removal by FeCl3-impregnated BC can be explained as

It can be assumed that Hg0reacts with Fe3+X on the sorbent surface(X is Cl,O).Adsorbed Hg0will react with Fe3+X to form the final oxidized mercury on the carbon surface.
3.4.Discussion on the removal mechanism
There are several factors that potentially affect the removal efficiency of sorbents,such as mercury speciation, flue gas composition,sorbent characteristics,and reaction temperature.The removal of Hg0by sorbents is by physical and chemical interactions.Hence,the most important factor is the sorbent properties,such as size,shape,surface area,pore volume and pore size.Physical adsorption is mainly caused by intermolecularvan der Waals forces and it is characterized by textural parameters,such as surface area,pore volume and pore size.The chemical adsorption relies on the combination of functional groups and chemical adsorption sites,so the surface functional groups strongly affect the mercury adsorption.
The bamboo activated sample with CO2possesses the most developed micropore structure and consequently highest total pore volume as well as BET surface area,but presents poor Hg0adsorptive performance at high temperature,140°C[21].In this study,excellent performance of ZnCl2-impregnated and FeCl3-impregnated BCs compared with BC at reaction temperatures 20,140 and 180°C suggests the enhancement of chemisorptions,indicating that Hg0reacts with atomic chlorine to form mercurous chloride and final mercuric chloride on the carbon surface.
3.5.Effect of SO2 and NO
Flue gas compositions significantly affect mercury adsorption by carbonaceous sorbents.Some authors concluded that SO2favors mercury adsorption particularly in the presence of O2[22],while other studies suggested that SO2or NO hindered mercury adsorption by activated carbons.For coal- fired and incinerator system,the acid gases produced will dominate the sorption chemistry on the surface.It is better to develop anti-SOx/NO-poisoning sorbents to overcome the impact of SOx and NO.As shown in Fig.6,BC2 sample was also active for Hg0removal in the presence of 2800 mg·(m3SO2)-1,better than BC4.Hg removal efficiency of BC2 and BC4 was 90.6%and 85.7%,respectively,lower than that of the samples without SO2.The inhibition effect of SOx for Hg0removal has been reported[23].It is considered that SOx competes with mercury for adsorption sites.The bond between S(VI)species(sulfuric acid and sulfates)and carbon surface has been proven stronger than that between mercury and the surface,so the mercury capture is inhibited[23,24].Surface S(VI)forms by direct adsorption of SO3or oxidation of chemically bound SO2.Therefore,mercury capture could be inhibited by competition with SO3adsorption and/or SO2oxidation.

Fig.6.The effect of SO2 and NO on mercury removal(the efficiency after 1 h at 140°C).
Since SO2can react with FeCl3to form H2SO4and FeCl2,the depletion of Fe3+by SO2may be another reason for the inhibition effect of SO2.The amount of H2O needed in the reaction can be supplied by the un-dehydrated feed gas.The SO2concentration in this study is 2800 mg·m-3,at least one order of magnitude higher than the concentration of Hg0.The following reaction may proceed and consume Fe3+,so Hg removal efficiency is lower in the presence of SO2.

It is reported that the effect of NO on Hg0removal is either promotable or inhibitory.In this study,Hg0removal efficiency of BC2 and BC4 decreases slightly in the presence of NO(130 mg·m-3).Some researchers attribute the inhibition effect of NO to scavenging of OH radicals by NO,hindering the oxidation of Hg0as follows.The competitive adsorption of NO,SO2and Hg0on the active sites of sorbents is possibly another reason for the inhibitory effect.

4.Conclusions
The bamboo charcoal after modification with common chemical reagents is excellent for elemental mercury removal at a relatively high reaction temperature(140°C).The BET surface area and average pore size of modified sorbents do not show noticeable priority than unmodified BC.The XPS spectra indicate that Fe atoms mainly exist in the form of Fe3+for FeCl3-impregnated BC.The mercury removal performance of ZnCl2-impregnated BC(BC1,BC2)and FeCl3-impregnated BC(BC3,BC4)is diverse.BC3 and BC4 present better performance for elemental mercury removal at 140°C(above 99.9%).BC1 and BC2 show considerable variation,with the maximum and final removal efficiency of 88%and 92%,respectively.
The performance of ZnCl2-impregnated and FeCl3-impregnated BCs for elemental mercury removal is excellent compared with BC at reaction temperatures 20,140 and 180°C,suggesting the enhancement of chemisorptions.It can be explained that Hg0reacts with atomic chlorine to form mercurous chloride and the final mercuric chloride on the carbon surface.Better performance of FeCl3-impregnated BC at these temperatures suggests the enhancement of non-chloride functional groups(Fe3+).It can be assumed that Hg0reacts with Fe3+to form the final oxidized mercury on the carbon surface.
The inhibition effect of SOx and NO for Hg0removal by BC2 and BC4 samples is observed.The competitive adsorption of NO,SO2and Hg0on the active sites of sorbents is a possible reason,and the depletion of Fe3+by SO2may be another reason for the inhibition effect of SO2.
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
