Production of biodiesel from palm fatty acid distillate using sulfonated-glucose solid acid catalyst:Characterization and optimization
Ibrahim M.Lokman ,Umer Rashid *,Yun Hin Tau fiq-Yap
1 Catalysis Science and Technology Research Centre,Faculty of Science,Universiti Putra Malaysia,43400 UPM Serdang,Selangor,Malaysia
2 Department of Chemistry,Faculty of Science,Universiti Putra Malaysia,43400 UPM Serdang,Selangor,Malaysia
3 School of Chemistry,Faculty of Applied Sciences,Universiti Teknologi MARA,40450 Shah Alam,Selangor,Malaysia
4 Institute of Advanced Technology,Universiti Putra Malaysia,43400 UPM Serdang,Selangor,Malaysia
Keywords:Palm fatty acid distillate(PFAD)Sulfonated-glucose solid acid catalyst Esterification Optimization PFAD methyl ester
ABSTRACT A palm fatty acid distillate(PFAD)has been used for biodiesel production.An efficient sulfonated-glucose acid catalyst(SGAC)was prepared by sulfonation to catalyze the esterification reaction.The effect of three variables i.e.methanol-to-PFAD molar ratio,catalyst amount and reaction time,on the yield of PFAD esters was studied by the response surface methodology(RSM).The optimum reaction conditions were:12.2:1 methanol-to-PFAD molar ratio,2.9%catalyst concentration and 134 min of time as predicted by the RSM.The reaction under the optimum conditions resulted in 94.5%of the free fatty acid(FFA)conversion with 92.4%of the FAME yield.The properties of the PFAD esters were determined according to biodiesel standards.
1.Introduction
The high cost of biodiesel production has become a major obstacle for commercialization[1].In order to reduce the cost of biodiesel production,cheaper starting materials are necessary to be studied to replace the highly expensive feedstock,such as virgin oil.In the past few years,scientists started to produce biodiesel from other waste oil sources,such as waste cooking oil,waste animal fat,grease oil,and Jatropha cake oil[2,3].Recently,the by-product of the palm oil's refinery or what is known as PFADhas received high recommendation as the potential biodiesel's starting material.Esterification of the PFAD with methanolin the presence of a catalyst will produce fatty acid methylesters(FAMEs)or known as biodiesel[4].Fig.1 shows the general chemical equation of the esterification reaction.

Fig.1.Esterification of fatty acid with methanol in the presence of an acid catalyst.
In 2009,Malaysia and Indonesia produced about 17.5 million and 20.9 million metric tonnes of crude palm oil,respectively[5].During the refining of the crude palm oil,a lower value by-product(PFAD)was generated in the fatty acid stripping and deodorization stages.As a result,almost 700,000 metric tonnes of PFAD were produced in Malaysia in the same year[6].Basically,PFAD is a common material in soap industries,oleo-chemical industries,animal feed industries and food industries[2].However,only a few developed factories use the PFAD as a starting material for biodiesel production.This is due to the FFA content,which normally interferes with and gives difficulties during the production process especially when using a homogeneous base catalyst due to the saponification process[7-11].On the other hand,the acid catalyst showed an excellent process in avoiding the saponification problem.
The heterogeneous solid acid catalysts have shown that they are excellent choices due to their unique properties,such as good stability and reusability;also,they can be separated easily from the product.It has made them very attractive catalysts as compared to homogeneous catalysts[12,13].The common heterogeneous solid acid catalysts such as sulfated zirconia-alumina(SZA),amberlyst-15(sulfonated polystyrenebased resin),tungstated zirconia-alumina(WZA),sulfated tin oxide(STO)and na fion NR50(per fluorinated alkane sulfonic acid resin)have been used as the catalysts in biodiesel production[14,15].Unfortunately,the high cost preparation of these catalysts has become a major obstacle[12,16].To overcome the problem,novel carbon-based heterogeneous solid acid catalysts which are synthesized from organic materials have been introduced.The common carbon-based catalysts are sulfonated activated carbon and biochar[17],sulfonated vegetable oilasphaltand petroleum asphalt[18],sulfonated sugar cane bagasse[19],sulfonated oil-cake waste[20]and sulfonated carbohydrate-derived solid acid catalysts[21].Chen&Fang[22]reported that the amorphous carbon with the-SO3H group on the structure comprised much higher catalytic activity than other solid acid catalysts and can act as an active acid catalyst for both the esterification and transesterification processes.Nevertheless,the heterogeneous carbon-based solid acid catalyst was reported to have a slightly lower catalytic activity than the homogeneous acid catalyst;this is due to the lack of active site density on the heterogeneous catalyst[23].In addition,an obvious limitation is due to the hydrophilic properties of active sites.Normally,esterification of high free fatty acid(FFA)feedstock will produce water molecules as a by-product,due to which,the hydrophilic property of active sites has potential to entrap the water molecules and lead the hydration of-OHgroup[24].This process will destroy the catalyst and reduce the catalytic activity.In this research,the application and catalytic activity of carbohydrate-derived solid acid catalysts were focused on and highlighted.
Although,the glucose-derived solid acid catalyst has been prepared and studied by Lou et al.[21],Zong et al.[25]and Nakajima and Hara[26].However,an intensive study of the particular catalyst to catalyze the esterification of the PFAD for biodiesel production has not been reported yet.In this work,the catalyst was prepared and characterized by infrared spectroscopy(IR),X-ray diffraction(XRD),ammoniatemperature programmed desorption(TPD-NH3),thermal gravimetric analyzer(TGA),BET surface area analysis and the scanning electron microscope(SEM).Meanwhile,the effect of the catalyst amount,methanol molar ratio and reaction time on the rate of the esterification of the PFAD was statistically studied by using the response surface methodology(RSM)technique.The obtained responses from a series of reactions were used to generate the ANOVA table and regression analysis.The properties of the PFAD methyl ester were analyzed according to the ASTM standard method.
2.Experimental
2.1.Materials and chemicals
PFAD was obtained from the Jomalina Oleochemical R&D,Sime Darby Sdn.Bhd.(Klang,Selangor).Commercial D-glucose was purchased from Sigma-Aldrich.The concentrated sulfuric acid(H2SO4),hydrochloric Acid(HCl)and potassium hydroxide(KOH)were obtained from J.T.Baker.Meanwhile,the methanol,ethanol and n-hexane were obtained from Merck chemicalcompany.The reference FAMEs standard such as methyl oleate,methyl palmitate,methyl linoleate,methyl myristate,methyl stearate and methyl heptadecanoate were purchased from Fluka,USA.
2.2.Catalyst preparation
The SGAC catalyst was prepared according to the modified method proposed by Zong et al.[25 23].Brie fly,15 g of D-glucose were heated at 400°C for 12 h in an inert environment to produce incomplete carbonized glucose.A black carbon material was milled to powder form and refluxed in 100 ml of concentrated H2SO4at 160°C for 12 h.The suspension was then diluted and washed with hot distilled water to remove the excess of sulfate ions.The SGAC catalyst was dried before the characterization analysis and catalytic activity studies.
2.3.Characterization
The SGAC catalyst was firstly characterized by infrared spectroscopy(IR,Perkin Elmer 1725 X)to determine the functional groups generated on the polycyclic carbon structure.The amorphous properties of the catalyst carbon structure were determined by powder X-ray diffraction(XRD,Shimadzu XRD6000).Scanning electron microscopy was performed with the SEM(JSM-6400,JEOL).The SEM device was fitted with the Shimadzu energy dispersive X-ray spectrometer(EDX,EDX-720)for the elemental composition analysis.The acid density and distribution were determined by temperature programmed ammonia desorption(TPD-NH3,Thermo Finnigan TPDRO 1100 series).The thermal analysis of the catalyst was performed using thermogravimetric analyzer(TGA,Mettler Toledo 990).N2adsorption-desorption isotherms were obtained from surface area analyzer(Thermo Finnigan Sorptomatic 1990 series).Prior to the measurement,the samples were degassed for 12 h at 150°C.The specific surface area of the samples was determined by using the BET method.
2.4.Esterification of the PFAD
The esterification reaction of the PFAD was carried out by using the conventional reflux technique.In a typical reaction,10 g of the PFAD was mixed with an amount of methanol and specified amount of the catalyst.The mixture was heated,stirred at 600 rpm and maintained at 65°C throughout the reaction process.Finally,the product was separated from the catalyst by centrifugation,followed by the methanol recovery process.The acid value of the feedstock and product was determined by using the classical titration method referred to as the standard method of AOAS Cd 3d-63.The FFA conversion was calculated according to Eq.(1).Meanwhile,the FAME yield of the final product was measured using Shimadzu gas chromatography equipped with a flame ionization detector(GC-FID,GC-14C).The methyl heptadecanoate was used as the internal standard.The methyl oleate,methyl palmitate,methyl linoleate,methyl myristate and methyl stearate were used as the reference standards.The high polar-capillary column BPX 70,SGE Company(length 30 m;ID 0.25 mm;diameter 0.25 μm)was used for the separation of the FAME components.The GC was programmed with the temperature range from 100 to 250 °C at 10 °C min-1.The temperature at the injector port was programmed at 230°C and the detector temperature was 270°C.Hexane was used as the solvent,and helium was used as the carrier gas.The FAME yield was calculated according to Eq.(2).

where AVf and AVp,respectively stand for the acid value of the feedstock and the product.

2.5.Experimental design
The response surface methodology(RSM)with a central composite rotatable design(CCRD)was used to design the experiment to determine the optimum operating conditions.Three identified variables were used to design 20 experiments of the quadratic model.The variables and their ranges were:(A)reaction time(60-180 min),(B)methanol-to-PFAD molar ratio(5:1-15:1)and(C)catalyst loading(1%-3%,by mass).The FFA conversion obtained from the reaction was chosen as the dependent variable for the RSM response.Table 1 depicts the experimental designs and their responses.The 20 designs of the experiment were produced to fit the CCRD model including 8 experiments at the factorial point,6 at the axial point and another 6 replicates at the center point.Each experiment was run in replicate to reduce the experimental error.

Table 1 Experimental design generated by RSM,and responses from each reaction
3.Results and Discussion
3.1.Analysis of PFAD
The chemical properties and characteristics of the PFAD were shown in Table 2.The physical and chemical properties of PFAD were analyzed by AOCS standard methods.The iodine value was used to estimate the total unsaturation in PFAD,the result showed that PFAD's iodine value was 0.548 g·g-1,which closely related to the high amount of acid content.The results from GC-MS analysis,showed that the PFAD feedstock contained saturated fatty acids i.e.palmitic acid(45.7%,by mass),myristic acid(1.9%,by mass),stearic acid(4.3%,by mass)and monosaturated fatty acid i.e.oleic acid(40.2%,by mass)and polyunsaturated linoleic acid(7.9%,by mass).The total FFA content of the PFAD feedstock was 86.3±1.75%(by mass)with an acid value of 172.6±3.53 mg KOH per milligram of the feedstock.The high amount of fatty acid content makes the conversion of PFAD simpler,which can be directly esterified with alcohol to produce methyl ester.In addition,triglycerides that exist in a small amount will be simultaneously transesterified to methyl ester during the reaction process.

Table 2 Physicochemical properties and characteristics of PFAD
3.2.Catalyst characterizations
The unique properties of the amorphous carbon are its ability to be incorporated with the hydrophilic molecules such as-COOH and-OH molecules.Such molecules normally give excess and provide the anchor sites for the-SO3H groups bonding[27].Fig.2(a)showed the IR spectrum of the SGAC catalyst.The presence of two peaks at the wave number of 1162.78 cm-1and a very sharp peak at 1029.27 cm-1were clearly attributed to the O-S-O symmetric stretching and SO3-asymmetric stretching modes of the-SO3H group,respectively.Zong et al.[25]and Nakajima and Hara[26]reported the same trends of the IR absorption of the glucose-derived solid acid catalyst.As reported by Okamura et al.[28],the carbonization of the glucose initiated the cleavage of the-C-O-C-and the dehydration of the water molecules.As a result,the amorphous carbon material with the polycylic aromatic carbon structure was formed.The XRD pattern of the SGAC catalyst is shown in Fig.2(b)with two broad and weak diffraction peaks at2θof10°-30°and 35°-50°,which are respectively assigned for the graphitic C(002)and C(101)planes.Typically,the first peak of the C(002)plane was assigned for the amorphous carbon that consists of disordered polycyclic aromatic carbon sheets.The weak peak of the C(101)plane was normally assigned for the graphitic structure of the carbon[29].
The specific surface area of the SGAC catalyst has been shown in Table 3.The prepared SGAC catalyst exhibited a larger surface area as high as 10.67 m2·g-1as compared to the previous reported literatures by Lou et al.[21],Zong et al.[25]and Okamura et al.[28],which were less than 5 m2·g-1.Theoretically,a larger surface area will provide more sites for anchoring the active groups on the carbon support.Thus,the amount of S contentand the density of the acid sites were improved up to 4.89%(by mass)and 4.23 mmol·g-1,respectively,with the density of the-SO3H groups at 1.88 mmol·g-1.
The TGA was used to analyze the thermal stability of the SGAC catalyst.As presented in Fig.2(c),three stages of mass loss at different temperatures were observed.The first mass loss was detected at around 100°C due to the evaporation of the water content which showed the tendency of the catalyst to absorb moisture in an open environment[26].The SGAC catalystlost5.4%(by mass)of its mass when the temperature increased from 132 to 285°C which was in acceptable agreement with the S content in the-SO3H groups(4.89%,by mass).The following mass loss from 386 to 738°C could be attributed to the continuing pyrolysis of incomplete carbonized glucose which has high oxygen content.The carbonization of SGAC catalyst was continued and undergoes the intermolecular dehydration process when heating exceeded from 400°C.The decomposition corresponds to the pyrolysis of the primary carbide through the deep carbonization activity[30].The acid site distribution and acid density of the-SO3H group were confirmed by the TPD-NH3in Fig.2(d).The graph demonstrated that the desorption of the ammonia occurred at two different events which were around 130-310°C culminating at 190 °C and 400-800 °C culminating at 630 °C,thus showing the presence of two types of Brønsted acid sites;weak Brønsted acid sites at lower temperature and strong Brønsted acid sites at higher temperature.The detailed morphological study of the catalyst was provided by the SEM image in Fig.2(e).The image at the magnification of 100×showed the irregular and the aggregation shape of the sample in micrometer dimensions[27].The image represents the range of the diameter particles of the SGAC catalyst ranging from 50 to 200 μm sizes.
3.3.Regression analysis and diagnostic plots

Fig.2.Characterization of the SGAC catalyst(a)IR spectroscopy,(b)XRD diffractogram,(c)TGA,(d)TPD-NH3 and(e)SEM image.
Regression analysis was used to study the relationship of the empirical or predicted model with the response collected by the data[31].The obtained quadratic model of the response surface of experimental design was analyzed using the ANOVA analysis and second order polynomial equation designed by the RSM to determine the optimum value of the variables[32,33].Table 4 depicts the sequential model sum of the square for this data.The quadratic model was suggested as the best model for this experimental design based on the response from the actual experimental data.Meanwhile,the model had to be of an insignificant lack of fits to ensure that the model was good and the error was lowered[33,34].The response surfaces in terms of 3D graph were obtained from the actual response.The FFA conversion was analyzed based on the response factor and three independent variables.The FFA conversion can be expressed as Eq.(3)in terms of the coded factor.


Table 3 Surface area,S content,density of sulfonic groups and total acid density of sulfonated and unsulfonated glucose-derived solid acid catalyst
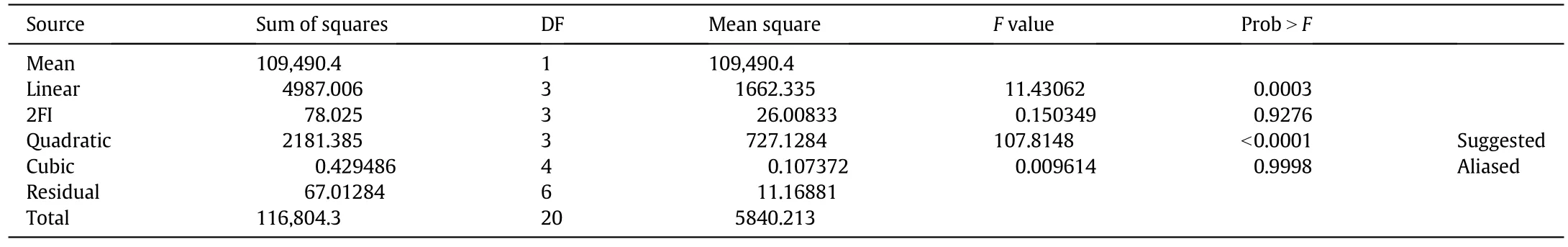
Table 4 Sequential model sum of squares
The ANOVA analysis in Table 5 was used to study the significance and the fitness of the quadratic model and the response towards the interaction of the variables in the reaction.The results showed that the variables had significantly affected the response of the model.The Prob>F-values less than 0.0500 indicate that the model terms were significant[33-35].Referring to Table 5,the variables,which significantly affected the response of the FFA conversion,were A,B,C,A2,B2,C2,and AC.Meanwhile,the F-value indicates the strength of the effect of the variables to the response.High F values indicate that the strength of the variables affecting the response was stronger[32].According to the response of biodiesel production from the PFAD,the interaction reaction time and the catalyst amount(AC)showed a high F-value as a result more interaction between both variables.The insignificant lack of fit model shown by the ANOVA analysis(Table 5)indicates that the model data was fit[35,36].Hence,this model was satisfactorily fitted to the experimental data and was used to estimate,predict,and analyze the relationship of all the variables involved and the optimum condition that can gave a high FAME yield.
Table 5 showed the precision and accuracy of the response generated by the RSM model from the actual experimental data.The coefficient of R2was 0.991 which showed that the estimated response of the variables was excellent and the model was adequate.Furthermore,the‘Pred.R-squared’of 0.9397 was in a reasonable agreement with the‘Adj.R-squared’of 0.9825.It was proportional to the calculated Adeq.Precision,this was to measure the signal to the ratio of noise.In this study,the ratio was 32.1653,indicating an adequate signal.Meanwhile,the result also shows that the co-efficiency of the variation(C.V.=3.51%)was lower,thus it specifies that the high degree of precision and the experimental value were in a good range[36].
Fig.3 showed the diagnostic plots of the predicted versus the actual data and the residual versus the predicted data,respectively.It seems that,the predicted values by the RSM were close to the response of the actual experiment;thus,the errors were smaller and distributed well[35,36].It was confirmed by the adjusted R2value,which was 0.9825,indicating the model with a 98.25%of variability(Table 5).Meanwhile,the responses for the plot of the residual versus the predicted conversion were scattered randomly within the limit.It is expected to produce a good estimation and has the ability to evaluate the correlation of the variables involved[36,37].
3.4.Relationship of the variables by 3D-dimensional graphs
Generally,heterogeneousacid catalyst has less activity as compared to the homogeneous catalyst.However,it is favorable for the low quality feed stocks having high FFAs,especially PFAD.The acid-catalyzed pathway for esterification of the PFAD consists of proton exchange processes.The carbonyl carbon of the FFA molecules will be protonated by the protons provided by the SGAC catalyst.The nucleophilic attack of CH3OH on carbonium ion formed tetrahedral intermediate species.Finally,the migration of the protons causes the breaking down of intermediate and allowing the formation of the FAME and reforming the protons[38].The detail mechanism of the SGAC catalytic action for esterification of the PFAD is shown in Fig.4.The esterification reaction rate can be maximized by the use of optimized reaction conditions of catalyst concentration,methanol-to-PFAD molar ratio,reaction temperature and reaction time.
The relationship of the methanol-to-PFAD molar ratio,catalyst loading,reaction temperature and reaction time was shown in 3D graphs in Fig.5.The correlation of the graphs showed that the FFA conversion was most significantly influenced by the methanol-to-PFAD molar ratio and the amount of the catalyst.The optimummethanol-to-PFAD molarratio was around 8:1 to 12:1,which showed the higher FFA conversion(Fig.5(a)).The excess of methanol significantly lowered the FFA conversion due to the dilution effect[34].The catalyst amount was very important to increase the rate of the conversion.The effect of the reaction time and catalyst amount towards the FFA conversion was studied and it was found to be significant as shown in Fig.5(b)and(c).It can be observed that the conversion of the PFAD increased significantly whilstincreasing the amount of the catalyst.As a result,2.9%(by mass)of the SGAC catalyst was enough to produce over 90%of the FAME yield.

Table 5 ANOVA analysis of response surface quadratic model
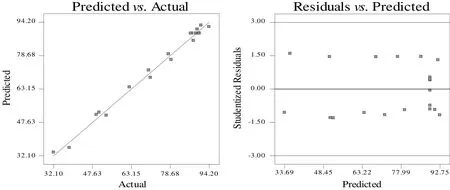
Fig.3.Diagnostic plots.
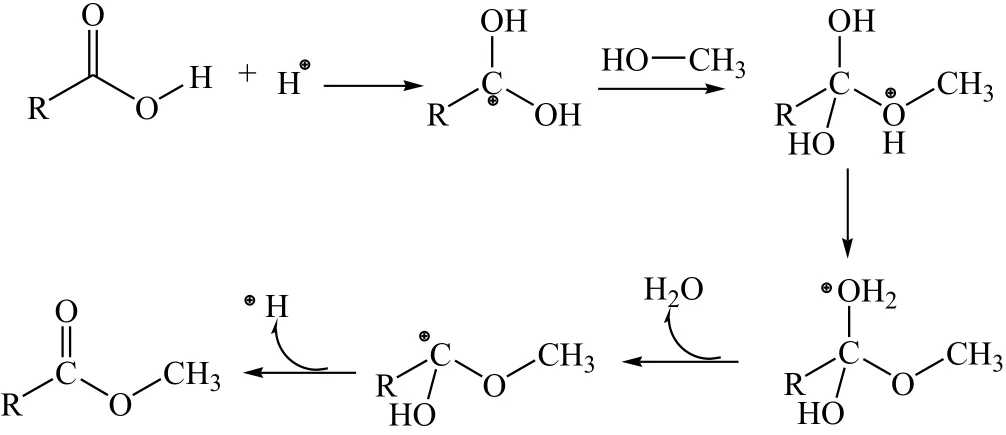
Fig.4.Mechanism acid-catalyzed esterification of the PFAD by SGAC catalyst.

Fig.5.3D-response surface plot(a)relationship of methanol and reaction time,(b)relationship of methanol and catalyst loading,and(c)relationship of catalyst amount and reaction time.
3.5.Biodiesel yield at the predicted optimum condition
The optimization of the biodiesel yield was performed by the numerical optimization method included in the RSM software.It has been used to seek an optimum condition for all the variables at which the maximum conversion was achieved[33,34].The RSM predicted that the FFA conversion would achieve 98.8%of the FFA conversion at the reaction temperature of 65°C within 134 min with a 12.2:1 methanol-to-PFAD molar ratio supported by a 2.9%(by mass)of the catalyst.As compared to the actual reaction using the predicted optimum condition from the RSM,it gave the conversion as(94.5±1.4)%.Meanwhile,the GC analysis revealed that(92.4±1.7)%of FAME yield had been produced with the same reaction condition.It revealed that the actual the FFA conversion and FAME yield were slightly less than the RSM's predicted data.Boey et al.[32]reported that(88.8±0.7)%methyl esters conversion was produced from the PFAD by using ferric-alginate beads as a catalyst at optimum reaction conditions(15:l of methanol-to-PFAD molar ratio,180 min of reaction time at 65°C).Meanwhile,80%of methyl ester content was successfully converted from the PFAD by using the carbon-based solid acid catalyst derived from sugar cane bagasse(reaction condition;20:1 methanol-to-PFAD molar ratio 11.5%(by mass)catalyst for 30 min reaction time at 190°C)as reported by Chin et al.[19].In this work,all reported results were demonstrated that the highest efficiency of the sulfonatedglucose solid acid catalyst for esterification ofPFADas compared to published work.
3.6.Properties of the PFAD methyl ester
The properties of the PFAD methyl ester that had been produced at the optimized condition are summarized in Table 6.They are comparative to the relevant methyl ester standard specifications ASTM D6751 and EN 14214.The basic properties of the products,such as the flash point,moisture content,viscosity and S content,have been confirmed in the range of the ASTM D6751 and EN 14214 standards except for the pour point,which was slightly higher than the limit by 2°C.This was due to the properties of the PFAD,which consisted of a high FFA contents.
4.Conclusions
The optimization of the SGAC catalyst for the esterification of PFAD using the RSM with the CCRD model was successfully studied.The study was conducted using a single step reaction by a conventional reflux system at the 65°C reaction temperature.The RSM approach showed the relationship of three variables that significantly affected the FFA conversion;including the methanol-to-PFAD molar ratio,catalyst loading and the reaction time.The amount of the catalyst was the most significant parameter that affected the rate of the conversion and the production yield.The optimum condition generated by the RSM had successfully managed to get as high as 94.5%±1.4%of the FFA conversion and 92.4%±1.7%of the FAME yield.From this result,the potential of the PFAD as the second-generation feedstock and the SGAC catalyst is getting high interest for the biodiesel industries.
Nomenclature
Adeq precision adequate precision
Adj R-squared adjusted R-squared
Actual value the value observed at that design point
Cor total totals corrected for the mean
C.V. coefficient of variation
DF degrees of freedom

Table 6 Quality assessment of the PFAD methyl ester
F value test for comparing treatment variance with error variance
Mean overall mean of the response
Pred R-squared predicted R-squared
Predicted value the value predicted at that design point using the current polynomial
Press predicted residual sum of squares
Prob>F probability of observed F value if the null hypothesis is true
Residual difference between the actual and predicted value for each point in the design
R-squared the multiple correlation coefficient
Std.dev. standard deviation
Studentized Residuals the residual divided by the estimated standard deviation of that residual
Acknowledgments
The authors are highly appreciative of Ministry of Science,Technology and Innovation(MOSTI),Malaysia for providing the eScience Project(Project No.06-01-04-SF1780;Vot No.5450746).The authors would like to thank Universiti Teknologi MARA and Malaysia's Ministry of Higher Education for granting a scholarship to one of the authors(Ibrahim M.Lokman).
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
