Influences of operating conditions on biocatalytic activity and reusability of Novozym 435 for esterification of free fatty acids with short-chain alcohols:A case study of palm fatty acid distillate
Sawittree Mulalee,Pongrumpa Srisuwan,Muenduen Phisalaphong*
Chemical Engineering Research Unit for Value Adding of Bioresources,Department of Chemical Engineering,Faculty of Engineering,Chulalongkorn University,Bangkok 10330,Thailand
Keywords:Biodiesel Oleic acid Novozym 435 Reusability Palm fatty acid distillate
ABSTRACT In the present study,the effects of operating conditions on biocatalytic activity and stability of Novozym 435 for repeated-batch biodiesel production from free fatty acid(FFA)were investigated.Thermal deactivation caused by increased operating temperature from 45 to 50°C could seriously affect the reusability of Novozym 435.The deactivation of Novozym 435 during the esterification of oleic acid with ethanol tended to be stronger than that in the system with methanol.Under the optimal conditions,considering both biocatalytic activity and stability of the enzyme,Novozym 435 could be reused for 13 cycles for biodiesel productions from oleic acid and absolute alcohols(methanol and ethanol)with FFA conversions of at least 90%.The presence of 4%-5%water in ethanol significantly affected the reusability of Novozym 435.Changes in the surface morphology of Novozym 435 during the esterification with various conditions were observed.It was revealed that the reduction in catalytic activity was related to the swelling degree of the catalyst surface.Additionally,biodiesel production from low cost renewable feedstocks,such as palm fatty acid distillate(PFAD)and 95%ethanolwas examined.The esterification of PFAD with 95%ethanol catalyzed by Novozym 435 in 10-repeated batch operation showed the similar results in FFA conversion as compared to those using oleic acid.Novozym 435 remained active and could maintain 97.6%of its initial conversion after being used for 10 batches.
1.Introduction
The energy supply shortage is currently one of the most important issues that continuously and directly affect humans.In Thailand,biodieselis one of the most attractive alternative fuels.Biodieselis an environmentally compatible product that can be produced from renewable resources,such as vegetable oils,free fatty acids(FFAs),animal fats[1,2]or waste cooking oil(WCO)[3]with short-chain alcohols,such as methanol,ethanol,propanol and butanol[4].The catalysts used in the production of biodiesel can be classified as alkaline catalysts(NaOH or KOH),acid catalysts(H2SO4)[5],solid acid and base catalysts[6]and biocatalysts(lipase enzymes).
Lipase can selectively and effectively catalyze both transesterification and esterification reactions with low energy consumption atmild operating temperatures of less than 50°C[7].Moreover,this process is more environmentally friendly than chemical processes[8].Because the use of enzymatic catalysts does not form soaps,it can esterify both FFA and TAG in one step without the need of a subsequent washing step[9].Since there is no discharge of chemicals and wastewater,the enzymatic process is considered to be a clean and environment friendly technique.However,the high cost of enzymatic catalysts is considered to be the limiting factor for their commercialization.Therefore,immobilization techniques are used to enhance the potential for industrial-scale enzymatic processes.Immobilization allows for the easy recovery of enzymatic catalysts and for reuse of the catalyst several times without significant losses in activity or stability[10].
Immobilized lipase from Candida antarctica lipase B(Novozym 435)is an attractive biocatalyst for the production of biodiesel from many types of oil-containing seed plants in Thailand,such as palm(Elaeis guineensis),physic nut(Jatropha curcas),papaya(Carica papaya)and rambutan(Nephelium lappaceum)[11].The operating conditions,such as the temperature,initial molar ratio of FFA to alcohol,mixing rate and enzyme concentration,have important roles in the enzymatic conversion of FFAs[12-14].Influence of alcohol structure on the enzymatic activity was also reported[15].
Oleic acid is a major component of various oils,such as palm oil,rapeseed oil and used frying oil[16].In our previous studies on enzymatic esterification using Novozym 435 in a batch solvent-free system,the optimal conditions for the conversion of oleic acid with methanol and ethanol were reported,and the conversion yields were greater than 90%[13].In this study,we focused on the study of effects of the operating conditions on the reaction progress and reusability of Novozym 435 for the esterification of oleic acid with short-chain alcohols.Moreover,for further development of“green technology”for a commercial biodiesel production from low-cost feedstocks,the esterification of palm fatty acid distillate(PFAD),a byproduct from palm oil refining and 95%ethanol using Novozym 435 as the catalyst was evaluated with repeated batch experiments.
2.Materials and Methods
2.1.Materials and chemicals
Novozym 435(lipase B from C.antarctica,EC 3.1.1.3),a nonspecific lipase immobilized on macroporous acrylic resin was purchased from S.M.Chemical suppliers Co.,Ltd,Bangkok,Thailand.The diameters of the particle beads are in a range of 0.3-0.9 mm with approximate density of 0.4 g·ml-1.The catalytic activity was 10000 PLU·g-1.All other chemicals used in this study were purchased from local suppliers in Thailand.
2.2.Enzymatic esterification reaction
The batch esterification process using 40 g of oleic acid and shortchain alcohols catalyzed by Novozym 435 was performed in a 250 ml Erlenmeyer flask.The optimal operating conditions obtained from our previous work were used,as follows:operating temperature of 45°C,FFA to alcohol molar ratio of 1:2,and enzyme loading of 5%(w/w of oleic acid)[13].To minimize the effect of external mass transfer limitations and maintain the enzyme activity,the mixtures were shaken at a constant rate of 250 r·min-1in a shaking incubator(Innova 4000,New Brunswick Scientific Co.,Inc.,Germany).Samples were collected from the mixtures during the reactions(0-8 h)for the determination of FFA conversion.Water,a reaction byproduct and residual alcohols were removed from the samples via thermal evaporation.The samples were then analyzed using the titration method to determine the FFA conversion.On the study of the reusability of Novozym 435,after each batch,the products and the remaining substrates were removed,and fresh substrates were added for the next cycle.
2.3.Biodiesel conversion analysis
The percentage of FFA conversion was determined by the titration method with 0.1 mol·L-1KOH solution using phenolphthalein as the indicator.The FFA conversions were calculated from the titration volumes of the KOH solution.The reported values were the average values of each duplicate set.
2.4.Characterization of Novozym 435
Scanning electron microscope(SEM)was performed to observe morphology changes of the biocatalyst(Novozym 435)after being used in the esterification reaction.Excess oil and solution at the surface of the biocatalysts was blotted out with Kimwipes paper.The samples of biocatalysts were then sputtered with gold and were examined formorphological structures by the scanning electron microscope JSM-5410LV(Tokyo,Japan).
3.Results and Discussion
3.1.Effects of operating conditions on catalytic activity and reusability of Novozym 435
3.1.1.Effect of operating temperature
Higher operating temperature results in higher initial conversion rate and according to the high rates,the reaction reaches equilibrium sooner.However,thermal deactivation of enzymes might occur and thereby negatively affect the activity,stability and reusability of enzymes.Our previous study showed that there was no significant difference in the FFA conversion and the reaction rate at temperatures between 45 °C and 60 °C[13].In the present study,the stability of Novozym 435 during the enzymatic esterification of oleic acid with short-chain alcohols,such as methanol(99.9%)and ethanol(99.9%)was investigated at the operating temperature of 45 °C and 50 °C.As shown in Fig.1A,with the use of methanol,the initial FFA conversion slightly increased as the temperature increased from 45 °C to 50 °C.However,it was found that the final FFA conversion was not significantly affected by temperature changes in this temperature range.The effect of the thermal deactivation of Novozym 435 at 50°C was more clearly observed in the esterification of oleic acid with ethanol as shown in Fig.1B.In the first batch with the fresh enzyme,the rates of FFA conversion and the final FFA conversions at the operating temperature of 45 °C and 50 °C were quite similar.However,from the second batch to the fifth batch,the rates of FFA conversion significantly decreased as the temperature increased from 45 °C to 50 °C.At the operating temperature of 50°C,the FFA conversion after 8 h was approximately 91%in the first batch,and it was less than 80%in the fifth batch,whereas no significant drop in FFA conversion was observed with the operating temperature at 45°C.The results indicated that for the long-term use of this biocatalyst,the optimal temperature for the esterification of oleic acid with methanolorethanol by Novozym 435 should be 45°C for maintaining high catalytic activity and reusability of the enzyme.
3.1.2.Effect of alcohols
The effect of alcohols on the enzyme activity in acyl transfer reactions includes reversible inhibition and irreversible inactivation[17,18].The type of alcohol might directly affect the reusability of enzymes.In this part,the effects of alcohols,methanol(99.9%)and ethanol(99.9%),on the reusability of Novozym 435 in the esterification of oleic acid were investigated.In Section 3.1.1,to reduce the effects of thermal deactivation on the activity and stability of Novozym 435,the reactions were performed at a constant operating temperature of 45°C.As shown in Fig.2,the FFA conversion rates to produce methyl oleate were higher than those of ethyl oleate.During the production of methyl oleate,the conversion rate slightly decreased as the number of enzyme reuse cycles increased.However,a gradual decrease in the FFA conversion rate with increasing number of Novozym 435 reuse cycles was more clearly seen during the production of ethyl oleate.Nevertheless,for both reactions,Novozym 435 could be reused for 13 repeated batches(104 h)with FFA conversions of at least 90%.Previously,it was reported that among all enzymes tested,Novozym 435 was the most effective biocatalyst for the methanolysis in a continuous process[19].However,the deactivation of Novozym 435 in the esterification could occur because of the interaction of alcohols with the surface of Novozym 435 through the adsorption of alcohols[20].The denaturing effect of alcohols on proteins,causing enzyme deactivation is well known.Progressive deactivation after several reuses of the biocatalyst during the esterification was also observed[20].In previous reports,85%of the initial Novozym 435 activity was maintained after 9 batches in the transesterification of vegetable oils and ethanol[21],and 90%of the activity of Novozym 435 was maintained over 7 batch reactions in the transesterification of vegetable oils and short-chain alcohols[22].
3.1.3.Effect of initial water content in alcohol

Fig.1.The effectof operating temperature on the reusability of Novozym 435 in the esterification of oleic acid with 99.9%methanol(A)and 99.9%ethanol(B).The cycle time is 8 h and the operating temperatures are 45°C()and 50°C().

Fig.2.The effect of type of alcohol on the reusability of Novozym 435 in the esterification of oleic acid at 45°C with 99.9%methanol()and 99.9%ethanol().
Influence of the initial water content on the esterification and transesterification reactions is another important issue.It was reported that transesterification reactions catalyzed by Candida rugosa,Pseudomonas cepacia,and Pseudomonas fluorescens lipases could not occur in the water-free system[9].However,Novozym 435 exhibited the highest activity with a low availability of water content[23,24].The presence of water during the reaction could have a negative effect on the conversion of FFAs due to the occurrence of a hydrolysis reaction during transesterification[25]and a reversible reaction effect on esterification[4].Methanol is mostly produced from coal or natural gas.It is available commercially in anhydrous form.Ethanol is considerably less toxic and contains more energy than methanol.Ethanol is mostly produced from renewable materials via fermentation processes.During the separation of ethanol by distillation,ethanol forms an azeotrope with water with a composition of 96.6%ethanol and 3.4%water(by volume).Therefore,hydrated ethanol(95%-96%,v/v)is more available at a relatively lower price than anhydrous ethanol(99.9%,v/v).Thus,in this study,the effect of the initial water content in ethanol on the reusability of Novozym 435 during the esterification of oleic acid was investigated.
The initial water content in ethanol was varied by using 99.9%(v/v),96.0%(v/v)and 95.0%(v/v)ethanol.During the first hour of the reaction,the initial FFA conversions during the production of ethyl oleate with 96.0%and 95.0%ethanol were slightly higher than that with 99.9%ethanol.However,the final conversion reached the same level of about 90%(Fig.3).A higher initial rate of esterification catalyzed by an enzymatic catalyst in a system containing some amount of water was previously reported during the production of biodiesel from soybean oil catalyzed by various lipases,such as Rhizopus oryzae,C.rugosa,P. fluorescens,C.antarctica and Burkholderia cepacia[26].It was suggested that a certain amount of water was required for many enzymes to work[26,27].
The presence of 4%-5%water in ethanol did not considerably affect the FFA conversion catalyzed by Novozym 435 in Batch 1 to Batch 9;however it exhibited a negative effect on the reusability of Novozym 435.As shown in Fig.3,when 95.0%ethanol and 96.0%ethanol were used,to retain a conversion of at least 90%,the cycles of reuse of Novozym 435 should not be greater than 10 and 11 batches,respectively,whereas the number of Novozym 435 reuse cycles in the system using anhydrous ethanol was about 13 cycles.Previously,in a solventfree system,the deposition of water on the surface of the lipase could reduce the conversion yield of biodiesel[28].A fast interaction between water and enzymes might deactivate enzymes and the presence of water as a byproduct of the reaction could lower the equilibrium conversion[4].However,it was suggested that the inhibition of the enzyme by water is reversible,which could be solved by the removal of water[19].It was reported that the enzyme recovered its catalytic activity after being dried to its initial water content[28].The reusability of enzyme could also be influenced by other factors such as the purity of alcohols and the contaminants of toxic organic compounds.
3.2.Effects of operating conditions on the surface morphology of Novozym 435
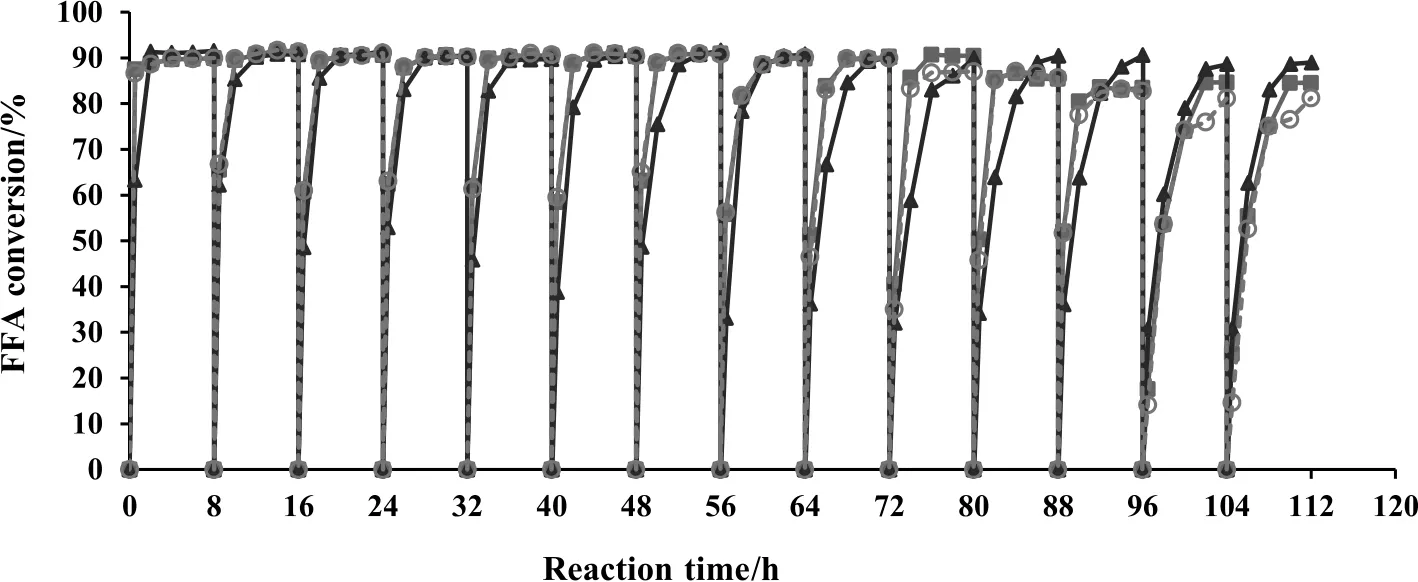
Fig.3.The effect of initial water content in ethanol on the reusability of Novozym 435 in the esterification of oleic acid at 45°C with 99.9%ethanol(),96.0%ethanol()and 95.0%ethanol().

Fig.4.SEM micrographs of Novozym 435 in the esterification of oleic acid with short-chain alcohols at various operating conditions.
Loss of the enzyme activity could also be caused by deactivation of the enzyme from the adsorption of alcohols on the surface.The experimental results in this study showed that in a solvent-free system,the deactivation of Novozym 435 during the esterification of oleic acid with ethanol tended to be stronger than that observed in the system with methanol.Under SEM observation of the surface morphology of fresh Novozym 435(Fig.4A)compared to that after being used in the systemusing 99.9%methanol at operating temperature of45°C for5 repeated batches(Fig.4B),a slight change in the surface morphology was noticed.On the other hand,the partial swelling of the catalyst surface was clearly observed after being used for 5 repeated batches in the system using 99.9%ethanol at 45°C(Fig.4D).A significant increase in the degree of swelling was observed on the surface of Novozym 435 as the operating temperature was increased to 50°C.Compared to the system using methanol at50°C for5-repeated batches(Fig.4C),considerable increase in the degree of swelling was observed in the system using ethanol at 50°C(Fig.4E).From the experimental results,it was noticed that the reduction in catalytic activity or the degree of deactivation of Novozym 435 was related to the swelling degree of the catalyst surface.From the SEM observation,it was also suggested that the reaction at a high operating temperature might enhance the adsorption of alcohols,especially in the system using ethanol,resulting in significant reduction in the reusability of Novozym 435.
Novozym 435 catalyzed the reaction with high activity under highly water-deficient conditions[23,24,29].Previously,it was suggested that the agglomeration of water might flood the enzyme pores,causing decreases in the reaction rates[30].In this study,it was also shown that the presence of water at 4%-5%in ethanol causes some negative effects on the reusability of the biocatalyst.Considerable swelling of the catalyst surface was also observed after Novozym 435 was being used for 13 repeated batches in the system using 95%ethanol at 45°C(Fig.4F).
3.3.A case study of palm fatty acid distillate(PFAD)
This study also focuses on producing biodiesel from low cost feedstocks such as palm fatty acid distillate(PFAD)(Patum Vegetable Oil Co.,Ltd.,Pathum Thani,Thailand)and 95%ethanol.The esterification of PFAD and 95%ethanol catalyzed by Novozym 435 in 10-repeated batch operation was performed under the optimalconditions,considering both catalytic activity and stability.According to the GC-MS results,PFAD using in this study composed mainly of free fatty acids(~93%)with palmitic acid(50.7%)and oleic acid(41.0%)as major components.PFAD is a byproduct obtained from palm oil refining process of the edible oil industry.PFAD is considered as one alternative low-cost feedstock for next-generation palm biodiesel in Southeast Asia,with a particular emphasis on Indonesia,Malaysia and Thailand.
The results as shown in Fig.5 revealed that the FFA conversions obtained from the system using PFAD and 95.0%ethanol as substrates were similar to those using oleic acid and 95.0%ethanol.The FFA conversion at 90.1%was obtained after 8 h in the first batch.Novozym 435 remained active and could maintain 97.6%of its initial conversion after being used for 10 batches.The conversion of FFAs after 10-repeated batch operation slightly decreased to 87.9%.The catalytic activity of Novozym 435 for esterification of PFAD and ethanol under the optimal conditions in this study was significantly more stable than those previously reported[31].Therefore,by using the optimal conditions,considering both biocatalytic activity and stability,the reusability of the enzyme could be enhanced.

Fig.5.The esterification of palm fatty acid distillate(PFAD)with 95.0%ethanol at 45°C.
4.Conclusions
The reaction progress and reusability of Novozym®435 for the production of biodiesel from oleic acid with short-chain alcohols,methanol and ethanol were studied.Novozym 435 gave the maximum FFA conversion of 94.6%in the production of methyl oleate and 90.5%in the production of ethyl oleate.The results indicated that temperature,type of alcohol,and water content considerably affected the reusability of Novozym 435.It was shown that under the optimal conditions,Novozym 435 could be reused in the production of methyl oleate and ethyl oleate for 13 cycles with FFA conversions of at least 90%.Changes in the surface morphology related to the degree of deactivation of Novozym 435 during the esterification with various conditions were observed.Furthermore,the esterification of PFAD and 95%ethanol catalyzed by Novozym 435 in 10-repeated batch operation showed the similar results in FFA conversion as compared to those using oleic acid.It was demonstrated that PFAD and 95.0%ethanol have high potentials as a source of low-cost feedstock for biodiesel production by enzymatic esterification.
Acknowledgments
We thank the National Research Council of Thailand(NRCT)for financial support.SM also received support from the 90th anniversary of Chulalongkorn University(Ratchadaphiseksomphot Endowment Fund).
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
