Ghezeljeh nanoclay as a new natural adsorbent for the removal of copper and mercury ions:Equilibrium,kinetics and thermodynamics studies
Majid Soleimani*,Zahra Hassanzadeh Siahpoosh
Department of Chemistry,Imam Khomeini International University(IKIU),Qazvin,Iran
Keywords:Montmorillonite Thermodynamic Kinetic Mercury Copper
ABSTRACT Heavy metal determination was carried out by applying the solid phase extraction(SPE)method in batch mode followed by atomic absorption spectroscopy(AAS)and inductively coupled plasma atomic emission spectroscopy(ICP-AES)from aqueous solutions using Ghezeljeh montmorillonite nanoclay as a new natural adsorbent.The Ghezeljeh clay is characterized by using Fourier Transform Infrared(FT-IR)Spectroscopy,Scanning Electron Microscopy-Energy Dispersive Spectrometry(SEM-EDS)and X-ray Diffractometry(XRD)and X-ray Fluorescence(XRF).The results of XRD and FT-IR of nanoclay confirm that montmorillonite is the dominant mineral phase.Based on SEM images of Ghezeljeh clay,it can be seen that the distance between the plates is Nano.The effects of varying parameters such as initial concentration of metal ions,pH and type of buffer solutions,amount of adsorbent,contact time,and temperature on the adsorption process were examined.The effect of various interfering ions was studied.The adsorption data correlated with Freundlich,Langmuir,Dubinin-Radushkevich(D-R),and Temkin isotherms.The Langmuir and Freundlich isotherms showed the best fit to the equilibrium data for Hg(II),but the equilibrium nature of Cu(II)adsorption has been described by the Langmuir isotherm.The kinetic data were described with pseudo- first-order,pseudo-second-order and double-exponential models.The adsorption process follows a pseudo-second-order reaction scheme.Calculation of ΔG0,ΔH0 and ΔS0 showed that the nature of Hg(II)ion sorption onto the Ghezeljeh nanoclay was endothermic and was favored at higher temperature,and the nature of Cu(II)ion sorption was exothermic and was favored at lower temperature.
1.Introduction
Exposure to heavy metals,even at trace level,is believed to be a risk for human beings.Unlike organic pollutants,heavy metals are nonbiodegradable,thus,how to efficiently and extremely remove undesirable metals from water systems is still a very significant however still challenging task for environmental engineers.Copper is a necessary nutrient in trace amount but at a higher level it is toxic to plants,animals and humans[1].Copper consumption in high amounts might bring about severe toxicological concerns because it can be deposited in the brain,skin,liver,myocardium and pancreas[2].Mercury(II)is one of the most serious contaminants of heavy metals in water and sediment,coming from chlor-alkali manufacturing industry,oil refinery,paint,pharmaceutical,paper and pulp and battery manufacturing industries[3].Diverse types of methods are available for removing heavy metals in water and wastewater including chemical precipitation,conventional coagulation,line softening,reverses osmosis,ion-exchange,photocatalytic reduction,electrodialysis and adsorption[4-12].Among these methods,adsorption is a highly effective,economical,and widely applied method[3].Clay minerals have drawn much attention due to their distinctive features,such as ion exchange properties,its large specific surface area,high sorption capacity,swelling,intercalation behavior and their lower cost compared to synthesized materials(e.g.silica gel,zeolite)[13].It is interesting to note at this point that Ghezeljeh montmorillonite(Geleh-Sar-Shoor)nanoclay(“Geleh-Sar-Shoor”means headwashing clay)was used in ancient Persia to clean the body,notably the hair,and to bathe dead bodies prior to funerals.The nanoclay is still used in some parts of Iran.This clay is readily obtainable,low-priced(its price is 0.1$per kilogram)and environment friendly with chemical and mechanical stability.The nanoclay was characterized using Fourier Transform Infrared(FT-IR),Scanning Electron Microscopy-Energy Dispersive Spectrometry(SEM-EDS),X-ray Fluorescence(XRF)and X-ray Diffractometry(XRD).Based on SEM images of this clay,it can be seen that the distance between the plates is Nano.This paper is a report on an investigation which attempted to measure and remove copper and mercury ions from aqueous solutions using batch equilibrium technique.The effect of different variables on adsorption of copper and mercury ions was investigated and optimum conditions were recognized.Langmuir,Freundlich,Dubinin-Radushkevich,and Temkin isotherm models were applied and isotherm coefficients were computed.Kinetic models such as first-order,second-order equations and intra-particle diffusion model and thermodynamics were also investigated and parameter values were derived by adsorption using the new natural adsorbent.So far Ghezeljeh montmorillonite nanoclay has not been used as adsorbent for the removal of heavy metals.
2.Theoretical Considerations
2.1.Clay
Clays are hydrous aluminum silicates which are classified as either 1:1 or 2:1 clay minerals.The sheets in these clays are held together by weak van de Waals forces making it easy for other chemicals to enter the interlayer region.There are three types of crystalline surfaces onto which metal adsorption can occuron the clays:a hydroxyl plane associated with the alumina octahedral layer,an oxygen plane on the silica tetrahedral layer and particle edges formed from the incomplete or irregular lattice structure.Many 2:1 clay minerals have permanent negative charge due to isomorphous substitution of aluminum(III)for silicon(IV)in the silica layer or magnesium(II)for aluminum(III)in the alumina layer[14].Montmorillonite is dioctahedral clay of the smectite group and is composed of alumino-silicate layers.The silica tetrahedra(T)(Si4+in tetrahedral coordination with O2-)and alumina octahedral(O)(Al3+in octahedral coordination with O2-)are interconnected(via the sharing of O2-at polyhedral corners and edges)in such a way that a sheet of alumina octahedral is sandwiched between two sheets of silica tetrahedral.Thus,the composition is T-O-T(2:1)[15].Most of the surface charges on montmorillonite are generated by isomorphous substitution or non-ideal octahedral occupancy.These permanent negative charges are distributed along the mineral basal surfaces and are balanced by absorbing aqueous cations,such as Na+,K+,Ca2+and Mg2+.These cations can be exchanged with other cations in solution and the exchange reactions are non-specific,stoichiometric and involve the formation of surface outer-sphere complexes.The edges of the silica tetrahedra and alumina or magnesia octahedra provide another type ofsorption site on montmorillonite.In montmorillonite,the edge sites account for a much smaller proportion of the exchange capacity.The adsorption of metals to these sites involves the formation of surface inner-sphere complexes similar to the interaction of these metals with the surfaces of oxide minerals[16,17].Removal of metal cations by clay minerals is controlled by parameters such as charge characteristics of the clay.Charge characteristics include the magnitude of the active sites and cation exchange capacity which has two components;namely,the permanent negative charge generated by isomorphous substitution within the octahedral and tetrahedral sheets of the silicate layers and the pH dependent charge arising from dissociation of edge hydroxyl groups.The pH-dependent charge may also be contributed by dissociation of other acid groups present such as humic acids particularly in un-purified clay minerals[18,19].The exchange processes depend on many factors,like the physicochemical characteristics of solid and cation(such as ionic radius,charge size,hard-soft acid-base properties,hydration volume and hydration enthalpy of cation),presence of competing ions,temperature,ionic strength and experimental conditions involving time of reaction,concentration of ions,and pH of the medium[18,20-26].Ofallthese factors,pH is considered the ‘master variable’controlling ion exchange,dissolution/precipitation,reduction/ oxidation,adsorption and complexation reactions[20].The metal species on the clay mineral surfaces seem to be adsorbates and/or precipitates of hydrolysis products.The knowledge of the heavy metal binding to the mineral structure is of fundamental importance to predict the mobility and long-term behavior of heavy metals in natural systems[27].
2.2.Metal sorption mechanisms onto montmorillonite
Although many studies on metal adsorption onto montmorillonite have been successfully modeled,there is no universal consensus on adsorption sites,surface reactions and modeling approaches.The associated proton and metal binding constants are generally inconsistent because the methods and models used in the studies vary considerably.It is thus difficult to compare the adsorption affinity of various metal cations with montmorillonite.With the heterogeneous nature of the clays it is likely that several metal sorption mechanisms take place simultaneously making the determination of metal/clay component interaction difficult on such material[13,27,28].In the lower pH range,the metallic cations were mainly bound through the formation of outer-sphere surface on the permanently charged basal surface sites(≡X¯),while in the higher pH range the adsorption occurred mainly on the variably charged edge sites(≡SOH)through the formation of inner-sphere surface complexes[27].
Eren and Afsin[29]have reported that the adsorption of Cu(II)by the bentonite samples is a complex procedure controlled by a number of environmental variables(pH,ionic strength,and the presence of Cl-,,).The isotherm studies propose that Cu(II)sorption onto the bentonite sample arises in two stages.The first stage includes adsorption on permanent charge sites and the second includes adsorption on variable charge sites.IR studies showed that Cu(II)cations substituted the original metalions in the interlayer or locate into hexagonal cavities of Si-O sheet in the Cu(II)-saturated bentonite samples.Structural modification of tetrahedral sheets due to the presence of Cu(II)cations either in hexagonal holes and/or in the previously vacant octahedral sites induces changes in the Si-Ovibration modes.The lower dehydroxylation temperature values for the Cu(II)-saturated bentonite samples than the parent materials presented the higher dehydroxylation rate,and the decomposed bentonite structure.The X-ray diffraction(XRD)spectra showed that the Cu(II)adsorption onto the bentonite samples led to changes in unit cell dimensions and symmetry of the parent bentonites[29].The results attained from IR and pH of final solution recommended that the surface complexation is a dominant adsorption mechanism for Cu(II)onto the palygorskite by Chen et al.[30].The Cu(II)adsorption has arisen not only ion exchange in exchangeable cations,but also specific adsorption,which can be defined by surface complexation model which describes a reaction between functional surface groups and Cu(II)ions[31-33].Strawn et al.[34]have reported adsorption of Cu(II)by smectites(montmorillonite and bedellite)as a function of varying ionic strength and pH.The X-ray adsorption fine structure(XAFS)and electron paramagnetic resonance(EPR)spectroscopic experiments on Cu(II)adsorbed on clays have exposed that Cu-Cu linkages in the multinuclear complexes were 0.265 nm apart having a coordination number near one.Brigatti et al.[28]have reported that Hg is specially sorbed as Hg-OH complexes by montmorillonite.Hg-OH2complexes are less strongly bound to the 2:1 layers,as verified by extended X-ray adsorption fine structure(EXAFS)and thermal analysis.On the contrary,Hg-O intercalates are more strongly bounded to the layer and mercury is released at higher temperatures.Sajidu et al.[13]have studied the interactions of chromium(III),copper(II),zinc(II),cadmium(II),mercury(II)and lead(II)on natural alkaline mixed clays using EXAFS spectroscopy.They have reported that copper(II)is chie fly sorbed through the phosphate groups of the fluoroapatite in the adsorbent mixture.Also,mercury(II)is adsorbed as hydrolysed linear O-Hg-O units on the clay surface at neutral pH,while it is reduced to mercury(I)at low pH and adsorbed as≡O-Hg-Hg-OHxcomplexes(x=1 or 2).The adsorption of metal ions largely depends on its hydrated radius.The larger the diameter the slower its mobility and the less likely its exchange would occur[35].Every metal in solution possesses a hydrated layer which has a characteristic thickness and degree of stability.It has been confirmed that ions with large radii often show large rejections while smaller ions are often favored by exchangers[36,37].The metal ions having smaller hydrated ionic radius have easier access to the mineral's surface and can diffuse more easily inside its pores.On the other hand,the presence of ions having a larger hydrated ionic radius results in a more rapid saturation of the adsorption sites[38].
3.Materials and Methods
3.1.Reagents and solutions
All the reagents were purchased from the German company of Merck:acids,bases,hydrogen peroxide,sodium acetate,sodium carbonate,sodium citrate,nitrate salts of copper,lead,chromium,cobalt,cadmium,mercury,sulfate salts of aluminum,nickel,manganese,zinc,magnesium,chloride salts of sodium,potassium,iron,calcium,and ammonium.Since the reagents were at the highest purity,they were used without any further purification.The element standard solutions were produced by diluting a stock solution of 1000 mg·L-1of the specified element using doubly distillated water.The Hg(II)stock aqueous solution(1000 mg·L-1)was obtained from the dissolution of Mercury(II)nitrate monohydrate(Merck,Darmstadt,Germany),acidified with nitric acid to avoid mercury precipitation.The working solutions of Hg(II)and Cu(II)were prepared daily by appropriate dilution of mercury and copper stock solutions which were prepared weekly.
A citrate-citric acid buffer solution was prepared using 0.1 mol·L-1citric acid solution at pH 2-3.Acetate buffer solution was prepared by combining appropriate volumes of 0.1 mol·L-1acetic acid and 0.1 mol·L-1sodium acetate at pH 4-6.Phosphate buffer solution was prepared using 0.1 mol·L-1phosphoric acid atpH 7.Carbonate buffer solution was prepared using 0.1 mol·L-1sodium carbonate at pH 7.Citrate buffer solution was prepared using 0.1 mol·L-1sodium citrate at pH 7.Ammonium buffer solution was prepared by mixing suitable amounts of 0.1 mol·L-1ammonia and 0.1 mol·L-1ammonium chloride at pH 8-10.The pH of the buffer solutions was adjusted by adding 1 mol·L-1NaOHor HCl,as needed.The nanoclay(adsorbent)was collected from Ghezeljeh,a village 18 km west of the city of Tafresh in Iran.This clay,to the best of our knowledge,has not been used as adsorbent for the elimination of heavy metals.
3.2.Instrumentation
A model 420A digital Orion pH meter(Gemini,the Netherlands)equipped with a combined glass electrode was employed for pH adjustments.An ultrasonic water bath(Bandelin,Berlin,Germany)was used to disperse and disaggregate clay.Agitation of the system was carried out on a mechanical shaker(Flask shaker SF1 Scientific model,STUART,Britain).X-ray diffraction(XRD)data were obtained using an Ital Structures diffractometer(GNR,Novara,Italy),with Cu Kα radiation(40 kV per 30 mA,λ=0.1542 nm).Fourier Transform Infrared(FT-IR)study was carried out using Tensor Bruker MIR-T27(Germany)having a standard mid-infrared deuterated triglycine sulfate(mid-IR DTGS)detector.Batch experiments were carried out in an Incubator Shaker(model 3020 DRS,FSA,Iran)at 200 r·min-1.
To quantitatively measure mercury and copper ions a Varian 220Z(Australia)atomic absorption spectrometer(AAS)with Vapor Generation Accessory(VGA)system and a Thermo Scientific ICP-AES(inductively coupled plasma atomic emission spectroscopy)(DUO 6500,England)spectrometer was used,respectively.Philips X-ray fluorescence(XRF)of the sample has been studied using XRF Analysis Instruments(Philips Magix Pro,Netherlands).A scanning electron microscope(SEM)(LEO 1450 VP,Thornwood,N.Y.,USA)with variable pressure secondary electron detector and energy dispersive spectrometer(EDS)operating at 30 kV(Oxford INCA software,High Wycombe,U.K.)were used for SEM-EDX analysis.
3.3.Preparation of the adsorbent
The adsorbent was prepared using the Galehouse method[39].Natural Ghezeljeh nanoclay was first treated with 0.1 mol·L-1of acetic acid to eliminate carbonates,and then 30%H2O2was used to exclude mineral and organic impurities.The Ghezeljeh nanoclay was carefully rinsed with doubly distilled water to eliminate traces of acetic acid and hydrogen peroxide.The treated Ghezeljeh nanoclay was dispersed and disaggregated in doubly distilled water through an ultrasonic water bath.The resulting suspension was transferred to a measuring cylinder and permitted to stand for 3 h,26 min,6 s for sedimentation.The fine fraction(<2 μm)was removed and then placed in an electric vacuum oven at 50°C for 72 h to be dried.Then,it was put in a desiccator for subsequent experimentation.Scanning electron microscopy(SEM)is a powerful technique applied in micro imaging of a variety of surfaces.Based on SEM images of this Ghezeljeh clay,it can be seen that the distance between the plates is Nano(Fig.1(a)).
3.4.Adsorption procedure
In a single component,adsorption experiments were carried out using batch method.First,a 25 ml solution containing metal ion was transferred into an Erlenmeyer flask.Then,5 ml of an appropriate buffer solution was added and followed by 0.5 min of agitation.Next,0.5 g of the Ghezeljeh montmorillonite nanoclay was added,and immediately the mixture was shaken in a temperature controlled shaker incubator at 200 r·min-1.Samples were withdrawn from the shaker at different time intervals and the suspensions were centrifuged at 3500 r·min-1for 5 min.The supernatants were then collected and analyzed for the metal ions.Blank samples containing metal ions without the adsorbent were also used in this study.The effects of the amount of adsorbent,pH of the buffer solutions,buffer type,volume of the standard solutions,initial metal ion concentration,and contact time between adsorbates and adsorbent were investigated.All experiments were performed at least three times and average values of the results are given here.The concentration of metal ions remaining in the solution was calculated by taking the difference of initial and finalmetalion concentrations.Adsorption process was quantified by calculating the sorption percentage(Ads,%)as defined by the following:

where C0is the initial concentration and Ceis the equilibrium concentration,mg·L-1.The amount of ions adsorbed per unit mass of adsorbed,qe(mg·g-1)is evaluated using the following expression:

where C0and Ce(mg·L-1)are the same as in Eq.(1),V(ml)is the volume of metal ions solution,and w(mg)is the mass of adsorbent.
4.Physicochemical Characterization
4.1.XRD study
X-ray diffracto grams were obtained for the 2θ angles ranging from 2°to 40°at room temperature.The Ghezeljeh nanoclay was treated with ethylene glycol,an organic compound which steadily intercalates itself into the lattice of the clay.The structural properties of the Ghezeljeh nanoclay were monitored before and after treatment with ethylene glycol.The X-ray diffraction analysis revealed that the Ghezeljeh nanoclay sample was chiefly composed of montmorillonite minerals(Fig.1(b))[40,41].
4.2.FT-IR study
To prepare the Ghezeljeh montmorillonite nanoclay sample for FT-IR spectroscopy,an electric vacuum oven was used to dry(at 50°C for 6 h)and cool the Ghezeljeh montmorillonite nanoclay.A FT-IR spectrum was recorded in the range of 400-4000 cm-1using the KBr pellet technique.FT-IR spectrum of untreated Ghezeljeh nanoclay(Fig.1(c))shows the bands at 3626 cm-1in OH stretching region,which are assigned to hydroxyl groups coordinated to octahedral cations(Al3+cations).The most intensive band at 1035 cm-1is attributed to Si-O inplane stretching and 529 cm-1is due to Si-O bending vibrations.The shoulderat1113 cm-1shows Si-O out-of-plane stretching vibration.The broad bands at3440 cm-1and 1639 cm-1are the stretching and bending vibrations for the hydroxyl groups of water molecules present in the clay[15].Montmorillonite had two characteristic FT-IR regions[42],(1)3500-3750 cm-1(due to the surface structural OH groups of layered aluminosilicates and adsorbed water)and(2)400-1150 cm-1(due to lattice vibrations).Consequently,the FT-IR analysis confirmed that Ghezeljeh montmorillonite nanoclay was mainly composed of montmorillonite minerals[15].

Fig.1.(a)SEM images and(b)XRD patterns of the Ghezeljeh nanoclay are treated with ethylene glycol(A),untreated(B).(c)FT-IR spectrum and(d)EDX spectrum of untreated Ghezeljeh nanoclay.
4.3.XRF and EDS study
The technique of XRF spectroscopy is analogous to EDS in that an X-ray spectrum is attained which represents an elemental fingerprint of the sample.The main difference between XRF and EDS is the excitation radiation.XRF uses an X-ray beam to produce characteristic X-rays,whereas EDS uses an electron beam.XRF gives the overall composition of a sample,Instead,the EDS data are an average of several local compositions which are dependenton the positions at which the analysis is performed.One of the advantages of XRF is the ability to detect major,minor,and trace levels of an element,whereas EDS is limited to major and minor elemental concentrations.Consequently,the detection limit for XRF is about 10 parts per million(ppm.)and EDS is around 1%[43-45].The chemical composition of the Ghezeljeh montmorillonite nanoclay was determined with XRF and EDS.Table 1 and Fig.1(d)demonstrate chemical composition of this nanoclay[46].
5.Results and Discussion
5.1.Effect of adsorbent amount
Ten quantity levels of the Ghezeljeh montmorillonite nanoclay were studied:0.05,0.1,0.15,0.2,0.25,0.5,0.75,1,1.25 and 1.5 g.The standardsolution was 30 ml composing of 25 ml of doubly distilled water containing 7.32 mg·L-1of mercury or 2.5 mg·L-1copper ions,and then 5 ml of buffer solution added at pH 7.The adsorption of the metal ions on the clay improved as the amount of the Ghezeljeh montmorillonite nanoclay was increased.However,the adsorption declined at adsorbent amounts higher than 1 g for mercury and 0.5 g for copper ions.Reduction in the adsorption may be due to the fact that when the adsorbent amount is less than 1 g for mercury and 0.5 g for copper ions,the metal ions can simply come into contact with the adsorption sites,but when the adsorbent content exceeds 1 g for mercury and 0.5 g for copper ions,the number of such sites per unit mass decreases,because of the aggregation and flocculation of adsorbent particles[47-49].Similar observations have been reported earlier(Pb(II)on sawdust[50],Cd(II)on river bed sediment[51],Cu(II)on palygorskite[30],etc.).

Table 1 XRF-analysis of the Ghezeljeh nanoclay
5.2.Effect of pH and type of buffer solutions
Optimizing the initial pH value of the solution is an important parameter that investigates for obtaining high sorption capacity.Clays are known to have a negative surface charge in solution,as pH changes,surface charge as well changes,and the adsorption of charged species is affected.At low pH values,there are excess of H3O+ions in solution,a competition exists between the positively charged hydrogen ions and metal ions for the accessible adsorption sites on the negatively charged clay surface.In this study,at room temperature,pH of Hg(II)solutions in the range of 2 to 8,and pH of Cu(II)solutions in the range of 2 to 10 were adjusted with appropriate volume of buffer solutions given in Section 3.1.At pH higher than 8,Hg(OH)2solid phase was formed,so the mercury maintenance capacity diminishes.Hg ions were optimally adsorbed on the Ghezeljeh montmorillonite nanoclay at pH 7 by using phosphate buffer solution.Therefore for subsequent runs of the experiment,pH 7 was used as the optimum pH level for phosphate buffer solution.With increasing pH,Cu(II)adsorption increases until pH(7-8),as the pH becomes further basic due to the precipitation of several Cu(II)ions,adsorption reduces.Subsequently,in all following works,the pH of Cu(II)solutions was kept as 7.5 by using ammonium buffer solution.
5.3.Effect of volume of the standard solution
Due to the low concentration of metal ions in real sample,these analytes should be taken in smaller volumes to attain high preconcentration factorthan by using sample solutions with large volumes,ten feed volumes of 30,60,90,120,300,400,600,800,1000 and 1200 ml were investigated.It was found that recovery was over 95%at quantities of 30-90 ml for Hg(II)and 30-1020 ml for Cu(II)ion solutions,but it declined to below 95%when the volume of the solution exceeded 90 ml for Hg(II)and 1020 ml for Cu(II)ion solutions.
5.4.Effect of contact time
To study the effect of contact time,the adsorption of Hg(II)and Cu(II)ions on to the Ghezeljeh montmorillonite nanoclay was measured after 5,10,15,20,30,40,50,100,150,200 and 300 min of shaking the standard solutions at room temperature.As Fig.2 shows,the adsorption capacity of metal ions increased with contact time up to 20 min after this time,there is no considerable change in metal ion removal.
5.5.Effect of initial metal ion concentration

Fig.2.Effect of shaking time on the adsorption efficiency of(a)Cu(II)and(b)Hg(II)on Ghezeljeh nanoclay.
In most cases,the increase of the initial metal concentration results in an increase in the amount of metal adsorbed per unit mass of adsorbentuntila plateau is reached and in a decline in the over all removal efficiency[55].Because at low initial metal concentrations the ratio of metal cations to adsorbent mass is low,thus adsorption does not depend on initial concentration.Increasing the initial concentration means that more metals are available and thus,more metal ions are adsorbed for constant adsorbent mass.At higher initial metal concentrations the driving forces to overcome the mass transfer resistance for the movement of the metals from the bulk solution to the mineral surface increase.However,each unit mass of adsorbent is subjected to a larger number of metal cations,which slowly fill up the sites until saturation is reached.In such a case,further increase of metal concentrations is not accompanied with an increase in the amount of metal adsorbed per unit mass of adsorbent.The determination of the maximummetal concentration where total saturation of the adsorbent arises is important for practical applications.Moreover,the level of surface precipitation intensely depends on the initial metal concentration.At low metal concentrations,the adsorbent surface coverage is low and the formation of surface complexes is the main mechanism.The increase of metal concentration favors the concentration of compounds and aggregates on mineral surface.More increase in metal concentration results in saturation of adsorption positions and surface precipitation is the chief uptake mechanism.The saturation of active sites is generally faster in the cases where minerals display low selectivity for a metal.However,this is not always the fact,since the metal complexes formed or trapped in the mineral's surface could accelerate the surface saturation even at low concentrations of metals that present high affinity for the specific mineral.Temperature increase results in changes related to both kinetics and equilibrium attributed to:(l)increase in the kinetic energy which assists the access of the metalions to the active adsorption locations,(2)increase in the surface activity of the mineral resulting in higher affinity or increase in the active adsorption locations,and(3)decrease in the mass transferresistance.The increase in temperature is accompanied with a decrease in the thickness of the boundary layer surrounding the mineral,so that the mass transfer resistance of the adsorbate in the boundary layer decreases,facilitating the diffusion of metals in the adsorbent.Therefore,the effective diffusion coefficient of ions in solid phase generally increases and an increase in external mass transference is observed.As temperature is increased,the retarding specific or electrostatic interactions become weaker and the ions become smaller because solvation is decreased.However,at very high temperatures physical damage to the mineral can occur,reducing its adsorption capacity.In most cases it is required to evaluate the adsorption capacity of minerals at room temperature,since at higher temperature the effective cost of the procedure increases[55].
When the initial concentrations of Cu(II)and Hg(II)were varied(10,20,30,40,50 mg·L-1),the amount adsorbed per unit mass(qe)increased with rise in metal concentration(Fig.3)and the extent of adsorption(%)decreased with an increase in metal concentration(Fig.4).The effects of temperature on the uptake of metal ions by the Ghezeljeh nanoclay adsorbent were monitored at four temperatures of 298,308,318 and 328 K.An increase in the temperature resulted in a decrease in the amount of Cu(II)adsorbed per unit mass of Ghezeljeh nanoclay adsorbent,which showed the interactions to be exothermic.Athigh temperature,the adsorbate-adsorbent complex becomes unstable and Cu(II)ions escape from the solid phase to the bulk solution[79].It is also likely that the instability of the complex may be accompanied by damage to the adsorption sites in the clay decreasing Cu(II)uptake at higher temperature.An increase in the temperature resulted in an increase in the amount of Hg(II)adsorbed per unit mass of Ghezeljeh nanoclay adsorbent,which showed the interactions to be endothermic.However,at high temperatures physical damage to the mineral can occur,reducing its adsorption capacity[55].Similar results have been reported earlier for metal ion adsorption on different adsorbents(e.g.,Cd(II)on activated carbon from coconut coir pith[52],Cu(II)on maple sawdust[53],Ni(II)and Cd(II)on bagasse fly ash[54],etc.).

Fig.3.Effect of initial concentration on the adsorption capacity of(a)Cu(II)and(b)Hg(II)on Ghezeljeh nanoclay.

Fig.4.Effect of initial concentration in different temperatures on the adsorption percentage of(a)Cu(II)and(b)Hg(II)ions.
5.6.Interference from other ions
In order to evaluate the feasible analytical applications of the preconcentration procedure presented the effect of several foreign ions:(Na+(75 mg·L-1),Ca2+(15 mg·L-1),Mg2+(100 mg·L-1),K+(120 mg·L-1),Zn2+(70 mg·L-1),Fe3+(25 mg·L-1),Mn2+(20 mg·L-1),Al3+(20 mg·L-1),Ni2+(80 mg·L-1),Cd2+(51 mg·L-1),Co2+(60 mg·L-1),Pb2+(4 mg·L-1),Cr3+(10 mg·L-1))which interfere with the determination of trace of copper and mercury ions on Ghezeljeh montmorillonite nanoclay was examined in the optimized conditions.Ions were considered to be interfering when they caused an error larger than±5%in the preconcentration and determination of analyte ion.None of the added ions caused interference.
6.Isotherms
Sorption isotherm is the equation or curve that connects the metal concentration that has been adsorbed on the solid phase with metal concentration in the solution at equilibrium for definite temperature.The application of experimental data on equations that describe the isotherms is valuable;subsequently it results in the estimate of the system's performance and the optimization of adsorbent use.Sorption equilibrium provides fundamental physicochemical data for estimating the applicability of sorption procedures as a unit process.Equilibrium models can be classified into empirical and mechanistic models.Empirical models cannot represent the mechanisms of the sorbate uptake but can be used to predict the experimental results,while the mechanistic models can characterize the system mechanisms.Consequently,mechanistic models define the fundamental interactions that happen between the metal ions in the solution and the charged surface.Empirical models are generally based on simple mathematical relationships between the liquid phase equilibrium concentration and the solid phase equilibrium concentration[55].In terms of equilibrium examination the Langmuir isotherm model is in several cases in good agreement with the experimental data,while the Freundlich model has also been found to fit the experimental data in several cases.Some researchers have also successfully applied other isotherm models,such as the Temkin and Dubinin-Radushkevich(DR)models to predict the adsorption equilibrium[56].Consequently,in this study,the isotherm data was analyzed using the Langmuir,Freundlich,Temkin,and Dubinin-Radushkevich equations.
6.1.Langmuir isotherm

Fig.5.Langmuir isotherm for adsorption of(a)Cu(II)and(b)Hg(II)on Ghezeljeh nanoclay.
Langmuir isotherm is often used to describe adsorption of solute from liquid solutions and this model assumes the monolayer coverage of the adsorption surface with finite number of equal surface sites are energetically,consequently,the Langmuir isotherm is specific to strong monolayer chemisorption[13].Langmuir equation:

Values of qmand KLare determined from a plot of(Ce/qe)vs.(1/Ce).Ceis the equilibrium concentration of metal ions on the adsorbent(mg·L-1),qeis the amount of metal ions adsorbed per unit mass of Ghezeljeh nanoclay at equilibrium concentration(mg·g-1),qm(mg·g-1)is the maximum adsorption capacity,and KLis Langmuir constant which is related to sorption energy;specifically,KLshows adsorption enthalpy which generally varies with temperature[57,58]and determines the direction in which the adsorbate-adsorbent equilibrium is shifted.Large values of KLensure that the equilibrium is predominantly driven toward the right,leading to the formation of the adsorbate-adsorbent complex[48].
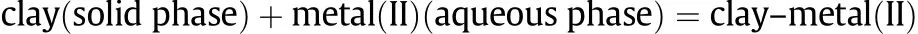
The Langmuir isotherm was applied to our experimental data and the results are shown in Fig.5 and Tables 2 and 3.One of the essential parameters of Langmuir equation is the equilibrium parameter or separation factor(RL)(Fig.6).RLcan be calculated by the following equation:

where C0(mg·L-1)is the highest initial solute concentration.The RLindicates the type of isotherm to be favorable(0<RL<1)or unfavorable(RL>1)or irreversible(RL=0)[40].This parameter indicated thatGhezeljeh montmorillonite nanoclay is a suitable adsorbent for the adsorption of Cu(II),and Hg(II)ions from aqueous solutions.
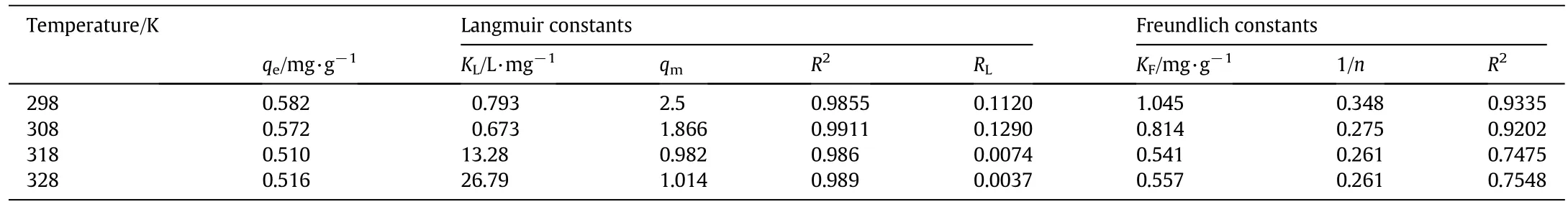
Table 2 Langmuir and Freundlich isotherm parameters for adsorption of Cu(II)onto Ghezeljeh nanoclay

Table 3 Langmuir and Freundlich isotherm parameters for adsorption of Hg(II)onto Ghezeljeh nanoclay
6.2.Freundlich isotherm
The Freundlich isotherm model assumes that the solid surface is heterogeneous,nonspecific and the energy of sorption is not constant.This model also assumes the multilayer adsorption,but no definite mechanism could be arrived at.The isotherm is valid for weak van der Waals type adsorption as well as for strong chemisorption.It is an empirical equation suitable for high and middle range of solute concentration but not for low concentrations.The linear form of the Freundlich equation is given in the following equation[59].

where qedefines the amount of metal species adsorbed at equilibrium in mg·g-1,Ceis the solute equilibrium concentration in mg·L-1,KFand n are Freundlich constants related to the adsorption capacity and intensity of adsorption,respectively.KFand n were determined from plot of lg qevs.lg Ce(Tables 2 and 3).A favorable adsorption has n values in the range of 1-10;for this study,we found a favorable value for n.
6.3.Dubinin-Radushkevich isotherm
The Dubinin-Radushkevich isotherm is more general since it does not assume a homogeneous surface or constant sorption potential.It is used to distinguish between the physical and chemical adsorption of metal ions on surfaces[57].The Dubinin-Radushkevich equation is given by Eq.(6):

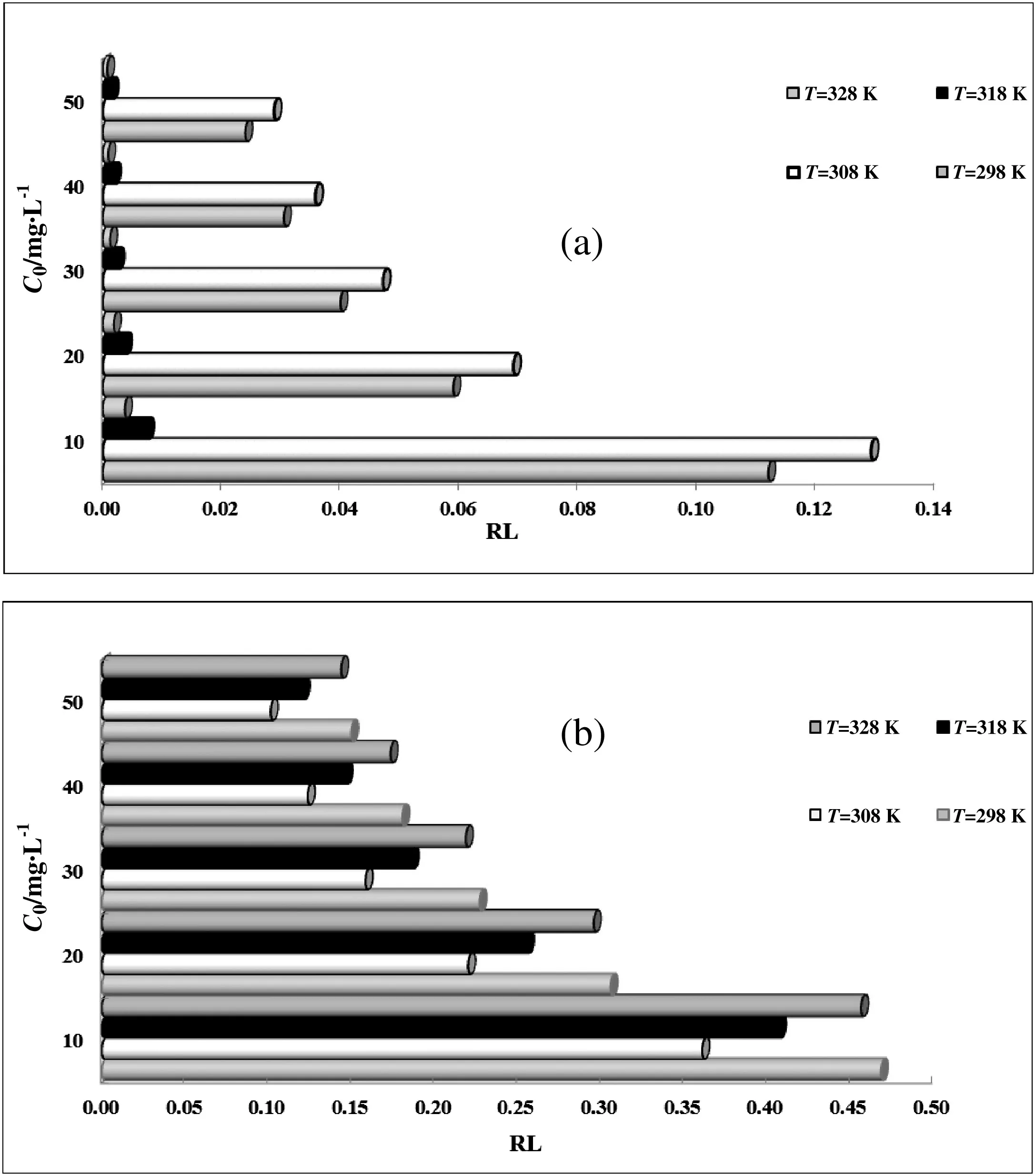
Fig.6.Separation factor for adsorption of(a)Cu(II)and(b)Hg(II)by Ghezeljeh nanoclay.
where qeand qmhave the same meaning as before,K·mol-2·J-2is a constant related to the adsorption energy,and ε is the maximum adsorption capacity given by Eq.(7):

R(J·mol-1·K-1)is the gas constant,and T(K)is the absolute temperature.The constant k gives the mean free energy E(kJ·mol-1)of sorption per molecule of the sorbate when it is brought to the surface of the solid from an in finite distance in the solution.E is used to evaluate the type of adsorption process.If E < 8 kJ·mol-1,adsorption process is of a physical nature whereas,if value 8 < E < 16 kJ·mol-1,the adsorption process can be explained by ion exchange mechanism(chemical adsorption).E can be calculated using Eq.(8)and indicates the mean energy for bringing a mol of adsorbate from infinity to the surface of the adsorbent[60].

Small values of E show that the adsorption process is a physical nature(Tables 4 and 5).
6.4.Temkin isotherm
Temkin's isotherm,considers the effects of the heat of adsorption that decreases linearly with coverage of the adsorbate and adsorbent interactions[47].The Temkin's isotherm has been used in the form as follows:

where B=RT/b,R is gas constant(8.314 J·mol-1·K-1),T is the temperature(K),KTis equilibriumbinding constant(L·g-1);b is related to heat of adsorption(J·mol-1).The sorption data can be analyzed using Eq.(10).Therefore,a plot of qeversus ln Ceenables one to determine the constants as shown in Tables 4 and 5.
Data from Fig.5 and Tables 2-5 show that Langmuir and Freundlich model acceptably fit the experimental data for Hg(II),but the equilibrium nature of Cu(II)adsorption has been described by the Langmuir isotherm.The similar studies of adsorption metal ions on various adsorbents are reported in the literature.Langmuir adsorption capacity for Cu(II)adsorption on natural clinoptilolite and synthetic zeolites has been shown to be 5.91 and 50.5 mg·g-1,respectively,by Alvarez-Ayuso et al.[61].The adsorption of Hg(II)on 2-mercaptobenzimidazole loaded natural clay follows Langmuir equation with an adsorption capacity of 12.49 mg·g-1[62].Adsorption of Cu(II)on raw kaolinite has been shown to follow Langmuirisotherm with a maximumadsorption capacity of 11.0 mg·g-1[63].The retention of Cu(II)on Na-bentonite and Cabentonite was favored by increase of pH with maximum Langmuir capacities of 30.0 and 7.7 mg·g-1,respectively[64].Lin and Juang[65]tried to modify the adsorptive properties of montmorillonite by introducing sodium dodecylsulfate before adsorbing Cu(II)and adsorption increased with the solution pH.However,Langmuir adsorption capacity of 0.02 mg·g-1for the modified montmorillonite did not show significant increase in the number of adsorption sites.De Leon et al.[66]have found a Langmuir monolayer adsorption capacity of 110.0 mg·g-1for adsorption of Cu(II)on a 1:10 phenanthroline-grafted Brazilian bentonite.Zeng and Jiang[16,67]tried to modify bentonite and montmorillonite with incorporation of various polymeric species for finding suitable adsorbents for Cu(II),and follow Freundlich equation.
7.Adsorption Kinetics
In order to test the kinetics of clay-metal interactions and evaluate the kinetic mechanism for the adsorption of metal ions onto Ghezeljeh montmorillonite nanoclay,and its potential rate-controlling steps that contain masstransport and chemical reaction processes,different kinetic models like pseudo- first-order,pseudo-second-order,and intraparticle diffusion were employed[68].A good correlation of the kinetic data explains the adsorption mechanism of the metal ions on the solid phase.
7.1.Pseudo- first-order model
Pseudo- first-order model is generally expressed as follows[69].

where qeis the amountofmetalions adsorbed per unit weight of adsorbent at equilibrium i.e.,adsorption capacity(mg·g-1),qtis the amount of adsorbent adsorbed(mg·g-1)at any time t and k1is the rate constant.The value of k1was calculated from the slope of the linear plot of lg(qe-qt)versus t(Tables 6 and 7).

Table 4 Dubinin-Radushkevich and Temkin isotherm parameters for adsorption of Cu(II)onto Ghezeljeh nanoclay

Table 5 Dubinin-Radushkevich and Temkin isotherm parameters for adsorption of Hg(II)Ghezeljeh nanoclay

Table 6 Adsorption kinetics models and intraparticle diffusion model parameters for copper(II)adsorption

Table 7 Adsorption kinetics models and intraparticle diffusion model parameters for Hg(II)adsorption
7.2.Pseudo-second-order rate model
Pseudo-second-order rate model is given as follows:

where k2is the rate constant.The values of k2can be determined from the plot of t/qtversus t;see Fig.7 and Tables 6 and 7.
7.3.Intra-particle diffusion model(Waber-Morris model)
The uptake of adsorbate by the sorbent from solutions involves several steps which include:bulk diffusion, film diffusion,intraparticle diffusion in the solid phase and within the pores,and finally adsorption on the sites.To determine the rate-controlling step,intraparticle diffusion model was applied to adsorption kinetic data as given by the following equation[70].

where qtis the amountofmetalions adsorbed onto Ghezeljeh montmorillonite nanoclay at time t,and kintis the rate constant for intraparticle diffusion.Fig.8 shows a plot of qtvs.t1/2.Significantly,the plots have a zero intercept,indicating that the diffusion of metal species into the pores is the dominating factor controlling the mechanism of the process[48].It may present multi-linearity which indicates two or more steps happening in the adsorption process.The first sharper portion(t≤20 min)is the external surface adsorption or instantaneous adsorption stage.The second portion is the gradual adsorption stage where the intraparticle diffusion rate is controlled.The third is the final equilibrium stage where intraparticle diffusion starts to slow down due to extremely low solute concentration in the solution.The parameters calculated are given in Tables 6 and 7.The value of kintwas higher at the higher concentrations.The multi-stepped adsorption observed for all the metal ions and best fitting obtained for the experimental data with high regression coefficient values indicates that pseudo second order kinetic model might play a significant role in the adsorption of metal ions onto Ghezeljeh montmorillonite nanoclay.Similar kinetic studies for metal ions on various adsorbents are reported in the literature.The clay-Cu(II)interactions have been shown to follow a second order mechanism with rate coefficients of 15.4×10-2(montmorillonite)[71,72].Lin and Juang[65]have proposed a pseudo- first order kinetics with a rate coefficient of 3.14×10-3min-1at 298 K.Wang et al.[73]proposed second order kinetics for adsorption of Cu(II)with the rate coefficient varying from 0.238 to 0.399 g·mg-1·min-1in the temperature range of 293 to 313 K.Chen et al.[30]have reported that adsorption Cu(II)onto the palygorskite followed pseudo-second-order kinetic model with a relatively small contribution of film diffusion.The equilibrium experimental data fit well with the Freundlich isotherm.
8.Determination of Thermodynamic Parameters
Three thermodynamic parameters free energy change(ΔG0),enthalpy change(ΔH0)and entropy change(ΔS0)were determined by the following equations:

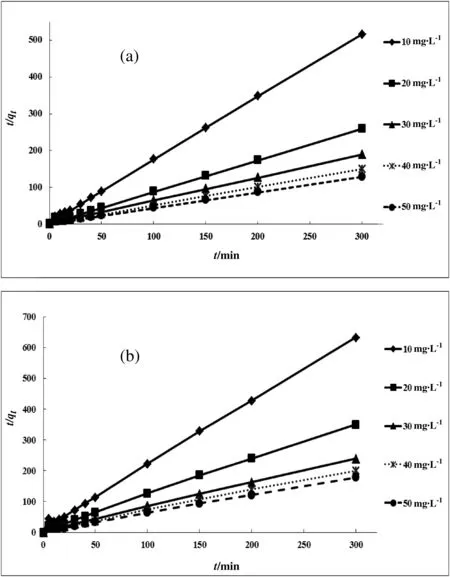
Fig.7.Pseudo second order kinetic model(a)Cu(II)and(b)Hg(II)adsorption on Ghezeljeh nanoclay.
where R is the universal gas constant,8.314 J·mol-1·K-1;T is the absolute temperature(K),and kLis the Langmuir constant(mol·L-1).ΔS0and ΔH0could be obtained from the slope and intercept ln kLversus 1/T according to Eq.(17).Values of ΔS0,ΔH0and ΔG0are shown in Tables 8 and 9.The negative values of standard enthalpy change(ΔH0)for the intervals of temperatures showed the exothermic nature of the adsorption process(for Cu(II)ion).While,some literatures showed that clay-Cu(II)interaction is endothermic[48,49,72].Eren and Afsin[29]have reported that Cu(II)sorption onto the bentonite sample occurs in two stages.The first stage involves adsorption on permanent charge sites and the second involves adsorption on variable charge sites.The endothermic nature of the processes can be accounted for by the partial dehydration of Cu(II)before its sorption on the bentonite samples.The endothermic and exothermic natures of the adsorption process are controlled by the adsorbate-adsorbent and adsorbate adsorbate forces.Ghezeljeh montmorillonite nanoclay-Cu(II)interaction is exothermic,because the adsorbate have a tendency to escape from the solid phase to the solution.Moreover,rise in temperature and excess energy supply promote desorption(migration of metal ions from the solid to the liquid phase).Such results are not uncommon for adsorption of metal ions from the aqueous phase[74].The effect of increasing temperature is quite different in the case of Hg(II)ion.The positive values of ΔH0for mercury ions confirm the endothermic nature of the sorption process and can be explained that the adsorption of Hg(II)ions has to displace more than one water molecule and therefore,the net results correspond to an endothermic process.It is clear from the results that the adsorbate-adsorbent force becomes strong in comparison to the adsorbate-adsorbate force as the temperature increases[55].It is possible that increasing temperature could have increased the driving force of the metal ions toward the active sites on clay[48,62].The magnitude of the adsorption enthalpy,ΔH,indicates moderately strong bonding between the metal ions and the clay minerals.The low enthalpy values of ΔH0< 20 kJ·mol-1indicate that the physisorption is involved in the process of adsorption.The estimated values of ΔH0for the present system were greater than 20 kJ·mol-1and hence,the process may involve a spontaneous sorption mechanism as ion exchange where chemical bonds are not of strong energies[75].The clay-metal interactions take place spontaneously and are accompanied by decrease in Gibbs energy.It is cleared from Tables 8 and 9 that ΔG0values were found to be negative at lower temperatures and concentration copper ion and became positive at higher temperatures and concentration copper ion.The increase of ΔG0values simultaneously with the temperature shows that the adsorption was unfavorable at higher temperatures.Decreasing the positive values of ΔG0for Hg(II)ion with the increase of sorption temperature confirms the better sorption at a higher temperature.The endothermic interactions are driven by entropy increase,which illustrates increased randomness at the solid solution interface indicating strong affinity of the adsorbent for Hg(II).The negative values of ΔS0for Cu(II)ion correspond to a decrease in degree of freedom of the adsorbed species.The negative value ΔS0obtained for Cu2+indicates a stable configuration of the metal ion on the adsorbent surface(more stable formation of Ghezeljeh montmorillonite nanoclay-Cu2+complex),while the positive value for Hg2+suggests some structural changes in the adsorbent and adsorbate after adsorption reaction[62,76].Yuvaz et al.[63]reported the thermodynamic parameters for adsorption of Cu(II),Ni(II),Co(II),and Mn(II)on Turkish kaolinite.ΔH and ΔS values were+39.52 kJ·mol-1and+11.7 J·K-1·mol-1for Cu(II),+37.27 kJ·mol-1and+10.7 J·K-1·mol-1for Ni(II),+21.52 kJ·mol-1and+5.4 J·K-1·mol-1for Co(II),and+36.73 kJ·mol-1and+10.1 J·K-1·mol-1for Mn(II).Echeverria et al.[77]have reported that ΔH,ΔS,and ΔG for adsorption of Ni(II)onto illite have values of+16.8 kJ·mol-1,+58 J·mol-1·K-1,and-1.04 kJ·mol-1,respectively.Lin and Juang[65]investigated the adsorption of Cu(II)and Zn(II)onto montmorillonite modified with sodium dodecyl sulfate.The values of ΔH,ΔS,and ΔG were reported as 7.05 kJ·mol-1,9.09 J·K-1·mol-1,and-9.66 kJ·mol-1,respectively,for Cu(II)and 7.39 kJ·mol-1,6.39 J·K-1·mol-1,and-9.17 kJ·mol-1,respectively,for Zn(II)at 298 K.Bhattacharyya and Gupta[78]reported the thermodynamic parameters for adsorption of Cd(II),Co(II),Cu(II),Pb(II),and Ni(II)on montmorillonite.ΔH and ΔS values were+50.7 kJ·mol-1and+180.7 J·K-1·mol-1for Cu(II),+40.2 kJ·mol-1and+147.5 J·K-1·mol-1for Cd(II),-15.1 kJ·mol-1and -47.1 J·K-1·mol-1for Co(II),-45.2 kJ·mol-1and-146.4 J·K-1·mol-1for Ni(II),and -75.5 kJ·mol-1and-235.9 J·K-1·mol-1for Pb(II).Manohar et al.[62]investigated the adsorption of Hg(II)onto 2-mercaptobenzimidazole loaded natural clay.The values of ΔH,ΔS,and ΔG were reported as 34.81 kJ·mol-1,189.82 J·K-1·mol-1,and-22.64 kJ·mol-1,respectively.Adsorption of Cd(II)on hematite[79],adsorption of Fe(III)on montmorillonite[78],adsorption of Cu(II),Zn(II),Cd(II)and Ni(II)on synthesized silicoantimonate ion exchanger[74],etc.,have already been reported as exothermic[78].Cu(II)-kaolinite interactions have endothermic nature with adsorption enthalpy of+39.5 kJ·mol-1driven by an entropy gain of+11.7 J·K-1·mol-1[63].Adsorption on sodium dodecyl sulfate-montmorillonite was also endothermic in nature with enthalpy of+7.05 kJ·mol-1and entropy of+9.09 J·K-1·mol-1,the decrease in Gibbs energy of-9.66 kJ·mol-1[65]makes the interactions spontaneous.Bhattacharyya and Sen Gupta[71,72]have also observed an endothermic path for Cu(II)adsorption on kaolinite,montmorillonite and their modified forms.Endothermic adsorption of Cu(II)on natural kaolinite has also been shown by other workers[73].Doula et al.[80]have observed earlier that Cu(II)interactions with Ca-kaolinite were exothermic in nature.Strawn et al.[34]have reported adsorption of Cu(II)by smectites(montmorillonite and bedellite)as a function of varying ionic strength and pH.The X-ray adsorption fine structure(XAFS)and electron paramagnetic resonance(EPR)spectroscopic experiments on Cu(II)adsorbed on clays have revealed that Cu-Cu linkages in the multinuclear complexes were 0.265 nm aparthaving a coordination number near one.

Fig.8.Intraparticle diffusion for(a)Cu(II)and(b)Hg(II)adsorption on Ghezeljeh nanoclay.
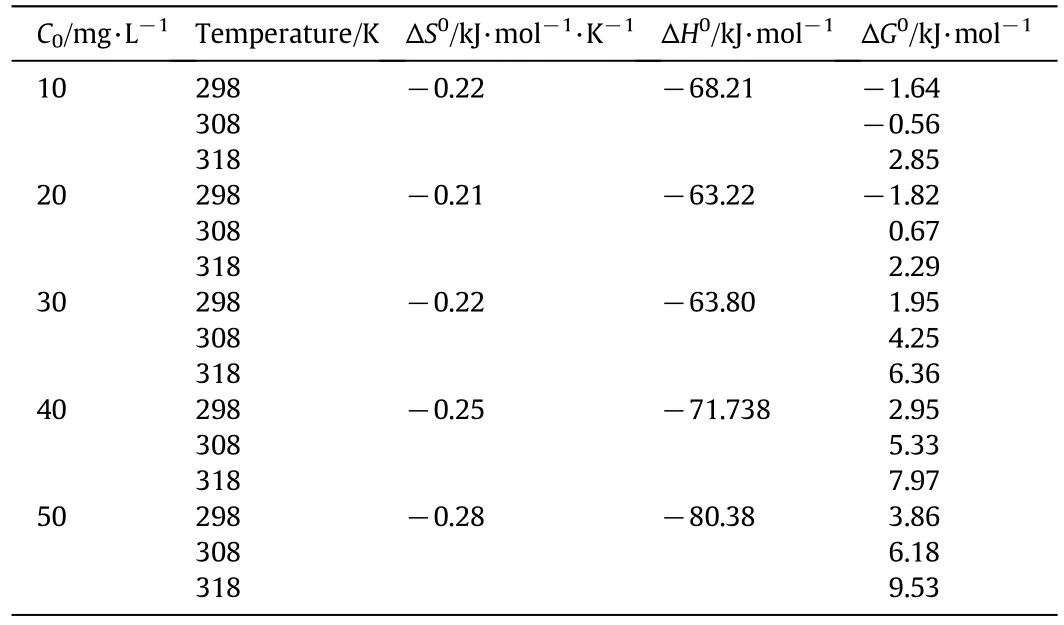
Table 8 Thermodynamic parameters of Cu(II)adsorption onto Ghezeljeh nanoclay
9.Conclusions
This study presented the extraction and removal of Hg(II)and Cu(II)ions from aqueous solutions using Ghezeljeh montmorillonite nanoclay as a new natural adsorbent.This nanoclay was characterized by using FT-IR,SEM-EDS,XRF and XRD.Based on SEM images of Ghezeljeh clay,it can be seen that the distance between the plates is Nano.The effects of varying parameters were examined.Both Langmuir and Freundlich isotherms yield good fits and the adsorption coefficients agree well with the conditions supporting favorable adsorption.Data show that Langmuir and Freundlich model acceptably fit the experimental data for Hg(II),but the equilibrium nature of Cu(II)adsorption has been described by the Langmuir isotherm.Adsorption of Hg(II)and Cu(II)ions onto Ghezeljeh nanoclay obeyed the pseudo-secondorder kinetic model.Calculation of ΔG0,ΔH0and ΔS0showed that the nature of Hg(II)ion sorption onto the Ghezeljeh nanoclay was endothermic and was favored at higher temperature,and the nature of Cu(II)ion sorption was exothermic and was favored at lower temperature.Previous reports indicate clay-Cu(II)interaction as an endothermic process.However,the clay-metal interactions are spontaneous.
Acknowledgments
The authors are grateful for the financial support for this work by the Imam Khomeini International University(IKIU),and Mines and Mining Industries Development and Renovation Organization of Iran(IMIDRO).
 Chinese Journal of Chemical Engineering2015年11期
Chinese Journal of Chemical Engineering2015年11期
- Chinese Journal of Chemical Engineering的其它文章
- N-methyl-2-(2-nitrobenzylidene)hydrazine carbothioamide—A new corrosion inhibitor for mild steel in 1 mol·L-1 hydrochloric acid
- A dual-scale turbulence model for gas-liquid bubbly flows☆
- Gas-liquid hydrodynamics in a vessel stirred by dual dislocated-blade Rushton impellers☆
- Convective mass transfer enhancement in a membrane channel by delta winglets and their comparison with rectangular winglets☆
- Cobalt-free gadolinium-doped perovskite Gd x Ba1-x FeO3-δas high-performance materials for oxygen separation☆
- Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid☆
