胃转流手术对糖尿病大鼠肝脏糖代谢相关因子表达的影响
李旭忠,颜 勇,周 舟,邱文娟,周联明,黄忠明,胡 承,张学利*
胃转流手术对糖尿病大鼠肝脏糖代谢相关因子表达的影响
李旭忠1,颜 勇2,周 舟3,邱文娟1,周联明2,黄忠明2,胡 承3,张学利2*
(1苏州大学研究生院,苏州 215000;2上海交通大学附属第六人民医院南院普外科,上海 201400;3上海交通大学附属第六人民医院内分泌代谢科,上海市糖尿病研究所,上海 200233)
通过建立2型糖尿病(T2DM)胃转流手术(GBP)大鼠模型,探讨对肝脏糖代谢相关因子表达的影响。将12只SD大鼠随机分为正常对照组(NCD,=4)、高脂组(HFD,=4),高脂手术组(HFD-GBP,=4);HFD组大鼠经高脂饲料喂养4周后,通过腹腔注射链脲佐菌素构建T2DM模型,比较两组体质量、血糖、口服葡萄糖耐量实验(OGTT)、胰岛素耐量实验(ITT)的变化。2周后,构建HFD-GBP组胃转流手术模型,连续观察2周,比较手术前后两组体质量、血糖浓度的变化,并分别提取3组大鼠肝组织的总RNA和蛋白,通过RT-PCR、Western印迹法检测法呢醇X受体(FXR)、磷酸烯醇式丙酮酸羧基激酶(PEPCK)、葡萄糖−6−磷酸酶(G6Pase)、葡萄糖转运体2(GLUT2)和成纤维细胞生长因子-21(FGF-21)的表达水平。与HFD组相比,HFD-GBP组大鼠体质量下降,血糖改善(<0.05),术后FXR和GLUT2 mRNA表达明显上调(<0.05),PEPCK、G6Pase和FGF-21 mRNA表达也增加;与NCD组相比,HFD和HFD-GBP两组FXR和PEPCK蛋白表达明显升高(<0.05)。在T2DM大鼠模型中,GBP能有效改善糖代谢;FXR、PEPCK和GLUT2可能参与GBP术后的血糖调节过程。
胃转流术;糖尿病,2型;法呢醇X受体;磷酸烯醇式丙酮酸羧基激酶
减重手术包括胃转流术(gastric bypass,GBP)、袖状胃切除术、胆胰转流术、回肠转位术等手术方式。目前GBP和袖状胃切除术在临床上应用相对较多[1]。近年来对接受减重手术的患者研究发现,对伴2型糖尿病(type 2 diabetes mellitus,T2DM)的肥胖患者行GBP术后,在减重的同时,能在一定时间内缓解糖尿病,并能减少并发症的发生[2−4]。基于减重手术对T2DM的疗效,国际糖尿病联盟(International Diabetes Federation,IDF)正式发表声明,认为以GBP为代表的减重手术可用于治疗T2DM。本研究拟通过建立T2DM GBP模型,並检测术后体质量、血糖和糖代谢相关因子的变化,进一步明确GBP术对糖代谢的影响,更为明确手术治疗糖尿病的机制提供新的线索。
1 材料与方法
1.1 实验器材和试剂
磷酸烯醇式丙酮酸羧基激酶(phosphoenolpyr-uvate carboxykinase,PEPCK)单克隆抗体(sc-32879)(Santa Cruz公司),法呢醇X受体(farnesoid X receptor,FXR)抗体(ab126602)(ABACAM公司)。中国三诺微量血糖仪及血糖试纸;Real-time PCR扩增仪(美国ABI7500公司);酶标仪(美国Beckman公司)。高脂饲料(上海斯莱克实验动物有限责任公司)。
1.2 实验动物和方法
普通级6周龄Sprague-Dawley(SD)大鼠(上海西普尔−必凯实验动物有限公司)。SD大鼠12只,随机分为正常对照组(normal chow diet,NCD)4只、高脂组(high-fat diet,HFD)4只、高脂手术组(high-fat diet followed by GBP,HFD-GBP)4只。HFD组高脂饲料喂养4周后,腹腔注射链脲佐菌素(streptozocin,STZ)35mg/kg,构建T2DM模型。2周后构建T2DM GBP模型。
1.3 手术方法
术前禁食16h,不禁水。1%戊巴比妥钠0.5ml/100g左或右腹腔注射麻醉,麻醉成功后,腹部备皮,将大鼠固定于手术台上,术野常规碘伏消毒后,铺无菌洞巾。取上腹正中切口,上至剑突,下至脐部,依次切开皮肤、皮下组织,钝性分离肌肉,打开腹膜。分离并以4−0丝线结扎胃周韧带与血管,于胃小弯处距食管胃交界处3~5mm向大弯侧斜行断胃,断端以5−0丝线连续缝合关闭,并以4−0丝线间断缝合加强,旷置远端胃大部,保证留下的胃小囊约占胃总容积的20%。旷置十二指肠+近端1/5空肠,即距离屈氏韧带10cm处离断空肠,空肠远端与残胃行侧端吻合,近端与胃肠吻合口以远15cm处行端侧吻合。各组关腹前用生理盐水擦拭腹腔,检查有无活动性出血。以3−0丝线关闭腹膜及腹肌,4−0丝线间断缝合皮肤。
1.4 检测项目和方法
术前每周禁食12h后,测定NCD组和HFD组大鼠体质量和血糖,高脂喂养4周后注射STZ(35mg/kg),每隔3d检测血糖变化,术前1周行口服葡萄糖耐量实验(oral glucose tolerance test,OGTT)、胰岛素耐量实验(insulin tolerance test,ITT)。手术当天测定各组空腹状态下大鼠体质量和血糖。术后观察2周,每周测定各组大鼠体质量和血糖。2周后,提取各组大鼠肝组织备用。通过逆转录−聚合酶链反应(reverse transcription- polymerase chain reaction,RT-PCR)和Western印迹法检测各组大鼠肝中FXR、PEPCK、葡萄糖−6−磷酸酶(glucose-6-phosphatase,G6Pase)、葡萄糖转运蛋白2(glucose transporter 2,GLUT2)和成纤维细胞生长因子−21(fibroblast growth factor-21,FGF-21)相对表达情况。
1.5 实时荧光定量法(RT-PCR)
在NCBI(National Center for Biotechnology Information)中查找目的基因的信使RNA(messenger ribonucleic acid,mRNA),应用Primer Premier5.0设计引物,NCBI-blast验证PCR引物。FXR引物为5¢-GCCTCTGCTCGATGTCCTACA-3'和5¢-GGAGGCAGGCGAAATGCT-3';FGF-21引物为5¢-ACCGGAGTCAGAACACAATTCC-3'和5¢-AACTCTAGATCTCATCCATTCCATCA-3¢;G6Pase引物为5¢-CCCAGACTAGAGATCCTGACAGAAT-3¢和引物5¢-GCACAACGCTCTTTTCTTTTACC-3¢;GLUT2引物为5¢-TGACCGAAGAGCTACCATTAACTATG-3¢和5¢-GTGTCGTATGTGCTGGTGTGACTSYBR-3¢;PEPCK引物为5¢-GCCTGTGGGAAAACCAACCT-3'和5¢-CACCCACACATTCAACTTTCCA-3¢;引物均由上海BioTNT公司合成。Premix EX Taq(TaKaRa公司),Light Cycler 480实时荧光定量PCR系统(Roche公司)。PCR反应预变性95℃ 5min,变性95℃ 5s,退火60℃30s,反应40个循环。每个基因重复操作3次,运用2−△△Ct法分析结果。
1.6 Western印迹法
进行配胶、蛋白变性及上样、蛋白电泳、转膜及封闭操作后,FXR一抗(1∶2000)、PEPCK一抗(1∶500),4℃孵育过夜后室温孵育1h,洗膜后二抗(1∶2500)室温孵育2h后洗膜,按ECL试剂盒(Pierce公司)说明书操作显影后运用分析软件Gel-Pro analyzer 4分析条带灰度值计算蛋白相对表达量。
1.7 统计学处理
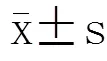
2 结 果
2.1 构建T2DM大鼠模型
HFD组高脂喂养大鼠4周,各组大鼠体质量、血糖均无统计学差异(图1A,1B;>0.05)。HFD组腹腔注射STZ后,HFD组血糖明显升高,糖耐量和胰岛素敏感性明显降低(图1C,1D;<0.05)。
2.2 手术前后大鼠体质量和血糖的变化
与术前(0周)相比,HFD-GBP组术后1周体质量及空腹血糖显著降低(<0.05),NCD和HFD组同时间点体质量和血糖与0周比较差异无统计学意义(表1,表2;>0.05)。
2.3 3组大鼠肝组织中FXR的表达变化
3组大鼠肝FXR mRNA相对表达量:NCD组为3325.0±589.8,HFD组5093.0±532.8,HFD-GBP组8835.0±165.6。FXR蛋白相对表达量:3组分别为1.201±0.634,6.230±3.265,3.723±0.808。FXR mRNA表达,NCD组与HFD组差异无统计学意义(=2.015,>0.05),NCD和HFD-GBP组差异有统计学意义(=8.994,<0.01);HFD和HFD-GBP组差异有统计学意义(=4.645;<0.05;图2A)。FXR蛋白相对表达NCD组与HFD组、HFD-GBP组差异均有统计学意义(=1.834,<0.05;=3.473,<0.05);HFD组和HFD-GBP组差异无统计学意义(=0.912,>0.05;图2B)。
2.4 3组大鼠肝组织中PEPCK的表达变化
3组大鼠肝PEPCK mRNA相对表达量:NCD组为47460±3643,HFD组为49850±1480,HFD-GBP组为68940±2667。PEPCK蛋白相对表达量:3组分别为1.538±1.907,为3.694±2.470,3.284±0.749。PEPCK mRNA表达,NCD组与HFD组差异无统计学意义(=2.015,>0.05),NCD和HFD-GBP组差异有统计学意义(=4.475,<0.05);HFD和HFD-GBP组差异无统计学意义(=0.693;>0.05;图3A)。PEPCK蛋白相对表达,NCD组与HFD组、HFD-GBP组差异均有统计学意义(=1.063,<0.05;=1.204,<0.05);HFD组和HFD-GBP组差异无统计学意义(=0.218,>0.05;图3B)。
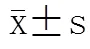
表1 手术前后大鼠体质量比较
NCD: normal chow diet; HFD: high-fat diet; HFD-GBP: high-fat diet followed by gastric bypass. Compared with pre-operation (0-week) in the same group,*<0.05
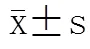
表2 手术前后大鼠空腹血糖比较
NCD: normal chow diet; HFD: high-fat diet; HFD-GBP: high-fat diet followed by gastric bypass. Compared with pre-operation (0-week) in the same group,*<0.05
2.5 3组大鼠肝脏组织中G6Pase,GLUT2及FGF-21 mRNA的表达变化
3组大鼠肝G6Pase mRNA相对表达量:NCD组为28 430±2599,HFD组10 400±739,HFD-GBP组为14610±3207。FGF-21 mRNA相对表达量:3组分别为337.4±24.1,172.9±78.6,254.3±6.1。GLUT2 mRNA相对表达量:分别为1041±59,864±52,3931±189。G6Pase和FGF-21 mRNA各组间差异无统计学意义(>0.05;图4A,4B)。GLUT2 mRNA表达,NCD组与HFD组无统计学差异(>0.05),但和HFD-GBP组相比较差异均有统计学意义(=3.463,<0.05;=4.185,<0.05;图4C)。
3 讨 论
减重手术用于治疗T2DM已逐渐被医学界所熟知,尤其对肥胖合并T2DM的患者效果更显著,术后在体质量下降的同时,血糖也得到了明显改善。Mingrone等[5]在随机对照研究中,将60例伴有T2DM的病态肥胖患者随机分为内科治疗组、GBP组及胆胰转流术组,研究术后T2DM缓解率,结果2年时内科治疗组缓解率为0%,GBP组及胆胰转流术组缓解率分别为75%和95%。Schauer等[6,7]分析随机对照研究150例伴有T2DM的肥胖患者,接受单独内科治疗、内科治疗+GBP及内科治疗+袖状胃切除术后1年和3年的血糖控制情况,发现减肥手术+内科治疗明显优于单独内科治疗。可见,一定时间内减肥手术可以治疗T2DM。虽然减重手术在治疗糖尿病中疗效显著,但是,减重手术治疗糖尿病的作用机制仍未揭晓。手术前后胃肠道激素、胆汁酸代谢、肠道菌群的变化,是目前研究的热点。Pournaras等[8]在人体和动物研究中得出GBP术后血清胆汁酸水平升高,可刺激肠道高血糖素样肽−1、多肽YY等激素分泌,进而达到改善T2DM的效果。Ryan等[9]在袖状胃切除术肥胖小鼠模型中发现,术后小鼠体质量下降的同时,循环中胆汁酸水平增加,肠道微生物菌群也发生了显著变化。Turnbaugh等[10,11]则将肥胖小鼠的肠道微生物移植到无菌小鼠中,无菌小鼠体质量增加。而从GBP术后小鼠中移植的微生物却减慢了体质量的增加[12]。可见,减重手术可通过影响胃肠道激素、胆汁酸代谢、肠道菌群变化等多方面的作用改善糖尿病。
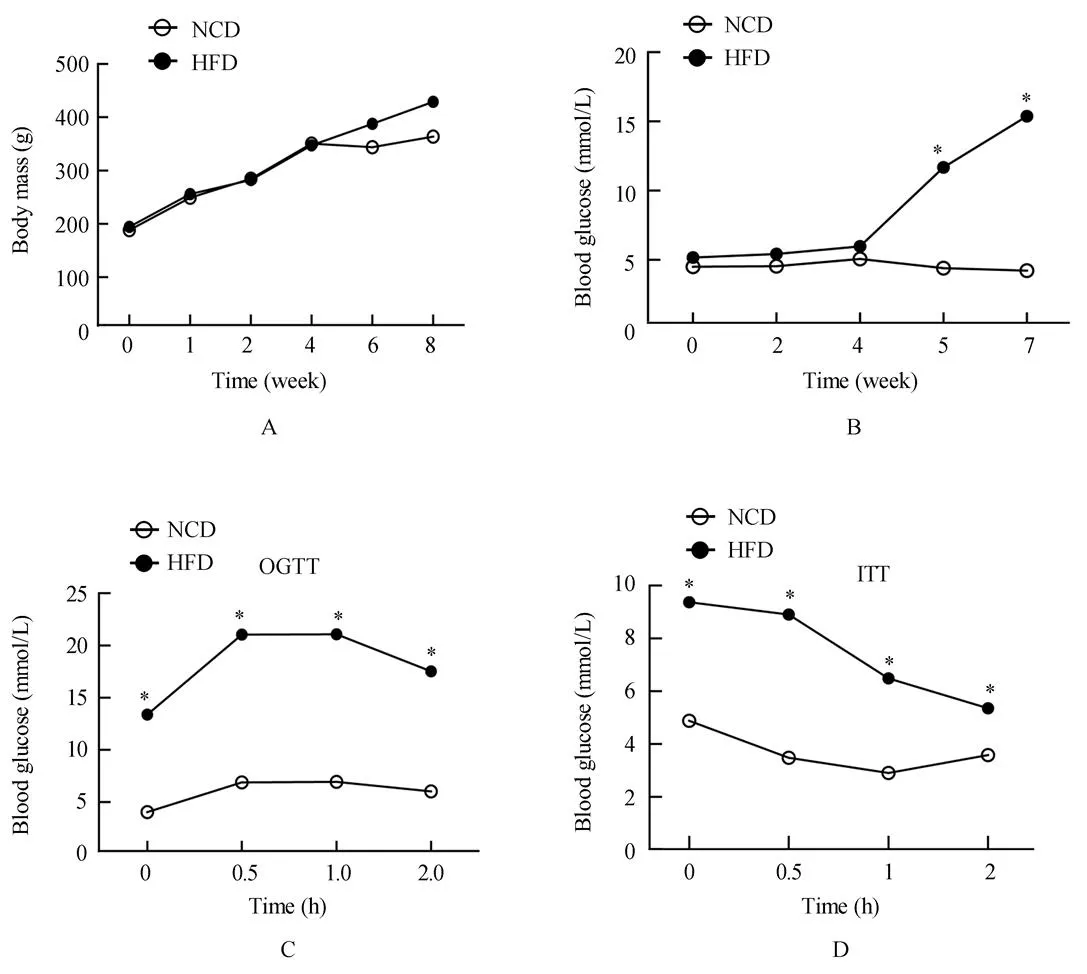
图1 T2DM大鼠模型的相关指标检测
Figure 1 Assessment of T2DM rat model
T2DM: type 2 diabetes mellitus; NCD: normal chow diet; HFD: high-fat diet; OGTT: oral glucose tolerance test; ITT: insulin tolerance test .Compared with NCD,*<0.05
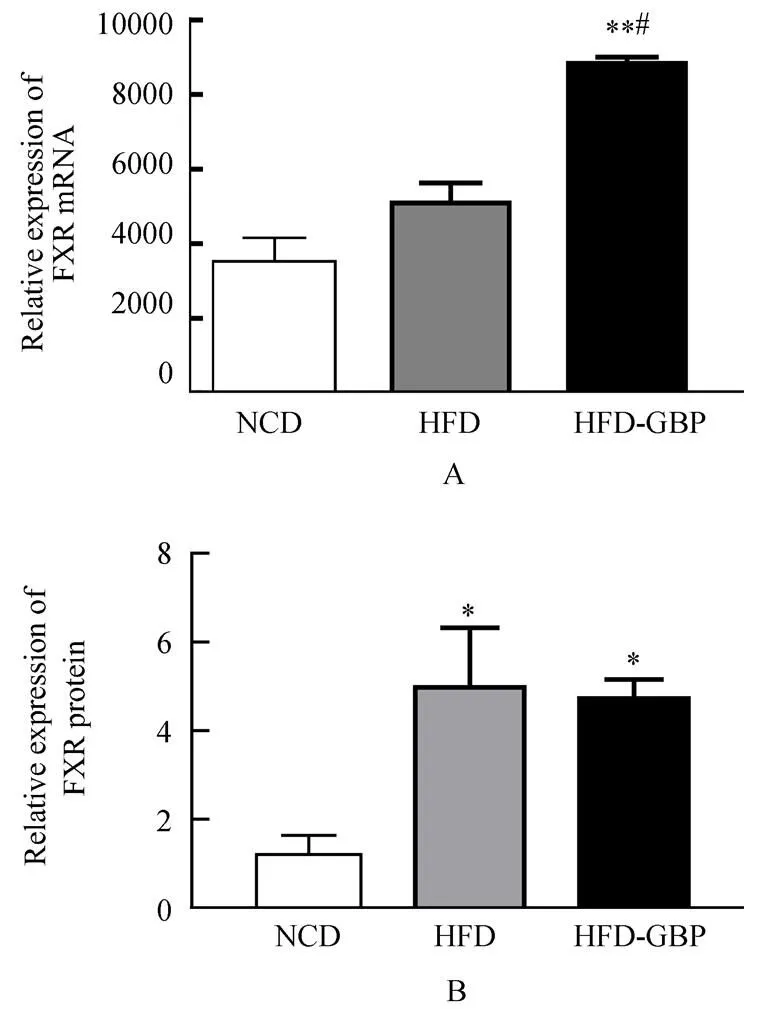
图2 3组大鼠肝组织中FXR表达
Figure 2 FXR expression in liver tissue in three groups
FXR: farnesoid X receptor; NCD: normal chow diet; HFD: high-fat diet; HFD-GBP: high-fat diet followed by gastric bypass.Compared with NCD,**<0.01; compared with HFD,#<0.05
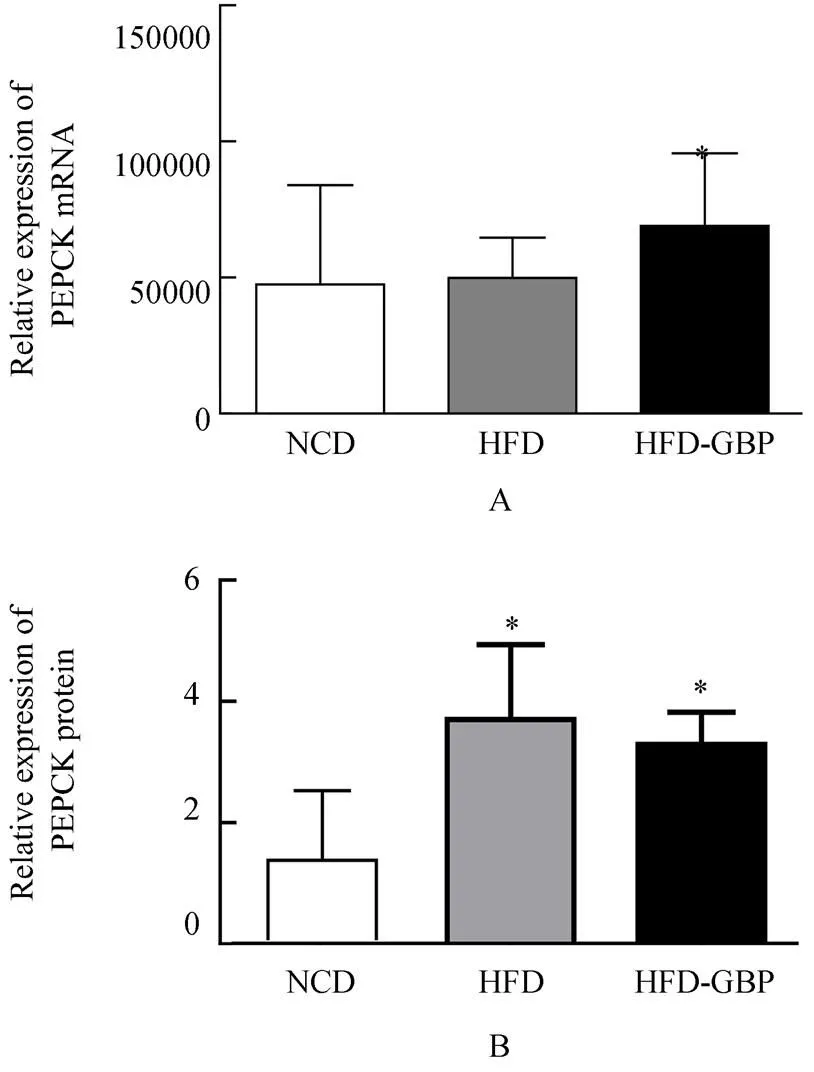
图3 3组大鼠肝组织中PEPCK表达
Figure 3 PEPCK expression in liver tissue in three groups
PEPCK: phosphoenolpyruvate carboxykinase; NCD: normal chow diet; HFD: high-fat diet; HFD-GBP: high-fat diet followed by gastric bypass. Compared with NCD,*<0.05
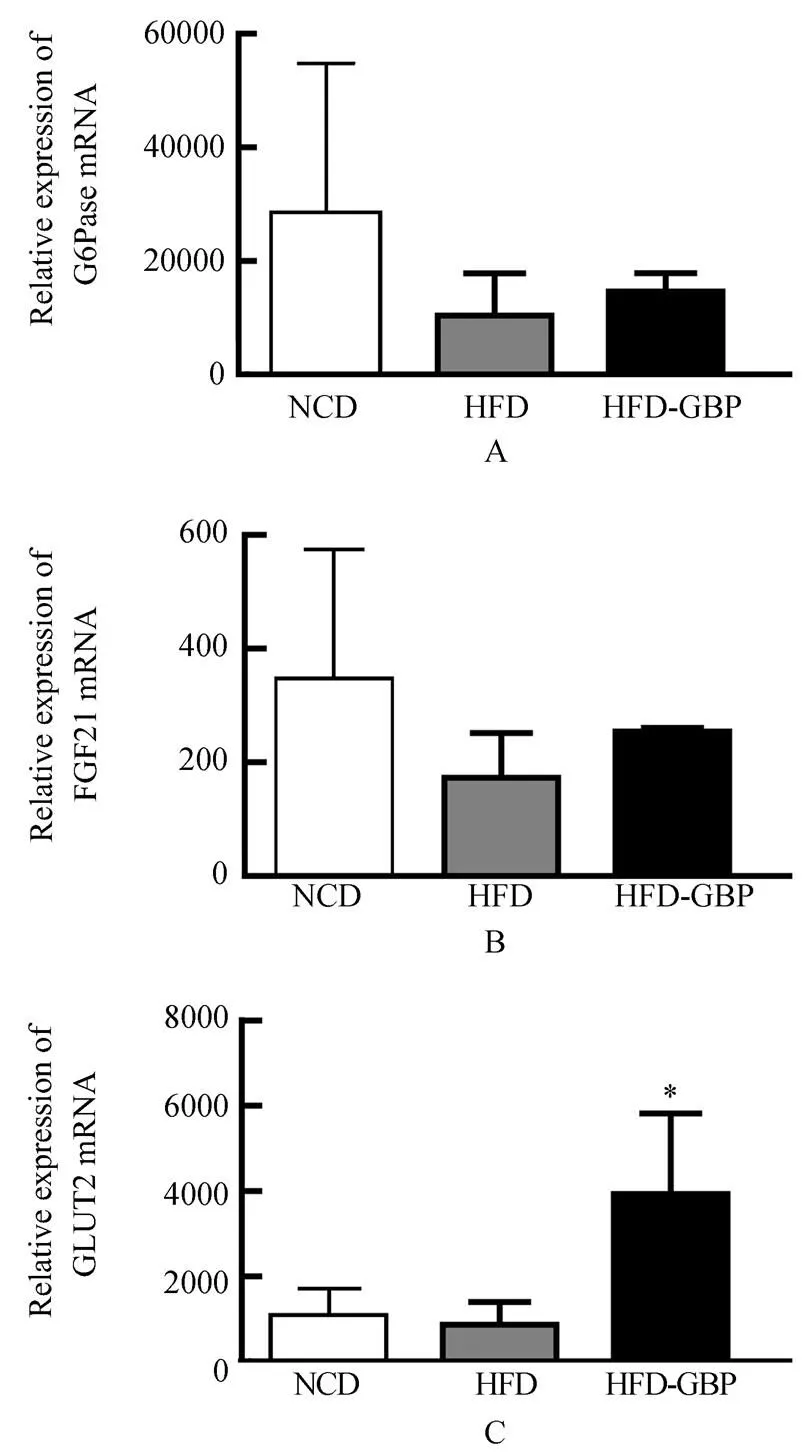
图4 3组大鼠肝组织中G6Pase、FGF-21和GLUT2 mRNA的表达
Figure 4 G6Pase, FGF-21 and GLUT2 mRNA expression in liver tissue in three groups
G6Pase: glucose-6-phosphatase; FGF-21: fibroblast growth factor-21; GLUT2: glucose transporter 2; NCD: normal chow diet; HFD: high-fat diet; HFD-GBP: high-fat diet followed by gastric bypass. Compared with NCD,HFD,*<0.05
本实验通过建立T2DM GBP模型观察GBP对糖代谢的影响,发现GBP在减轻体质量和改善血糖水平的同时,肝中相关糖代谢因子也发生变化。FXR在肝、小肠等器官中高表达。生理情况下,胆汁酸可激活FXR,因此也被称为胆汁酸受体。近年来研究发现胆汁酸−FXR信号通路可能在减重手术治疗糖尿病中发挥重要作用[9]。本研究发现,GBP在早期改善糖尿病的同时,术后肝组织中FXR的表达水平显著提高,说明FXR对糖代谢发挥重要作用。PEPCK是糖异生中的关键酶,主要在肝中表达。Ma等[13]发现胆汁酸通过FXR途径抑制糖异生限速酶PEPCK的表达,而Stayrook等[14]研究发现FXR能通过激活过氧化物酶体增殖物激活受体α而上调PEPCK的表达。本研究初步探索发现,与非手术相比,GBP后在改善糖代谢的同时,肝中PEPCK的表达增加。因此,可推断PEPCK也参与术后糖代谢稳态。GLUT2是介导葡萄糖摄取的膜蛋白,是肝、胰岛β细胞、小肠等器官或细胞最主要的葡萄糖转运体。在新西兰肥胖小鼠[15]以及Zuker肥胖糖尿病大鼠[16]等多种糖尿病动物模型中,胰岛β细胞GLUT2的表达减少。在这些模型中胰岛β细胞GLUT2的减少与葡萄糖刺激下胰岛素分泌能力下降密切相关。我们目前发现GBP后的T2DM大鼠肝中GLUT2 mRNA表达上调。但GLUT2是如何参与术后糖代谢,还需进一步研究。实验研究[17]发现T2DM患者行GBP术后血清中胆汁酸水平升高,血清中FGF-21也增加,由此推断术后FGF-21对糖代谢发挥有利影响。
由于本实验受样本量少及研究时间短等因素限制,研究尚不深入。总之,GBP已成为一种有效的干预T2DM的手术方式,其作用机制值得我们去探究,期望在糖尿病的治疗方式上有更新的突破。
[1] Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011[J]. Obes Surg, 2013, 23(4): 427−436.
[2] Adams TD, Davidson LE, Litwin SE,. Health benefits of gastric bypass surgery after 6 years[J]. JAMA, 2012, 308(11): 1122−1131.
[3] Pories WJ, Swanson MS, MacDonald KG,. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus[J]. Ann Surg, 1995, 222(3): 339−350; discussion 350−352.
[4] Morino M, Toppino M, Forestieri P,. Mortality after bariatric surgery: analysis of 13 871 morbidly obese patients from a national registry[J]. Ann Surg, 2007, 246(6): 1002−1007; discussion 1007−1009.
[5] Mingrone G, Panunzi S, De Gaetano A,. Bariatric surgeryconventional medical therapy for type 2 diabetes[J]. N Engl J Med, 2012, 366(17): 1577−1585.
[6] Schauer PR, Bhatt DL, Kirwan JP,. Bariatric surgeryintensive medical therapy for diabetes—3-year outcomes[J]. N Engl J Med, 2014, 370(21): 2002−2013.
[7] Schauer PR, Kashyap SR, Wolski K,. Bariatric surgeryintensive medical therapy in obese patients with diabetes[J].N Engl J Med, 2012, 36(17): 1567−1576.
[8] Pournaras DJ, Glicksman C, Vincent RP,. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control[J]. Endocrinology, 2012, 153(8): 3613−3619.
[9] Ryan KK, Tremaroli V, Clemmensen C,. FXR is a molecular target for the effects of vertical sleeve gastrectomy[J]. Nature, 2014, 509(7499): 183−188.
[10] Turnbaugh PJ, Ley RE, Mahowald MA,. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature, 2006, 444(7122): 1027−1031.
[11] Turnbaugh PJ, Backhed, F, Fulton L,. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome[J]. Cell Host Microbe, 2008, 3(4): 213−223.
[12] Liou AP, Paziuk M, Luevano JM Jr,. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity[J]. Sci Transl Med, 2013, 5(178): 178ra41.
[13] Ma K, Saha PK, Chan L,. Farnesoid X receptor is essential for normal glucose homeostasis[J]. J Clin Invest, 2006, 116(4):1102−1109.
[14] Stayrook KR, Bramlett KS, Savkur RS. Regulation of carbohydrate metabolism by the farnesoid X receptor[J]. Endocrinology, 2005, 146(3): 984−991.
[15] Chankiewitz E, Peschke D, Herberg L,. Did the gradual loss of GLUT2 cause a shift to diabetic disorders in the New Zealand obese mouse (NZO/HI)[J]? Exp Clin Endocrinol Diabetes, 2006, 114(5): 262−269.
[16] Király MA, Bates HE, Kaniuk NA,. Swim training prevents hyperglycemia in ZDF rats: mechanisms involved in the partial maintenance of beta-cell function[J]. Am J Physiol Endocrinol Metab, 2008, 294(2): E271−E283.
[17] Lips MA, de Groot GH, Berends FJ,. Calorie restriction and Roux-en-Y gastric bypass have opposing effects on circulating FGF21 in morbidly obese subjects[J]. Clin Endocrinol (Oxf), 2014 May 19. doi: 10.1111/cen.12496. [Epub ahead of print].
(编辑: 周宇红)
Effect of gastric bypass on expression of glycometabolism-related factors in liver of type 2 diabetes mellitus rats
LI Xu-Zhong1, YAN Yong2, ZHOU Zhou3, QIU Wen-Juan1, ZHOU Lian-Ming2, HUANG Zhong-Ming2, HU Cheng3, ZHANG Xue-Li2*
(1Graduate School, Soochow University, Suzhou 215000, China;2Department of General Surgery, Shanghai Jiaotong University Affiliated Sixth People’s Hospital South Campus, Shanghai 201400, China;3Department of Endocrinology and Metabolism, Sixth People’s Hospital Affiliated to Shanghai Jiaotong University, Shanghai Institute of Diabetes, Shanghai 200233, China)
To establish a rat model of type 2 diabetes mellitus (T2DM) following by gastric bypass (GBP) surgery, and then determine the effect of GBP on the expression of glycometabolism-related factors in the liver.Twelve 6-week-old SD rats were randomly divided into normal chow diet group (NCD,=4) and high-fat diet group (HFD,=8). In 4 weeks after high-fat diet, the rats of the latter group were injected with streptozocin (STZ) intraperitoneally to induce T2DM model. Body mass and blood glucose were measured, oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were carried out in the NCD and HFD groups, and the results were compared between them. In 2 weeks later, 4 rats of the HFD group underwent GBP surgery and named as HFD-GBP group. After another 2 weeks, body mass and blood glucose were measured again. The expression of farnesoid X receptor (FXR), phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), glucose transporter 2 (GLUT-2) and fibroblast growth factor-21 (FGF-21) at mRNA and protein levels in the liver of the 3 groups of rats were detected by RT-PCR and Western blotting respectively.Compared with the HFD group, the HFD-GBP group had significantly decreased body mass and improved blood glucose (<0.05), obviously up-regulated FXR and GLUT2 at mRNA level (<0.05), and increased mRNA expression of PEPCK, G6Pase and FGF-21. The protein expression levels of FXR and PEPCK were significantly elevated in HFD and HFD-GBP groups than in the NCD group (<0.05).GBP surgery effectively improves glucose metabolism in T2DM rats. FXR, PEPCK and GLUT2 may participate in the blood glucose regulation after the surgery.
gastric bypass; diabetes mellitus, type 2; farnesoid X receptor; phosphoenolpyruvate carboxykinase
R587.1
A
10.11915/j.issn.1671−5403.2015.01.017
2014−11−05;
2014−11−13
张学利, E-mail: lejing1996@aliyun.com

