Neuraxialmodulation for treatmentof VT storm
Roderick Tung,Kalyanam Shivkumar
UCLA Cardiac Arrhythmia Center,UCLA Health System,David Geffen SchoolofMedicine atUCLA,Los Angeles,CA 90095-1679, USA.
Neuraxialmodulation for treatmentof VT storm
Roderick Tung✉,Kalyanam Shivkumar
UCLA Cardiac Arrhythmia Center,UCLA Health System,David Geffen SchoolofMedicine atUCLA,Los Angeles,CA 90095-1679, USA.
In the hyperadrenergic state of VT storm where shocks are psychologically and physiologically traumatizing, suppression of sympathetic outflow from the organ levelof the heartup to higher braincenters plays a significant role in reducing the propensity for VT recurrence.The autonomic nervous system continuously receives inputfrom the heart(afferentsignaling),integrates them,and sends efferentsignals to modify ormaintain cardiac function and arrhythmogenesis.Spinalanesthesia with thoracic epidural infusion of bupivicaine and surgicalremoval of the sympathetic chain including the stellate ganglion has been shown to decrease recurrences of VT.Excess sympathetic outflow with catecholamine release can be modified with catheter-based renaldenervation.The insights provided from animal experiments and in patients that are refractory to conventional therapy have significantly improved our working understanding of the heartas an end organ in the autonomic nervous system.
neuraxial,ventricular,tachycardia,denervation,autonomic
Introduction
Ventricular tachycardia(VT)storm,defined as>3 episodes of VT within a 24 hourperiod,hashigh morbidity and mortality.VT storm is commonly managed with antiarrhythmic therapy,treatment of reversal causes(ischemia and electrolyte imbalance),and catheterablation.Additionally,medicaloptimization ofconcomitantheartfailure is necessary with pharmacologic and/or mechanical support,as refractory arrhythmias may be a symptom of decompensated pump function. In the hyperadrenergic state of VT storm where shocks are psychologically and physiologically traumatizing, suppression ofsympathetic outflow from the organ level ofthe heartup to higherbrain centersplaysa significant role in reducing the propensity for VT recurrence.For this reason,sedation with generalanesthesia is recommended notonly for reducing pain and morbidity from shocks,butalso physiologically decreases excessive sympathetic tone.
The autonomic nervoussystem continuously receives inputfrom the heart(afferentsignaling),integratesthem, and sendsefferentsignalsto modify ormaintain cardiac function and arrhythmogenesis[1](Fig.1).In this review, we discuss the rationale and evidence behind neuraxial modulation for the treatmentof VT with thoracic epidural anesthesia,sympathectomy,and renaldenervation.
Thoracic epidural anesthesia
For patients presenting with VT or ventricular fibrillation storm,β-blockers and other antiarrhythmics not contraindicated are frequently used as primary therapy. Sympathetic tone can be further reducedby intubation and sedation and thoracic epiduralanesthesia(TEA). The institution of TEA can be performed atthe bedsidein standard fashion by anesthesiologists.In 2005, intrathecal clonidine was shown to reduce ischemiainduced ventricular arrhythmias in a canine model[2].
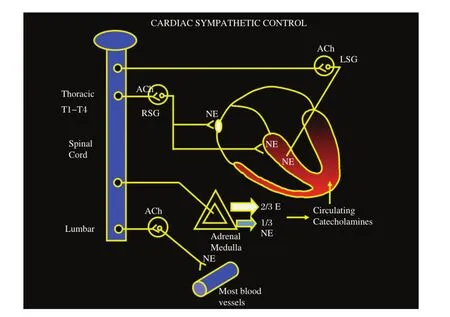
Fig.1Cardiac sympathetic regulation via the autonomic nervous system with the heart as an end organ under multiple levels of control.ACh:acetyl choline;LSG:left stellate ganglia;NE:norepinephrine;RSG:right stellate ganglia.
In the same year,our group reported the successful managementofelectricalstorm with use of TEA.using 1 m L bolus 0.2 5%bupiv acain e followed b y continuous infusion at2 mL/hratthe T1-T2 interspace confirmed with fluoroscopy[3](Fig.2).TEA is performed via a paramedian approach using a 17 gauge Tuohy epiduralneedle and a 19 gauge Flex-Tip plus epidural catheter(Arrow International Inc,Reading,PA,USA). No adverse hemodynamic changes were noted and complete suppression of VT was observed prior to catheter ablation.In our initialseries of 8 patients that underwent TEA,>80%burden in VT was observed in 6 patients[4](Fig.3).
Sympathetic denervation and stellate ganglionectomy
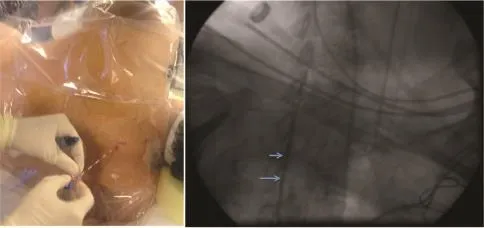
Fig.2Placement of thoracic epidural catheter for spinal anesthesia confirmed with contrast injection on fluoroscopy.Blue arrows indicate contrast in epidural space.
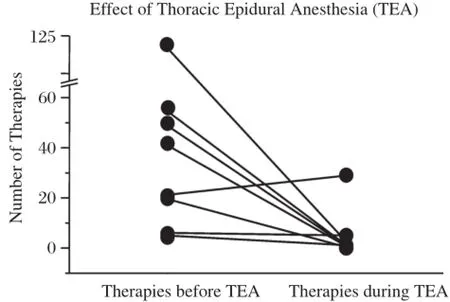
Fig.3 Reduction in ventricular tachycardia(VT)burden pre and post thoracic epidural anesthesia(TEA).
Efferent sympathetic preganglionic neurons that regulate cardiac function reside within the intermediate zone of the thoracic spinalcord atthe levelof T1-T4. The sympathetic chain lieson eitherside ofthe vertebrae and consist of paravertebral ganglia and interconnecting nerves extending from the cervical to the lumbar levels[5].
Within the sympathetic chain,preganglionic axons synapse on neurons within thestellate ganglion(fusion of inferior cervical and T1 ganglia)and ganglia at spinallevels T2-T4.The leftand rightstellate ganglia (LSG and RSG)are the predominant ganglia from which postganglionic fibers to the heartarise.
Sympathetic nerve fibers arrive at the base of the heart,and penetrate the myocardium giving offsmaller branches thatinnervate the entire heart with intrinsic cardiac ganglia as they extend to the apex.Denervation with sympathectomy can be safely performed with single-lung ventilation through a video assisted thoracoscopic approach(Fig.4).
Leftcardiac sympathetic denervation(LCSD)interrupts the major source ofnorepinephrine release in the heart.Schwartz et al.demonstrated that LCSD decreased the propensity for ventricular fibrillation in an animalmodelofleftanteriordescending occlusion[6]. A significant reduction in the number of recurrent arrhythmias has been shown in a cohortofpatientswith long-QT syndrome and catecholaminergic polymorphic VT who received LCSD for secondary prevention[7].In our initial report,amongst 9 patients thatunderwent LCSD,5 patientshad eithercomplete orpartialresponse[4].
More recently,ourgroup has been investigating the physiologic and therapeutic differences between left and bilateral sympathectomy[8].Clinical reports of bilateralcardiac sympathetic denervation(BCSD)to treatsevere ventricular arrhythmiasdate back to 1961, described by Estesand Izlar[9].In priorstudies,stimulation orresection ofthe RSGaccelerated orslowed arrhythmias originating from the right border of an anterior infarct[10], and RSG resection in a canine modelof ischemia and ventricular arrhythmias was as potentas LSG resection reducing the incidence ofventriculararrhythmias[11].
In ourearly experience,three of the six patients had undergone previous LCSD butdeveloped arrhythmia recurrence.RCSD after prior LCSD was effective in suppressing these arrhythmias.In a series of 6 patients, 4 patients had a complete response with BCSD and 1 patienthad a partialresponse[12].
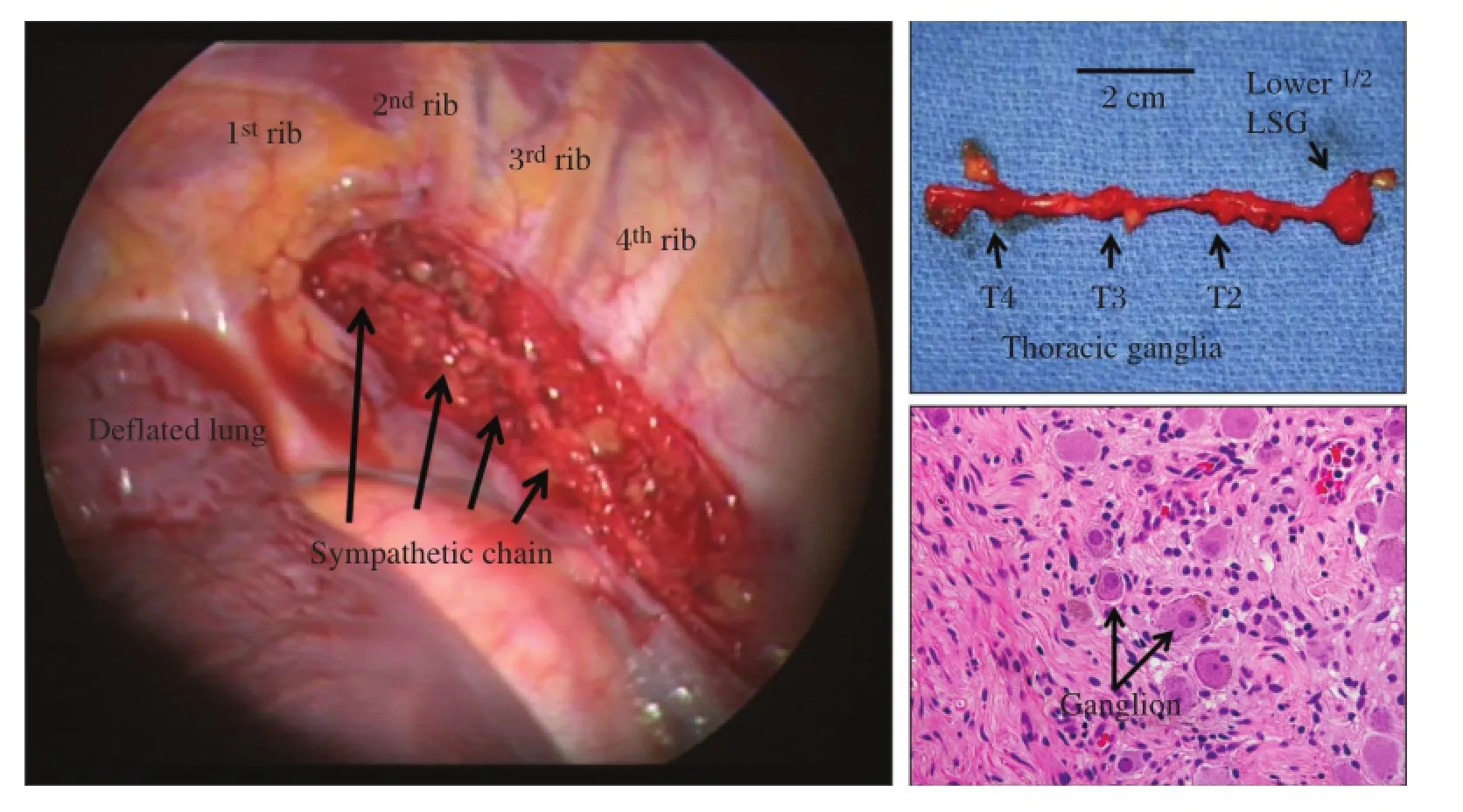
Fig.4 Thoracoscopic view of sympathetic chain with stellate ganglion and T2-T4 thoracic ganglia.Histopathologic confirmation of neural tissue is performed postsurgically.Arrows in the right lower panel indicates ganglion.Arrow indicates ganglion cell.
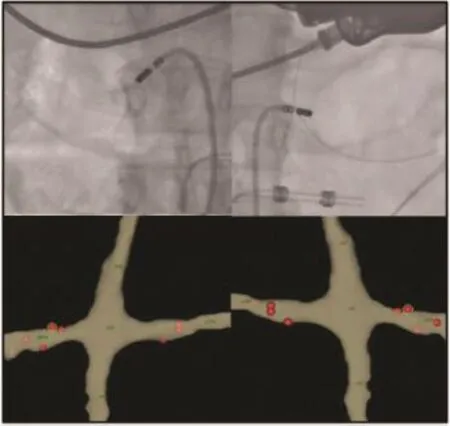
Fig.5 Flouroscopic views on bilateral renal denervation using an ablation catheter with lesions guided by electroanatomic mapping.
With longer follow up,we found that patients that underwent BCSD had significantly improved freedom from VT recurrence compared to those with unilateral denervation[13].Amongst 41 patients that underwent CSD(14 LCSD,27 BCSD),the numberof ICD shocks was reduced from a mean of 19.6±19 preprocedure to 2.3±2.9 postprocedure(P<0.001),with 90%of patients experiencing a reduction in ICD shocks.At mean follow-up of 367±251 days postprocedure, survivalfree of ICD shock was30%in the LCSDgroup and 48%in the BCSD group.Shock-free survivalwas greater in the bilateral group than in the LCSD group (P=0.04).
Renal denervation
Catheter-based renaldenervation(RDN)is currently being studied as a treatment option for drug-refractory hypertension.Although the results from Symplicity-HTN3trial did not meet the prespecified endpoint[14], posthoc analysis demonstrated thatpatients with more extensive ablation,with higher BP preprocedurally, non-African American,and non-use of vasodilators may representa subgroup more likely to derive clinical benefit[15].Ablation within the renalarteries,by altering efferentand afferentsignaling,hasthepotentialto improve blood pressure,as well as heartfailure,atrial,and ventricular tachyarrhythmias.By decreasing norepinephrine spillover,the propensity for VT may be decreased.
Ablation is performed within the renalarteries from proximalto the firstbifurcation in a spiralfashion sparing the ostium(Fig.5).The renalnerves run on the adventitia in a complex interlaced network,necessitating transmural lesions,although the precise location and number of ablation lesions required is currently notknown.Contrary to electrophysiologic ablation,a standardized endpointhas notreached consensus for renaldenervation,although blunting of a hypertensive response with high frequency stimulation hasbeen used.
Multiple case reports have highlighted the potential role of renal denervation for the treatmentof refractory VT[16,17].Ventricular fibrillation thresholds were decreased in an animalmodelwhen surgicalrenaldenervation was performed[18].In a multi-centercase series of 4 patients[19], RDN was well tolerated acutely and demonstrated no clinically significant complications during follow-up of 8.8±2.6 months(range 5.0-11.0 months).The number of VT episodes was decreased from 11.0± 4.2(5.0-14.0)during the month before ablation to 0.3 ±0.1(0.2-0.4)per month after ablation.
Neuraxialmodulation has emerged as a promising therapeutic strategy forpatients with refractory ventriculararrhythmias.Arrhythmogenesisrequiresa substrate and a trigger and autonomics may largely accountfor the timing of clinicalpresentation.The uniting pathophysiologic basis forthese interventions is suppression ofexcessive sympathetic activation.In currentpractice, TEA can be instituted by anesthesiologists and thoracic sympathectomy via a minimally-invasive thoracoscopic approach can be performed by thoracic surgeons.The role of renal denervation warrants further study but holds promise.The insights provided from animal experiments and in patients thatare refractory to conventional therapy have significantly improved our working understanding of the heartas an end organ in the autonomic nervous system.
[1]Zipes DP,Barber MJ,Takahashi N,etal.Influence of the autonomic nervous system on the genesis of cardiac arrhythmias.Pacing Clin Electrophysiol 1983;6(5 Pt 2):1210-1220.
[2]Issa ZF,Ujhelyi MR,Hildebrand KR,et al.Intrathecal clonidine reduces the incidence of ischemia-provoked ventricular arrhythmias in a canine postinfarction heart failure model.Heart Rhythm 2005;2(10):1122-1127.
[3]Mahajan A,Moore J,Cesario DA,et al.Use of thoracic epidural anesthesia for management of electrical storm: a case report.Heart Rhythm 2005;2(12):1359-1362.
[4]Bourke T,Vaseghi M,Michowitz Y,et al.Neuraxial modulation for refractory ventricular arrhythmias:value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation.Circulation 2010;121(21): 2255-2262.
[5]W.J.Functional anatomy of the peripheral sympathetic and parasympathetic system.In:The Integrative Action of the Autonomic Nervous System:Neurobiology of Homeostasis.UK:Cambridge University Press;2006.
[6]Sch wartz PJ,Billman GE,Ston e HL.Auto nomic mechanisms in ventricular fibrillation induced by myo-cardial ischemia during exercise in dogs with healed myocardial infarction.An experimental preparation for sudden cardiac death.Circulation 1984;69(4):790-800.
[7]Collura CA,Johnson JN,Moir C,etal.Leftcardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery.Heart Rhythm 2009;6(6):752-759.
[8]Ajijola OA,Vaseghi M,Mahajan A,et al.Bilateral cardiac sympathetic denervation:why,who and when? Expert Rev Cardiovasc Ther 2012;10(8):947-949.
[9]Estes EH Jr,Izlar HL Jr.Recurrent ventricular tachycardia.A case successfully treated by bilateral cardiac sympathectomy.Am J Med 1961;31(3):493-497.
[10]Martins JB.Autonomic controlof ventricular tachycardia: sympathetic neural influence on spontaneous tachycardia 24 hours after coronary occlusion.Circulation 1985; 72(4):933-942.
[11]Puddu PE,Jouve R,Langlet F,et al.Prevention of postischemic ventricularfibrillation late after rightorleftstellate ganglionectomy in dogs.Circulation 1988;77(4): 935-946.
[12]Ajijola OA,Lellouche N,Bourke T,et al.Bilateral cardiac sympathetic denervation for the management of electrical storm.J Am Coll Cardiol 2012;59(1):91-92.
[13]Vaseghi M,Gima J,Kanaan C,etal.Cardiac sympathetic denervation in patients with refractory ven tricular arrhythmias or electrical storm:intermediate and longterm follow-up.Heart Rhythm 2014;11(3):360-366.
[14]Bakris GL,Townsend RR,Liu M,et al.Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3.J Am Coll Cardiol 2014;64(11):1071-1078.
[15]Kandzari DE,Bhatt DL,Brar S,et al.Predictors of blood pressure response in the SYMPLICITY HTN-3 trial.Eur Heart J 2014,doi:10.1093/eurheartj/ehu441.
[16]Chen SJ,Chen WJ,Su L,et al.Renal denervation for‘‘resistant ventricular tachycardia’’:a potential treatment option?Chin Med J(Engl)2013;126(21):4199-4200.
[17]HilbertS,Rogge C,Papageorgiou P,etal.Successfulsingle-sided renal denervation in drug-resistant hypertension and ventricular tachycardia.Clin Res Cardiol 2014;11, doi:10.1007/s00392-014-0790-3.
[18]Linz D,Wirth K,Ukena C,et al.Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs.Heart Rhythm 2013;10(10):1525-1530.
[19]Remo BF,Preminger M,Bradfield J,et al.Safety and efficacy of renaldenervation as a noveltreatmentof ventricular tachycardia storm in patients with cardiomyopathy.Heart Rhythm 2014;11(4):541-546.
✉Corresponding author:Roderick Tung,MD,UCLA Cardiac Arrhythmia Center,UCLA Health System,David Geffen School of Medicine at UCLA,100 UCLA MedicalPlaza,Suite 660,Los Angeles, CA 90095-1679,USA.Tel/fax:+1-310-206-2235/+310-825-2092,E-mail:rtung@mednet.ucla.edu.
Received 10 November 2014,Accept 18 December 2014,Epub 28 December 2014
The authors reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140161
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Myocardin in biology and disease
- Optimalantiarrhythmic drug therapy for electricalstorm
- Optimalprogramming managementofventricular tachycardia storm in ICD patients
- Trigger elimination ofpolymorphic ventricular tachycardia and ventricular fibrillation by catheter ablation:trigger and substrate modification
- Prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza
- Portalvein arterialization promotes liver regeneration after extended partialhepatectomy in a rat model
