Optimalprogramming managementofventricular tachycardia storm in ICD patients
Zhiyong Qian,Jianghong Guo,Zhiyong Zhang,Yao Wang,Xiaofeng Hou,Jiangang Zou
Department of Cardiology,First Affiliated Hospital,Nanjing Medical University,Nanjing,Jiangsu 210029,China.
Optimalprogramming managementofventricular tachycardia storm in ICD patients
Zhiyong Qian,Jianghong Guo,Zhiyong Zhang,Yao Wang,Xiaofeng Hou,Jiangang Zou✉
Department of Cardiology,First Affiliated Hospital,Nanjing Medical University,Nanjing,Jiangsu 210029,China.
Ventriculartachycardia storm(VTS)is defined as a life-threatening syndrome ofthree ormore separate episodes of ventricular tachycardia(VT)leading to implantable cardioverter defibrillator(ICD)therapy within 24 hours. Patients with VTS have poor outcomes and require immediate medical attention.ICD shocks have been shown to be associated with increased mortality in severalstudies.Optimalprogramming in minimization of ICD shocks may decrease mortality.Large controlled trialsshowed thatlong detection time and high heartrate detection threshold reduced ICD shock burden withoutan increase in syncope or death.As a fundamentaltherapy of ICD,antitachycardia pacing(ATP)can terminate most slow VT with a low risk of acceleration.For fast VT,burst pacing is more effective and less likely to resultin acceleration than ramp pacing.One algorithm ofoptimalprogramming management during a VTS is presented in the review.
implantable cardioverter defibrillator,optimalprogramming,ventricular tachycardia storm
Introduction
Implantable cardioverter defibrillator(ICD)has revolutionized the preventive treatment of patients at risk for sudden cardiac death and has been widely used for these high-risk individuals[1-3].However,ICD does not target the pathological substrate responsible for ventricular arrhythmias.Consequently,a certain percentage of ICD recipients experience multiple episodes ofventricular tachycardia(VT)and/orventricularfibrillation(VF)in a syndrome called‘‘electricalstorm’’(ES).ES is a devastating eventand associated with an adverse prognosis and reduced quality of life[4].The review focuses on VT storm(VTS)and optimalprogramming strategies for VTS in ICD patients.
Although there is stillno consensus on formaldefinition of VTS,it is generally accepted that three or more separate episodes of VT leading to ICD therapies [antitachycardia pacing(ATP)or shock]over a 24 hour period constitute VTS[4-6].The end of a VTS is defined as termination of ES without VT recurrence in two weeks[7].No study to date has examined the threshold burden of ventricular arrhythmias which would resultin an adverse outcome.Whether ventricular arrhythmias should rely on ICD therapies and how to define the time intervalof separate episodes of VT are still unsettled.The definition of VTS remains empiric and is subject to amendment pending the results of large outcome studies[6].
Occurrence of VTS and its implication
In patients who have ICDs forsecondary prevention of sudden cardiac death,10%-20%willexperience aVTS within two years of ICD implant[8].In contrast, the incidence is lower for primary prevention patients at4%overan average of20.6 months[6].Possible triggering factors are acute heartfailure,acute myocardial ischemia,electrolyte disturbance,acute infection,and abnormalsympathetic activity.However,there are still other undetermined causes which were reported to accountfor 64%of VTS events[9].
A meta-analysis showed that ICD for secondary prevention,monomorphic VT as triggering arrhythmia, lower ejection fraction and class I anti-arrhythmic drugs were associated with ES,which could be used to define high risk populations for ES[10].Advanced age and male genderin ES patients had a trend towards increased prevalence,but with no statistical significance.One study observed thatthe combination ofleft ventricular ejection fraction<25%and QRS duration>120 ms was a powerfulpredictor of the occurrence of ES[11].
Many studies have consistently found that VTS is associated with highermortality in both secondary and primary prophylaxis patients.A recentmeta-analysis enrolling 5,912 cases showed that ES was a strong mortality risk factor,which accounted for a 3.2-fold increased risk of death and was associated with a 3.4-fold increased risk forthe composite endpointofdeath, heart transplantation,and hospitalization for heart failure[10].However,whether VTS directly contributes to increased mortality orismerely a markerofprogression of the underlying disease remains elusive[5-6].In fact,it has also been shown that repeated ICD shocks delivered fortreatmentofrefractory VTmightcontribute to the progression of heartfailure and cardiac injury, which in turn increases mortality[12].
The occurrence of VTS seriously impairs the quality oflife and psychologicalstatus ofthe patients[4-6].As a clinicalemergency,physicians should intervene early to preventthe recurrence of ventricular arrhythmias. The comprehensive management of VTS includes seeking and eliminating the triggering factors using sedation,anti-arrhythmic drugs,ICD programming, catheterablation and/orcardiacsympatheticdenervation. Optimization of programming to preventunnecessary shocks is paramountto these patients.
Optimal programming strategies of VTS in ICD patients
The detection and treatmentofventriculararrhythmias by an ICD involves a series ofsequentialsteps,each of which provides an opportunity to preventunnecessary shocks(Fig.1).These steps include heartrate detection,number of intervals to detect(NID),tachycardia detection,supraventricular tachycardia(SVT)-VT discrimination,VT confirmation,ATP,reconfirmation, and shock.During a VTS,optimal programming to minimize unnecessary shocks is discussed in detail based on the recenttrials(Table 1).
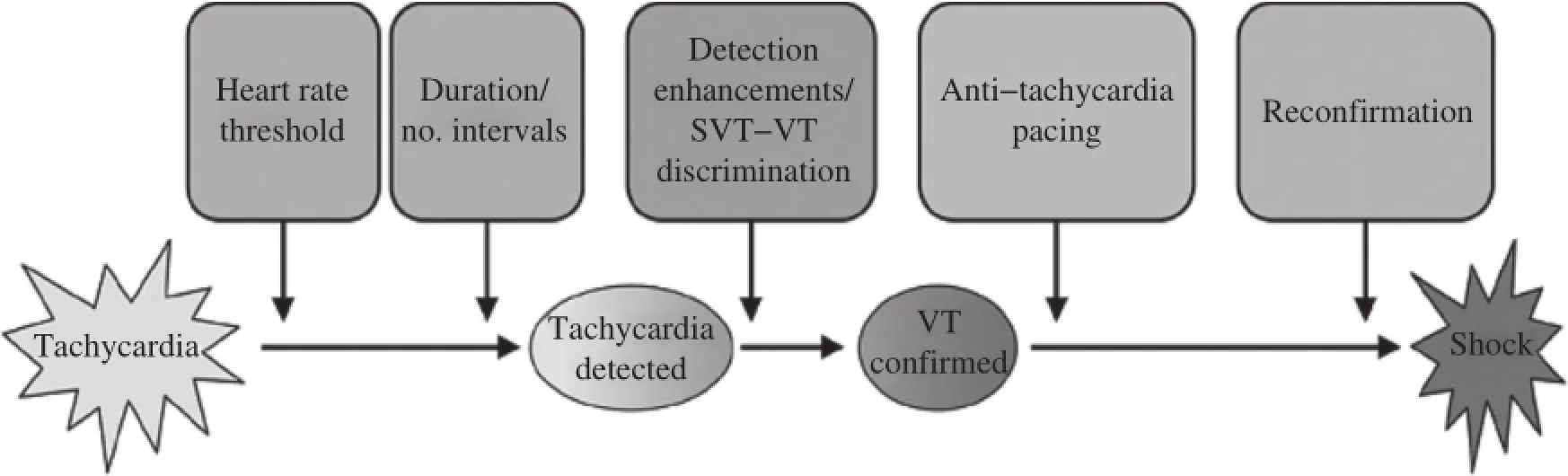
Fig.1Overview of detection and treatment of ventricular tachyarrhythmias by implantable cardioverter defibrillator(ICD).The detection and treatmentofventriculartachyarrhythmias by an ICD involves a sequence ofevents which provide opportunities for optimalprogramming. SVT:supraventricular tachycardia;VT:ventricular tachycardia.
Prolongation of arrhythmia detection time
The use of prolonged arrhythmia detection time is one programming strategy that has been widely evaluated[13-16].The prospective,multicentre,nonrandomized RELEVANT(Role of Long Detection Window Programming in Patients with Left Ventricular Dysfunction,Non-ischemic Etiology in Primary Prevention Treated with a Biventricular ICD)study compared a long VT/VF detection vs.standard ICD programming(NID 30/40 vs.12/16)and showed that longerdetection timereduced overallICDtherapy burden and heartfailure hospitalizations withoutany increase of syncope ordeath in primary prevention ofnon-ischemic heartfailure patients[13].The PROVIDE(Programming Implantable Cardioverter Defibrillators in Patients with Primary Prevention Indication to Prolong Time to FirstShock)study reported thatin a large cohortofpatients,a combination ofprogrammed parametersincluding higher detection rate,longer detection intervals,empiric ATP, and optimized SVT discriminators were associated with a significantreduction of ICDshock therapy with reduction in all-cause mortality and without increasing arrhythmic syncope[14].The MADIT-RIT(Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy)study was a large randomized controlled trialand included 1,500 patients[15].In this study,patients were randomly assigned to the delayed (with a 60-second delay at170 to 199 bpm,a 12-second delay at 200 to 249 bpm,and a 2.5-second delay at≥250 bpm)and the conventionalgroups.The results showed thatprogramming with a prolonged delay was associated with reductions in inappropriate therapy and all-cause mortality.The recent ADVANCE III (Avoid Delivering Therapies for Non-sustained Arrhythmias in ICD Patients III)trialhas demonstrated thatthe use of a long detection interval(30 outof 40 intervals),combined with ATP during charging,significantly reduces the rate of appropriate therapies(ATP and shocks)and inappropriate shocks in comparison with the standard detection interval(18 outof 24)in single,dual and triple chamber ICDs,regardless of indication[16].The ADVANCE III trial confirmed and
reinforced the results of MADIT-RIT in a larger and broader primary prevention ICD population.A metaanalysis including the above four trials concluded that the use of long detection time could significantly decrease the burden of inappropriate shock therapy (RR 0.50)and all-cause mortality(RR 0.77)without significantincrease in the risk ofsyncope[17].
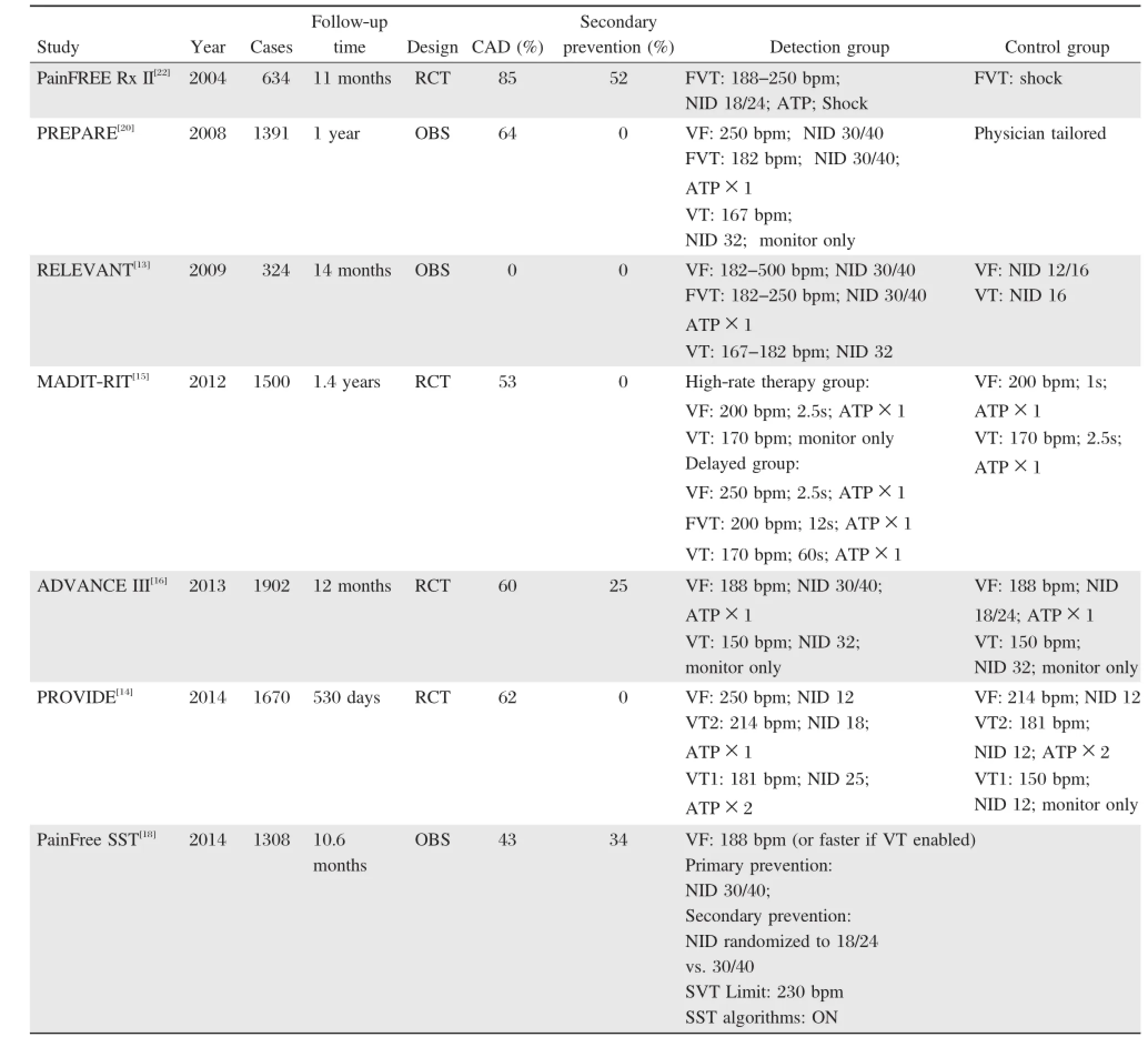
Table 1.Clinical trials of shock reduction programming
The PainFree SST trialwas designed to evaluate the inappropriate shock free rate atone-year postimplant in primary and secondary prevention patients with single,dual and triple chamber ICDs by SmartShock Technology(SST)[18].The primary results showed that over 98%of the patients were free of inappropriately shocked episodes during theirfirstyearafterimplantation and no difference was detected between primary (1.6%with inappropriate shocks)and secondary(2.3%) prevention patients.A cohort of secondary prevention patients were randomized in a 1:1 fashion to either a standard interval(NID=18/24)or prolonged interval (NID=30/40)detection of VT/VF≥188 bpm(VF zone).In this large randomized trialinvolving high-risk secondary prevention patients,longer detection intervals did notincrease the risk ofsyncope,ensuring the safety ofthisprogramming strategy.Prolonged intervaldetection programming did not impact the rates of inappropriate shocks,which were low using advanced discrimination algorithms in both groups at one year.
In conclusion,the increase of detection time could preventboth inappropriate shocks(shocks for rhythms other than VT or VF)and unnecessary shocks(shocks for self-terminating episodes)in patients with VTS. However,we should closely evaluate the status ofcardiac function to determine if each individual could tolerate possible risk resulted from the delayed therapy ofmalignantventriculararrhythmias.Additionally,VT may recur immediately after successful ATP,which may lead to misclassification of unsuccessful ATP, and subsequentunnecessary shocks may be delivered[5]. In some ICDs,we can decrease the numberofintervals fordeclaring an end ofepisode to obtain an early definition ofreturn to sinus rhythm.
Increasing heart rate detection threshold
According to the heartrate,programmable zones are strictly defined.ICDs usually provide three zones,VT, fast VT(FVT),and VF.The description ofarrhythmia characteristics in ICD patients with primary and secondary prevention indications could guide the settings of programmable zones[19].A shorter VT cycle length (CL,303±54 ms vs.366±71 ms)and a longer SVT CL(363±70 ms vs.323±75 ms)are found in patients with primary preventive ICDs compared with those with secondary prevention indications (Fig.2).These data indicate that ICD patients ofprimary prevention have faster VT and smalleroverlap of SVT and VT;as a result,these patients may benefit from higher rate detection zones.On the contrary, patients with a secondary prevention indication will benefitfrom slowerdetection zones and SVT-VT discrimination algorithms because of the greater overlap between SVT and VT.Severaltrials have investigated the strategy combined with longer detection time in primary prevention patients.
In the PREPARE(Primary Prevention Parameters Evaluation)study,detection rate was set to 182 bpm (FVT)and VF 250 bpm[20].In the MADIT-RIT trial, detection rate for ventricular arrhythmias was set to 200 bpm with a 2.5-second delay in the high-rate therapy group[15].Similarly,the PROVIDE study defined VT,FVT and VF zones as 181 bpm,214 bpm,and 250 bpm,respectively[14].Consistently,these highheartrate detection groupshad significantreductions in ICD shocks withoutan increase in syncope ordeath.In Mayo Clinic,the recommended detection rate of primary prevention patients is set to 200 bpm with a 5-to 9-second delay for ICD therapies[5].
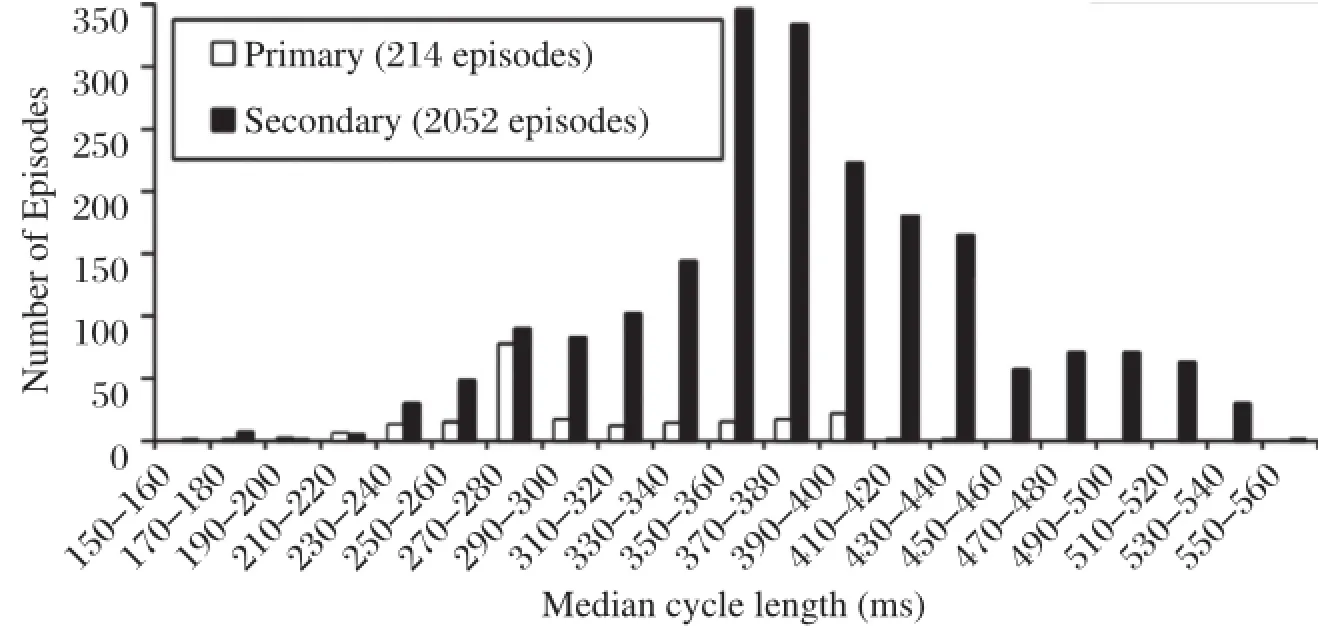
Fig.2 Rates of ventricular arrhythmias detected in 978 patients in whom an ICD was implanted for primary and secondary prevention.Patients with primary preventive ICDs have a shortercycle length ofventricular tachycardia compared with secondary preventive patients and may benefit from higher detection zones.(Cited from:Wilkoff BL,etal.J Cardiovasc Electrophysiol.2004;15(9):1002-1009[19]with permission)
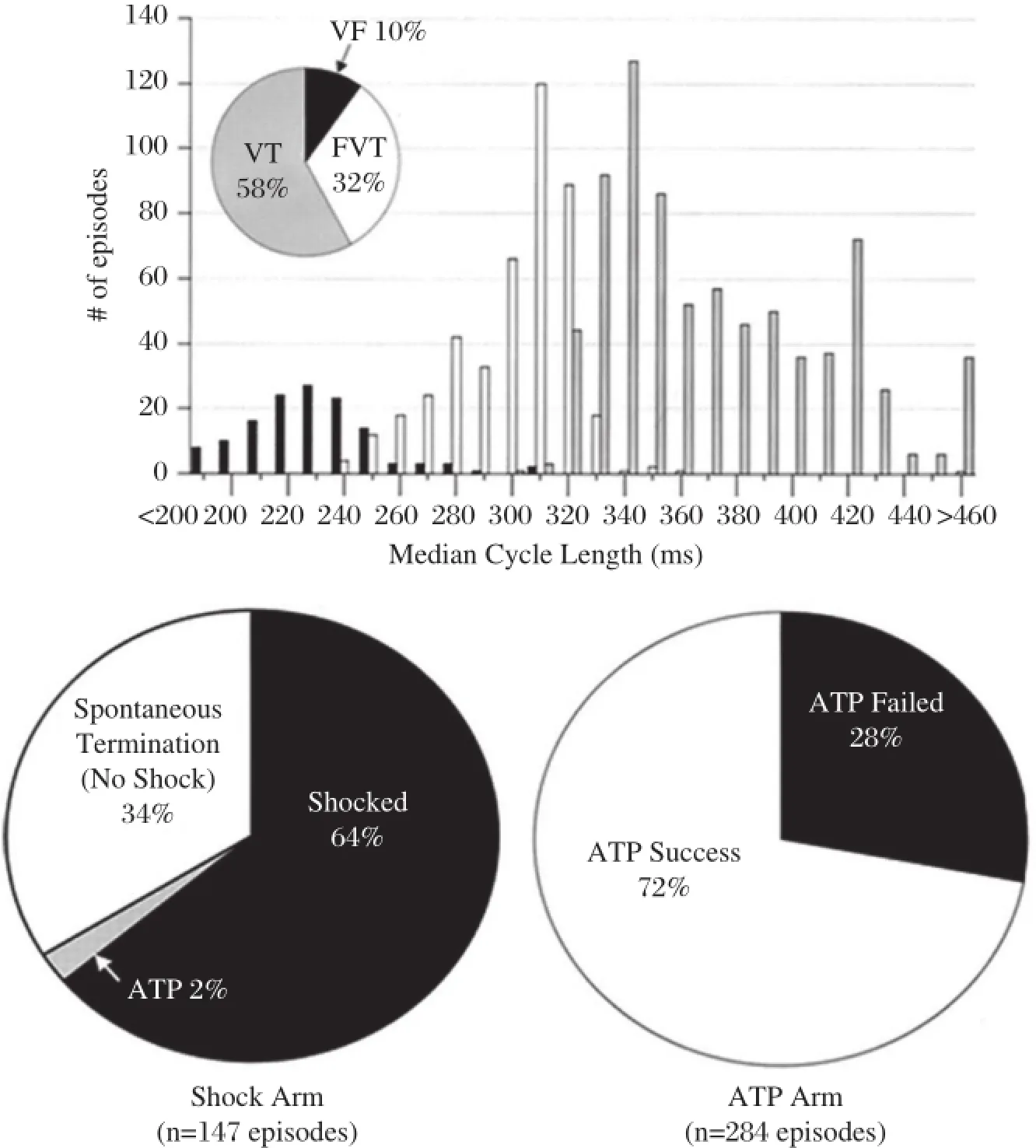
Fig.3Results of the PainFree Rx II(Pacing Fast Ventricular Tachycardia Reduces Shock Therapies)trial.The upper graph shows distribution of ventricular arrhythmias by detection zone and median cycle length.The pie charts show percentages of terminating therapy for FVT episodes in each arm.ATP could terminate FVT in 72%of episodes.FVT:fast ventricular tachycardia;ATP:antitachycardia pacing.(Cited from: Wathen MS,et al.Circulation.2004;110(17):2591-2596[22]with permission)
Increasing the efficacy of ATP
ATP israpid pacing ata CL shorterthan VT thatterminates reentrant VT by penetrating the circuit and blocking the reentry.ATP can reduce unnecessary shocks,improve quality oflife,and lengthen pulse generatorlife.Previous studies have demonstrated that ATP terminated 85%-90%of slow VT(<188-200 bpm) with a low risk of acceleration(1%-5%)[21].In PainFREE Rx II(Pacing Fast Ventricular Tachycardia Reduces Shock Therapies)trial,patients were randomized to ATP followed by shock orshock alone forthe treatmentof FVT(188-250 bpm)[22].The results showed that a single 8-pulse burst of ATP was successful in terminating FVT in 72%of episodes and resulted in a significantreduction in shocks with improved quality of life and low rates of VT acceleration and syncope (Fig.3).However,recentstudies showed thatefficacy of ATP with longer NID was notas high as previously reported.In ADVANCE III trial,ATP efficacy for FVT (CL 240-320 ms)was 54%[generalized estimating equation(GEE)adjusted].One latestreportshowed that a new automatic ATP algorithm(an initial ATP train based on heart rate history,subsequenttrains based on post-ATP intervaland shocks applied attimerexpiry) improved ATP efficacy for FVT to 59%(GEE adjusted)[23].
ATP mode selection
ATP was usually classified as burst if all paced beats were delivered atthe same CL,and ramp ifthe beat-tobeatpacing CL was shortened within each pacing train. Burst and ramp pacing sequences have similar efficacyin slow VTs[21].For FVT with CL<300 ms,burst is more effective and less likely to resultin acceleration than ramp.One study has investigated the efficacy of four ATP modes,including burst,ramp,scan(if the pacing CL was shortened between each pacing train), and ramp/scan(if the pacing CL was shortened both between and within each pacing train).As a result,when the VT rate was>200 bpm,ATP was less successful,
however,burst and scan exhibited greater efficacy (81.2%and 87.1%respectively)compared with ramp (57.1%)[24].
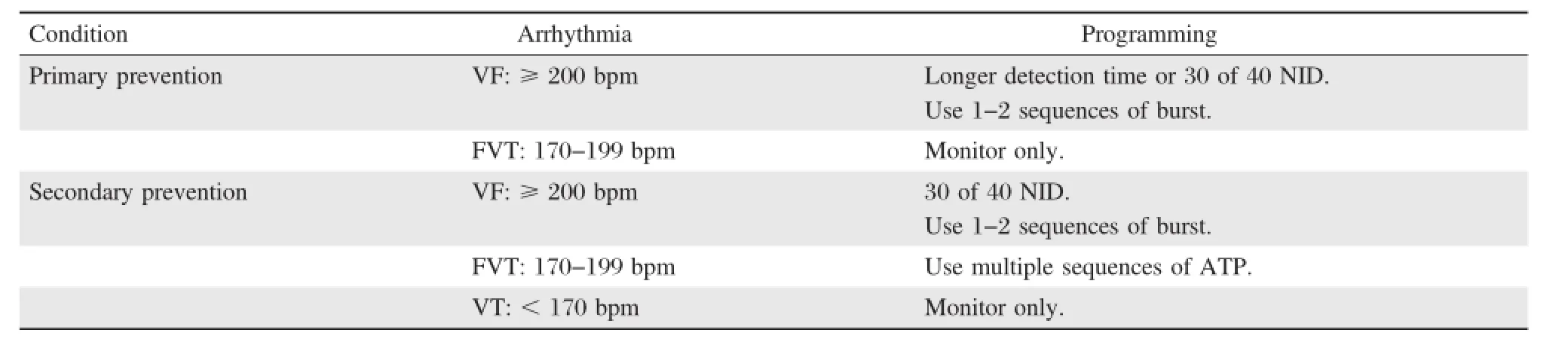
Table 2.Suggested ICD programming for specific ICD indications
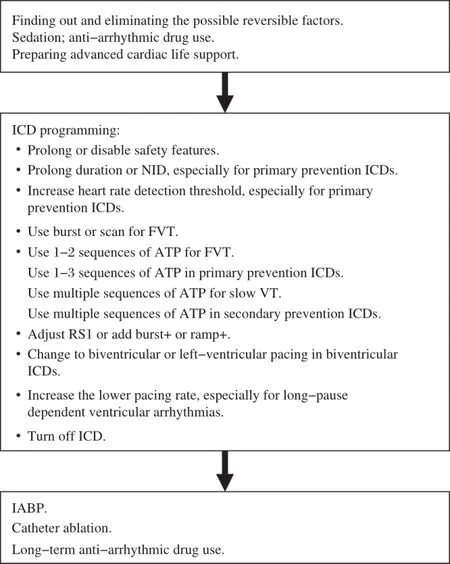
Fig.4A flowchart of optimal programming strategies during a ventricular tachycardia storm.NID:number of intervals to detect;VT: ventricular tachycardia;FVT:fast ventricular tachycardia;ATP:antitachycardia pacing;IABP:intra-aortic balloon pump.
Multi-site ATP
Several studies have reported greater success with biventricular ATP.One retrospective and observational study showed thatin heartfailure patients with biventricular(BiV)ICD,ATP efficacy of left ventricular (LV)and BiV pacing was higherthan rightventricular (RV)ATP[25].BiV-ATP and LV-ATP were safer than RV-ATP forslow VT(150-188 bpm).The ADVANCE CRT-Dtrialwasa randomized and controlled multicenter trial aimed at comparing the efficacy and safety of BiV-versus RV-ATP in heartfailure patients treated with cardiac resynchronization therapy-defibrillator[26]. The results showed that BiV-ATP seemed to be more effective and safer in ischemic patients than RV-ATP.
Multi-sequence ATP
The majority ofFVTswere successfully treated by one ortwo ATP attempts.Only a smallminority ofpatients were responsiveto>3 ATPs.Programming a high numberof ATP attempts in the FVT zone is both safe and efficientand could preventshocks in ICD recipients[27]. Onelateststudy demonstrated thatasecond burstpacing increased the effectivenessof ATP(GEE-adjusted,65% vs.75%)for FVT(CL 250-320 ms)and therefore, reduced the need for high-energy shocks[28].
Adjusting R-S1 interval(%RR)
Generally,burst CL should be 85%-90%of the VT CL for FVTs and 70%-80%forslow VTs[21].When an unsuccessful ATP occurred,analysis of the return CL is helpfulto optimize ICD programming[5].The CL of the drive train could be shortened or an extrastimulus added atthe end ofthe drive train(forexample,‘‘burst +’’mode)in orderto penetrate the circuit.If the CL of VT is unaffected,increasing the numberofpaced beats could facilitate penetrating the circuitby peeling away refractoriness.
Anti-arrhythmic drug therapy
Intensive therapy of the anti-arrhythmic drugs,especiallyβ-blockers and amiodarone,could help decrease the VT rate and increase the ATP efficacy[29].
Increasing the lower pacing rate temporally
In some patients,overdrive pacing by increasing the lower pacing rate of the ICD may avoid the long pause after a ventricular ectopic beat,shorten the QT interval and suppress recurrent VT/VF,particularly if dual chamber pacing is available[5,30].
Overall,during a VT storm,ICD programming should focus on minimizing shocks.Table 2 shows the suggested optimalprogramming for shock reduction based on ICD indications.Importantly,safety features that a shock is applied after a programmable time window independently from ATP should be prolonged or disabled[5].Additionally,ICD therapies are often turned off in hospitalized patients.Catheter ablation might be the only option when a VTS isrefractory to programming and drug therapies.A flowchartof optimalprogramming managementduring a VTS is exhibited in Fig.4.
Conclusion
VTS,a life-threatening emergency,isassociated with poor prognosis in ICD patients and requires immediate medical attention.Optimal programming aiming at reducing inappropriate or unnecessary shocks may provide up to a 30%relative decrease in mortality with no apparentincrease in the risk ofsyncope[31].During a VTS,ICD programming focuses on minimizing the frequency ofshocks.Long detection time and high heart rate detection threshold are key methods,especially for primary prevention patients.ATP therapy should be intensified in many ways including ATP mode,number ofsequences,and pacing sites.However,thecomprehensive managementofVTS including optimalpharmacological therapy,sedation,triggerorsubstrate ablation and denervation is comparably essential.
The optimalprogramming strategies from the present clinicaltrialsare mostly aimed atICDpatientsforprimary prevention of sudden cardiac death.Future studies are needed to clarify the roles of differentprogramming strategies on secondary prevention patients.
Acknowledgment
We thank Dr.Jian Cao(Senior principal scientist, Medtronic)for his diligentreview of this manuscript.
[1]Moss AJ,Zareba W,HallWJ,etal.Multicenter Automatic Defibrillator Implantation TrialIIInvestigators.Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction.N Engl J Med 2002;346(12):877-883.
[2]Bardy GH,Lee KL,Mark DB,et al.Sudden Cardiac Death in Heart Failure Trial(SCD-HeFT)Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure.N Engl J Med 2005;352(3): 225-237.
[3]Zipes DP,Camm AJ,Borggrefe M,etal.ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of SuddenCardiac Death:a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines(writing committee to d evelop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death):developed in collaboration with the European Heart Rh ythm Asso ciation and the Heart Rhythm Society.Circulation 2006;114(10):e385-484.
[4]Borne RT,Varosy PD,Masoudi FA.Implantable cardioverter-defibrillator shocks:epidemiology,outcomes,and therapeutic approaches.JAMA Intern Med 2013;173(10): 859-865.
[5]Madhavan M,Friedman PA.Optimal programming of implantable cardiac-defibrillators.Circulation 2013;128(6): 659-672.
[6]Gao D,Sapp JL.Electrical storm:definitions,clinical importance,and treatment.Curr Opin Cardiol 2013; 28(1):72-79.
[7]Greene M,Newman D,Geist M,et al.Is electrical storm in ICD patients the sign of a dying heart?Outcome of patients with clusters of ventricular tachyarrhythmias. Europace 2000;2(3):263-269.
[8]Exner DV,Pinski SL,Wyse DG,et al.Electrical storm presages nonsudden death:the antiarrhythmics versus implantable defibrillators(AVID)trial.Circulation 2001;103(16):2066-2071.
[9]Brigadeau F,Kouakam C,Klug D,etal.Clinical predictors and prognostic significance of electrical storm in patients with implantable cardioverter defibrillators.Eur Heart J 2006;27(6):700-707.
[10]Guerra F,Shkoza M,Scappini L,et al.Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors:a meta-analysis.Europace 2014;16(3): 347-353.
[11]Arya A,Haghjoo M,Dehghani MR,etal.Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator.Am J Cardiol 2006;97(3):389-392.
[12]Joglar JA,Kessler DJ,Welch PJ,etal.Effects of repeated electricaldefibrillations on cardiac troponin Ilevels.Am J Cardiol 1999;83(2):270-272.
[13]Gasparini M,Menozzi C,Proclemer A,etal.A simplified biventricular defibrillator with fixed long detection intervals reduces implantable cardioverter defibrillator(ICD) interventions and heart failure hospitalizations in patients with non-ischemic cardiomyopathy implanted for primary prevention:The RELEVANT study.Eur Heart J 2009; 30(22):2758-2767.
[14]Saeed M,Hann a I,Robotis D,et al.Programming implantable cardioverter-defibrillators in patients with primary prevention indication to prolong time to first shock:results from the PROVIDE study.J Cardiovasc Electrophysiol 2014;25(1):52-59.
[15]Moss AJ,Schuger C,Beck CA,et al.MADIT-RIT Trial Investigators.Reduction in inappropriate therapy and mortality through ICD programming.N Engl J Med 2012;367(24):2275-2283.
[16]Gasparini M,Proclemer A,Klersy C,etal.Effectof longdetection interval vs.standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery:the ADVANCE III randomized clinical trial.JAMA 2013;309(18):1903-1911.
[17]Scott PA,Silberbauer J,McDonagh TA,et al.Impact of prolonged implantable cardioverter-defibrillator arrhythmia detection times on outcomes:A meta-analysis. Heart Rhythm 2014;11(5):828-835.
[18]Schloss EJ,Auricchio A,Kurita T,et al.PainFree SST trialprimary results:low shock rates in patients with dual and triple chanmber ICDs using novel detection algorithms.Heart Rhythm 2013;10(5)Supplement:AB28-4.
[19]Wilkoff BL,Hess M,Young J,et al.Differences in tachyarrhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol 2004;15(9):1002-1009.
[20]Wilkoff BL,Williamson BD,Stern RS,et al.PREPARE Study Investigators.Strategic programming of detection and therapy parameters in implantable cardioverterdefibrillators reduces shocks in primary prevention patients:results from the PREPARE(Primary Prevention Parameters Evaluation)study.J Am Coll Cardiol 2008; 52(7):541-550.
[21]Sweeney MO.Antitachycardia pacing for ventricular tachycardia using implantable cardioverter defibrillators: Substrates,methods and clinical experience.Pacing Clin Electrophysiol 2004;27(9):1292-1305.
[22]Wathen MS,DeGrootPJ,Sweeney MO,etal.Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverterdefibrillators:Pacing fast Ventricular Tachycardia Reduces Shock Therapies(PainFREE Rx II)Trial results. Circulation 2004;110(17):2591-2596.
[23]Yee R,FisherJD,Birgersdotter-Green U,etal.Performance and safety ofa new automatic antitachycardia pacing algorithm based upon electrophysiologic firstprinciples.Heart Rhythm 2014;11(5)Supplement:AB27-4.
[24]Dewland TA,Carter N,Jones P,et al.Influence of ventricular arrhythmia rate and device programming on antitachycardia pacing efficacy:results from the ALTITUDE study.Heart Rhythm 2014;11(5)Supplement:AB27-3.
[25]Haghjoo M,Hajahmadi M,Fazelifar AF,et al.Efficacy and safety of different antitachycardia pacing sites in the termination of ventricular tachycardia in patients with biventricular implantable cardioverter-defibrillator. Europace 2011;13(4):509-513.
[26]Gasparini M,Anselme F,Clementy J,et al.ADVANCE CRT-D Investigators.BIVentricular versus right ventricular antitachycardia pacing to terminate ventricular tachyarrhythmias in patients receiving cardiac resynchronization therapy:the ADVANCE CRT-D Trial.Am Heart J 2010;159(6):1116-1123.e2.
[27]Martins RP,Blangy H,Muresan L,et al.Safety and efficacy of programming a high number of antitachycardia pacing attempts for fast ventricular tachycardia:a prospective study.Europace 2012;14(10):1457-1464.
[28]Anguera I,Dallaglio P,Sabate X,et al.The benefit of a second burst antitachycardia sequence for fast ventricular tachycardia in patients with implantable cardioverterdefibrillators.Pacing Clin Electrophysiol 2014;37(4):486-494.
[29]Connolly SJ,Dorian P,Roberts RS,et al.Comparison of beta-blockers,amiodarone plus beta-blockers,or sotalol for prevention of shocks from implantable cardioverter defibrillators:the OPTIC Study:a randomized trial. JAMA 2006;295(2):165-171.
[30]Kurisu S,Inoue I,Kawagoe T,etal.Temporary overdriving pacing as an adjunct to antiarrhythmic drug therapy for electrical storm in acute myocardial infarction.Circ J 2005;69(5):613-616.
[31]Tan VH,Wilton SB,Kuriachan V,et al.Impact of programming strategies aimed at reducing non-essential implantable cardioverter defibrillator therapies on mortality-a systematic review and meta-analysis.Circ Arrhythm Electrophysiol 2014;7(1):164-170.
✉Corresponding author:Jiangang Zou,MD,PhD,Department of Cardiology,First Affiliated Hospital,Nanjing Medical University,300 Guangzhou Road,Nanjing,Jiangsu 210029,China.E-mail:jgzou@ njmu.edu.cn.
Received 06 November 2014,Accepted 12 December 2014,Epub 05 January 2015
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140146
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Myocardin in biology and disease
- Optimalantiarrhythmic drug therapy for electricalstorm
- Trigger elimination ofpolymorphic ventricular tachycardia and ventricular fibrillation by catheter ablation:trigger and substrate modification
- Neuraxialmodulation for treatmentof VT storm
- Prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza
- Portalvein arterialization promotes liver regeneration after extended partialhepatectomy in a rat model
