Prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza
Nahed Al Laham,Mansour Elyazji,Rohaifa Al-Haddad,Fouad Ridwan
1Department of Laboratory Medicine,Faculty of Applied Medical Sciences,Al Azhar University-Gaza,Palestine;
2Department of Biological Sciences,Faculty of Science,Al Aqsa University-Gaza,Palestine;
3Department of Biological Sciences,Faculty of Science,Al Azhar University-Gaza,Palestine.
Prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza
Nahed Al Laham1,✉,Mansour Elyazji2,Rohaifa Al-Haddad3,Fouad Ridwan3
1Department of Laboratory Medicine,Faculty of Applied Medical Sciences,Al Azhar University-Gaza,Palestine;
2Department of Biological Sciences,Faculty of Science,Al Aqsa University-Gaza,Palestine;
3Department of Biological Sciences,Faculty of Science,Al Azhar University-Gaza,Palestine.
Gastroenteritisis considered as one ofthe leading causesofillness and death in children under5 years age,especially in developing countries.It is one of the major public health problems among childhood in Gaza strip, Palestine.This study was conducted to determine the prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza.A totalof 150 stoolsamples were collected and investigated for parasitic,viraland bacterialpathogens at Al Azhar microbiology laboratories by using standard microbiological and serologicalprocedures.Out of the 150 study samples,the overallpercentage of positive stoolsamples with a known enteric pathogen was 60.6%.The prevalence ofdifferententeric pathogens causing community gastroenteritis among symptomatic cases(88.5%)was significantly higher than the prevalence in asymptomatic carriage (11.1%).The mostprevalentisolated enteric pathogens were Entamoeba histolytica(28.0%)and Giardia lamblia (26.7%).Rotavirus was found in 3.1%of symptomatic cases but not detected in asymptomatic carriage.However, adenovirus type 40 and 41 were not detected in any of the study samples.The bacterial enteric pathogens Shigella and Enterohemorrhagic Escherichia coli O157:H7(EHEC)have comparable occurrence as rotavirus(3.1%),meanwhile,Salmonella was not isolated.Mixed infection with more than 1 pathogen was found(11.4%)only among symptomatic cases.Children aged 3-year-old showed the highest prevalence of community gastroenteritis.This study demonstrates a high prevalence of parasitic enteropathogens and a relatively low prevalence of bacterial and viral enteropathogens among kindergarten children living in Gaza city,moreover,children aged 3 years old showed the highest prevalence of isolated enteropathogens.
community gastroenteritis,enteropathogens,Gaza,kindergarten
Introduction
Worldwide,diarrhealdiseases are continued to be a common health problem that increase the financial burden on the health systemsparticularly in developing countries.Acute gastroenteritis is considered as one of the leading causes of illness and death in children under the age of 5 years[1-3],and is characterized by acute onset of diarrhea,which may or may not be accompanied by nausea,emesis,fever,abdominalpain and dehydration.Acute gastroenteritis is prevalent worldwide and associated with high rates ofmorbidity and mortality in developing countries[4-7].In Gaza,acute gastroenteritis is considered as a common infectionamong children and is known to cause high morbidity and mortality rates if they do notreceive propertreatmenton time[8,9].
Gastroenteritis transmission is spread by person to person or ingestion of contaminated food and drink.Ingestion of food containing toxins produced by bacterialcontaminants causes rapid onsetofvomiting and/or diarrhea.Water is also contaminated by bacteria,viruses,or protozoa thatcause gastroenteritis[7,10].A person continues to excrete enteropathogens after an episode of acute gastroenteritis or even be an asymptomatic carriage without previous disease. Asymptomatic carriers of parasitic,viral or bacterial pathogens have epidemiological importance due to their influence as a source of infection[10].
The etiologic agents include a wide variety of bacteria,viruses and parasites.The commonest are rotaviruses,Salmonella,Shigella,Campylobacter jejuni, enteropathogenic Escherichia coli(EPEC),enterotoxigenic E.coli(ETEC)and Yersinia enterocolitica.Parasitic agents,especially Cryptosporidium,Entamoeba histolytica,and Giardia lamblia are also etiologicalagents of diarrhea in children[11].Severe clinicaldiseases and mortality are associated with these intestinalpathogens which are known to cause malnutrition and impairment of physical developmentin children and affecttheir growth and learning[12].
Gaza Strip(geographic coordinates 31°25ˊN,34° 20ˊE)is a narrow region(365 km2)of land along the Mediterranean coast,just 41 km long and 6-12 km wide.It is one of the most heavily populated areas(4,383 persons/km2)of the world with a total population about1.6 million people[13].In the present work,we attempted to investigate the prevalence of differententeric pathogens causing community gastroenteritis among kindergarten children in Gaza.
Subjects and methods
Study population and sample collection
This study was performed from the beginning of January to the end of June 2011.One hundred fifteen written requests were sentto the parents or guardians of the symptomatic cases to have their approval to involve their child in the study.Thankfully,96 (response rate 83.5%)were agreed to participate,and they represent15 kindergartens of the differentlocalities and population clusters.Also a response rate of 72.0%(54/75)was achieved forthe asymptomatic carriage(apparently healthy controls)of same kindergartens.The study protocol and data handling were approved by the ethical committee at the Biology Department,Al Azhar University-Gaza.The study was performed in accordance with the ethicalstandards established in the 1964 and 1975 Declaration of Helsinki,and the modifications thereafter.A simple questionnaire that included personal,demographic and past history of diseases and treatment was constructed and conducted in Arabic language.The questionnaire was distributed to the parents who agree to include their children in this study prior to sample collection for filling and return with collected sample. Fresh stool samples were collected from cases and controls in labeled plastic vials without preservatives. Diarrhea was defined as the excretion of at least 2 loose stools in a 24-hour period[14].Any child suffering from any chronic disease or on antibiotics treatment was excluded from participation.Stool samples were transferred to the microbiology laboratory in ice box and processed within 4 hours of collection.
Microbiological methods
Standard microbiologicalprocedures and guidelines as described by the American Clinical Laboratory Institute(CLSI)were used.For the identification of parasites,fresh stool specimens prepared with saline and/or iodine were directly examined.Briefly,wet mount preparation of stool in 0.9%saline and/or iodine was examined microscopically for the presence of protozoa cysts and trophozoites or helminthes ova or larva and adult stage.Moreover,flotation and sedimentation techniques were performed on allsamples that gave negative resultusing direct microscopy examination.
Detection of bacterial agents
Fresh stoolsamplesweredirectly plated onto salmonellashigella(SS),Hecktoen enteric(HE),and Sorbitol MacConkey agar(SMAC)agars(HiMedia,India)and incubated for 18 to 24 hours at 37°C.Moreover, approximately 1 g of each sample was inoculated into 10 mL of selenite cysteine broth(SCB)(HiMedia, India),and incubated for 18 to 24 hours at 37°C. Approximately 0.5 mL ofinoculated SCB was subcultured onto SS and HEagarsand incubated asmentioned above.Suspected colonies on the primary and subculture plateswere furtheridentified by standard laboratory procedures as colony morphology,Gram staining,and biochemical tests including oxidase,urease and API20E system.Polyvalentantisera against Shigella and E.coli O157:H7 were used forconfirmation.
Detection of viral agents
Enzyme immunoassay was used for the detection of group A rotavirus and adenovirus serotype 40 and 41according to the instructionsofthe manufacturer.A total of100μL ofstoolspecimen was diluted in appropriate amounts of sample buffer included in the commercial GastroVir-Stripcolor kit(Cori BioConcept,Belgium). The GastroVir-Stripcolor kitwas performed to detect group A rotaviruses and the most human adenovirus serotypes.
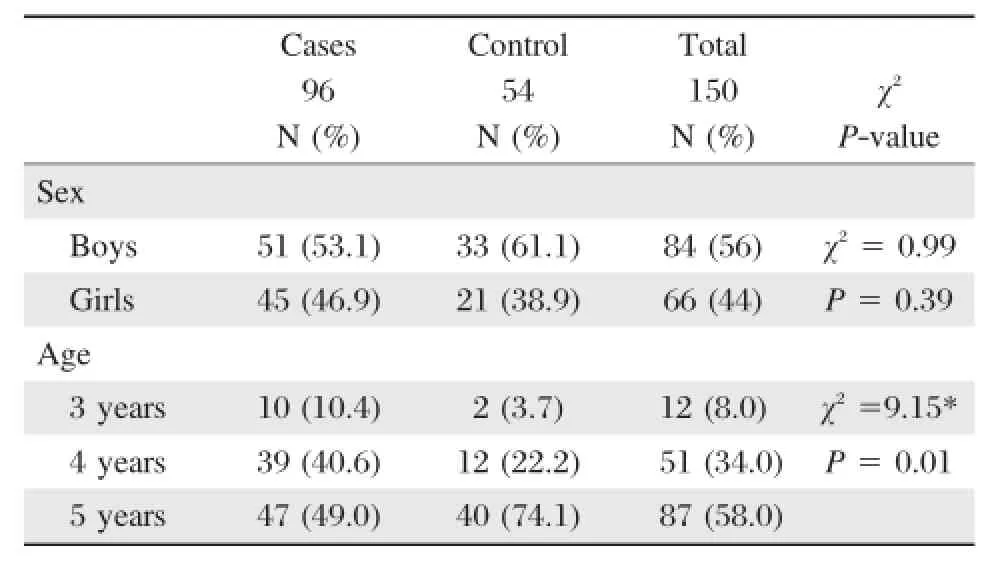
Table 1 Sex and age characteristics of the study samples
Statistical analyses
Data were tabulated,encoded and statistically analyzed using the Statistical Package for the Social Sciences (SPSS)version 15 software(IBM Corporation,Somers, NY).Discrete variables were expressed as percentages. Data were compared using Pearson Chi-square and the rank-sum test(Wilcoxon-Mann-Whitney test)asappropriate.The levelofstatisticalsignificance was setat P<0.05.
Results
The presentstudy was a cross-sectionaldescriptive study that included 150 kindergarten children from Gaza city.The stoolsamples(Table 1)were collected from children from both genders(56%male and 44% female).The ages of children enrolled in this study were 3(8%),4(34%)and 5(58%)years old.According to the study protocol,96 diarrhealstoolsamples were collected from 51 boys(53.1%)and 45 girls(46.9%) and they considered assymptomatic cases.On the other hand the asymptomatic carriage(controls group) included 33 boys(61.1%)and 21(38.9%)girls.
According to the rank-sum test(Wilcoxon-Mann-Whitney test)significant difference was reported between cases and controls in terms of the age of the children.Cases are significantly youngerthan the control;mean ranks were 68.50 and 87.94 respectively,P =0.003.
The highest percentage of diarrheal stool samples (10/12,83.3%)was collected from the 3-year-old group,followed by the 4-and 5-year-old group(39/ 51,67%and 47/87,54%,respectively).There was a significantdifference between the percentage of diarrhea in the 3-year-old group and other age groups(P =0.01).
Overall(Table 2),there are 91 samples(60.7%) positive for parasitic,bacterial,and/or viral enteric pathogens.The parasitic etiologic agents had the highestprevalence rate(54.7%)in comparison to the bacterial(4%)and viral(2%)etiologic agents.More than halfofsamples collected were positive forone ormore parasitic agent.The highest isolated enteric pathogen was E.histolytica(28%),followed by G.lamblia (26.7%).However,adenovirus and Salmonella were notisolated from alltested samples.The overallprevalence of positive stools from symptomatic cases was significantly higher(88.5%)than that isolated from asymptomatic controls(11.1%).E.histolytica was the mostprevalentin diarrhea cases(43.8%),followed by G.lamblia(35.4%).Rotavirus,Shigella spp.and E.coli O157:H7 has 3.1%for each(Table 2,and Fig.1).Alltypes ofenteric pathogens including viral, bacterial and parasitic agents were isolated fromsymptomatic cases samples,while only parasitic agent G.lamblia was isolated from asymptomatic controls (Table 2).
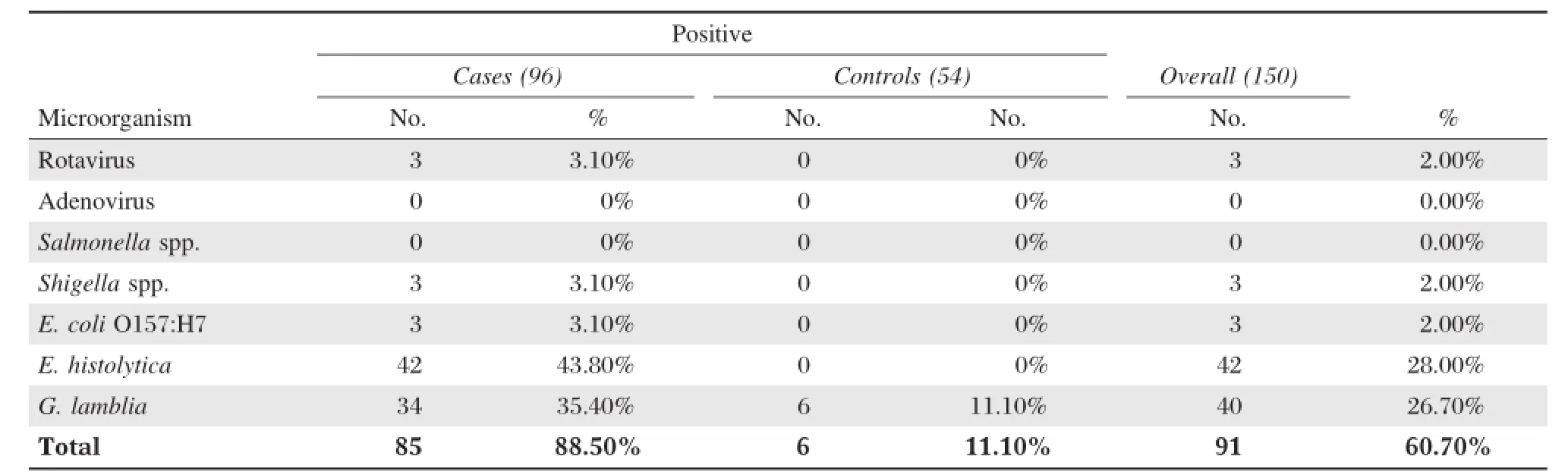
Table 2 Enteric pathogens isolated from symptomatic cases and asymptomatic carriage(controls)
Fig.1 Prevalence of different parasitic,viral and bacterial enteropathogens among symptomatic cases.
Table 3shows the numberand percentage ofpositive samples among different age groups.In 3 years age group children,E.histolytica was the mostprevalent(75%)isolated pathogen.Rotavirus and Shigella were found in only 8.3%,while E.coli O157:H7 and G.lamblia were not detected in this age group. Children from the 4-year-old group also have E.histolytica as the commonest(33.3%)isolated enteric pathogen followed by G.lamblia(27%).However, Rotavirus was isolated from 3.9%of the children and Shigella spp.was recovered from 1.9%,which represents the lowest isolated enteric pathogen in the 4-year-old group.Whereas,the enteric pathogen E.coli O157:H7 was not detected.Interestingly,in the 5-year-old group,the prevalence profile was different where G.lamblia infection was the most frequent (29.9%)isolated enteric pathogen followed by E.histolytica(18.4%),E.coli O157:H7(3.4%)and Shigella (1.1%).Nevertheless,rotavirus was notdetected.
Parasitic pathogens had the highest prevalence in comparison to the other enteric pathogens,where it accounted for more than half of the screened samples (89/150;59.3%).Overall[34(85.0%)from symptomatic casesand 6(15.0%)from asymptomatic controls]. E.histolytica was detected in 42(28%)samples outof the totalgrand,where allof them(100%)were from cases(Table 4).Statistically significantprevalences were reported for G.lamblia and E.histolytica among symptomatic cases as compared to asymptomatic controls(P<0.0002 and P<0.0001,respectively).
Viral pathogens had the lowest prevalence(2%) among enteropathogens thatwere isolated in this study. Most of the cases(93/96;96.9%)and all control samples(54/54;100%)were negative.Rotavirus was isolated from 3(2%)samples;all of them were reported in symptomatic cases.The difference in the presence and absence ofrotavirus in cases and controls was notsignificant(P=0.189).However,the second viral pathogen adenovirus was not isolated from any sample.
Bacterial pathogens had also lower prevalence in comparison to the parasitic pathogens(6/150,4%). Regarding the prevalence of bacterial pathogens in cases and control samples,Shigella spp.and E.coli O157:H7 were isolated from 3(2%)samples;all of them were reported in symptomatic cases.The difference in the presence and absence of Shigella spp.and E. coli O157:H7 in casesand controlswasnotsignificant(P =0.189).Finally,Salmonella was notisolated from any sample(Table 4).
Table 5 shows that E.histolytica was detected in 90%,43.6%and 34%of cases in the 3-,4-and 5-year-old groups,respectively.The prevalence of E. histolytica in the 3-year-old group was significantly higher than that of the other age groups.G.lamblia was detected in 0.0%,33.3%and 44.7%of cases in the 3-,4-and 5-year-old groups,respectively. However,G.lamblia was found in 8.3%and 12.5% of controlsamples(asymptomatic)collected from the 4-and 5-year-old groups,respectively.A statistical difference was found between the prevalence of parasitic infection among cases and control samples in all age groups with P value of 0.007,<0.0001 and<0.0001 in the 3-,4-and 5-year-old groups,respectively.Rotavirus was isolated from 10%,5.1%and0.0%of cases in the 3-,4-and 5-year-old groups, respectively.
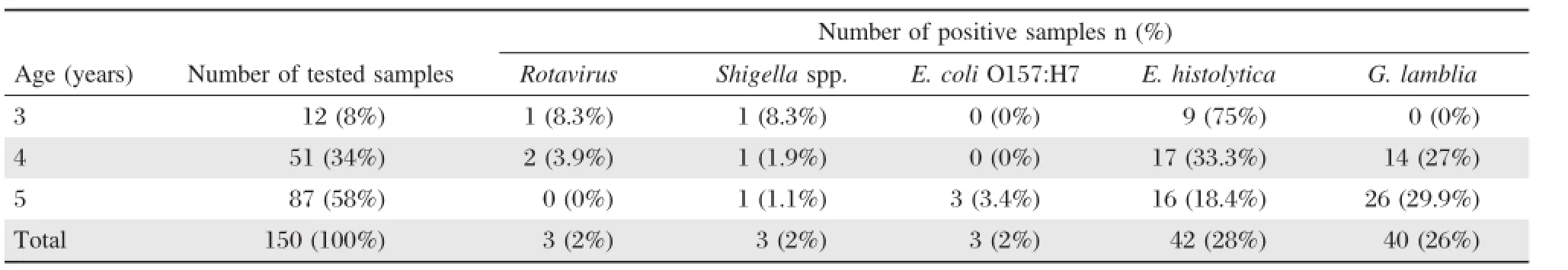
Table 3 Distribution of enteric pathogens isolated from stools according to age
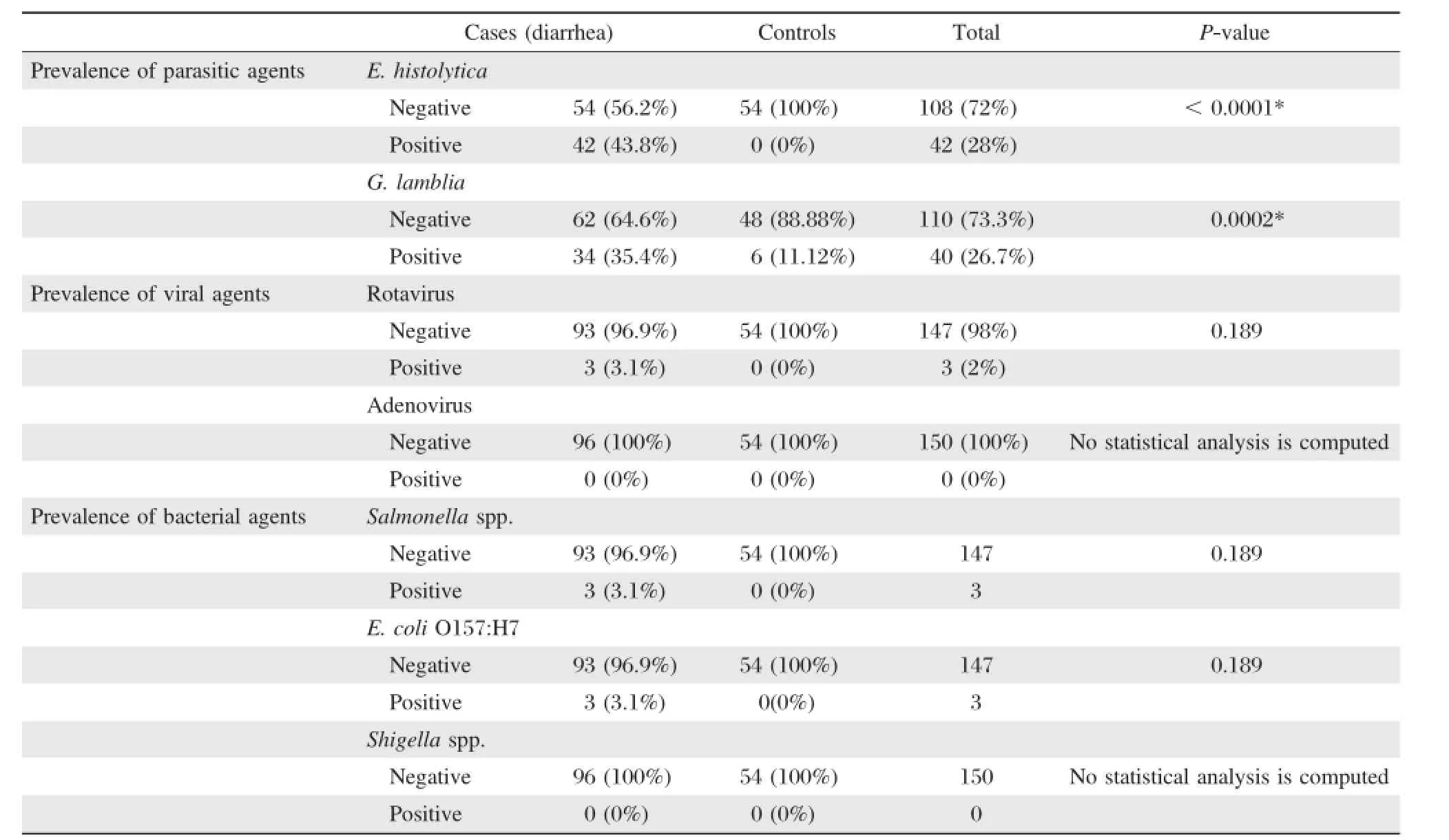
Table 4 Prevalence of parasitic,viral and bacterial pathogens among symptomatic cases and asymptomatic carriage(controls)
The prevalence ofrotavirusin the 3-year-old group was higherthan thatofthe otherage groups.Meanwhile,rotavirus was not detected in controls.There was no significantdifference in the prevalence of rotavirus infection between cases and controls in the 3-and 4-year-old groups(P>0.05).Shigella spp.was isolated from 10%,2.6%and 2.1%of cases in the 3-,4-and 5-yearold groups,respectively.
The prevalence of Shigella spp.in the 3-year-old group was higher than that of the other age groups. There was no statisticaldifference in the prevalence of Shigella spp.infection between cases and controls in allage groups(P>0.05).E.coli O157:H7 was only isolated(6.4%)from cases in the 5-year-old group. Therefore,all the asymptomatic control samples (100%)in all age groups were negative for bacterial infections.
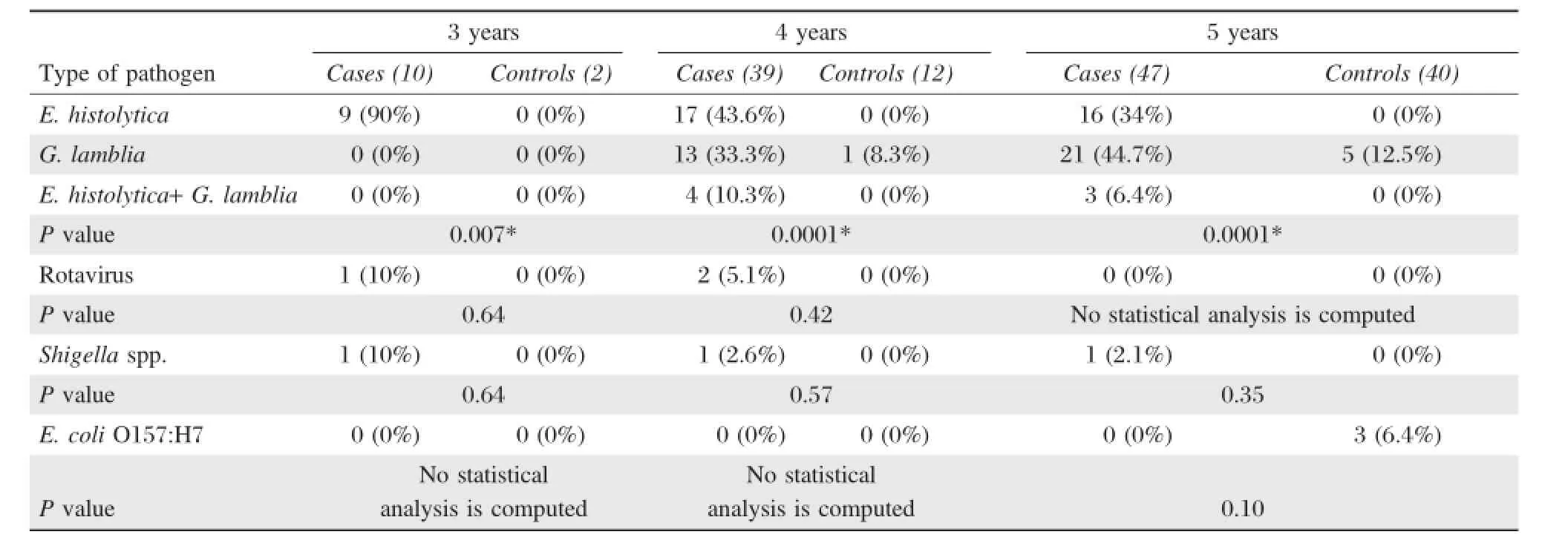
Table 5 Prevalence of different enteric pathogens among symptomatic cases and asymptomatic carriage (controls)according to age group
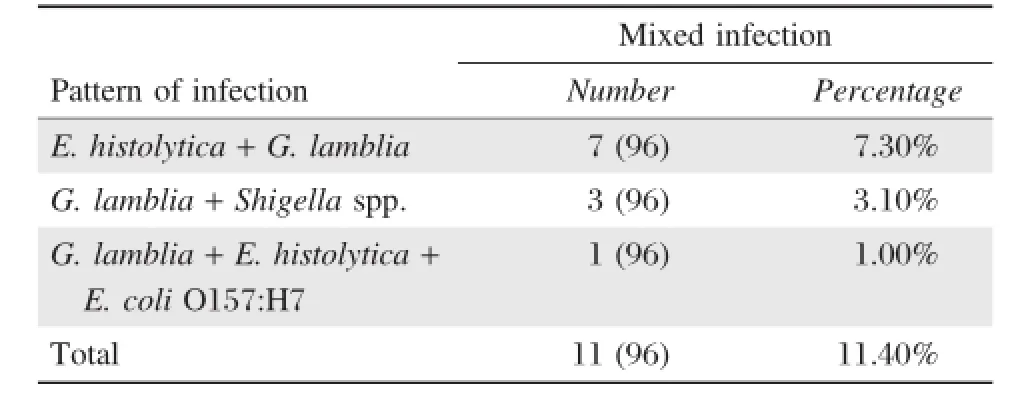
Table 6 Prevalence of mixed infections among symptomatic cases*
Mixed infection with more than one enteric pathogen was detected in 11.4%(11/96)of the symptomatic cases(Table 6).Mixed infection with E.histolytica and G.lamblia;G.lamblia and Shigella;or G.lamblia, E.histolytica and E.coli O157:H7 were detected in 7 (3.7%),3(3.1%)and 1(1.0%)samples,respectively. Nevertheless,all mixed infections were only isolated from the symptomatic cases.
Discussion
Diarrhealdiseases are a majorpublic health problem for children in Gaza strip,as in other developing communities around the world.The WHO reported that diarrheal diseases caused by viruses,parasites and enteropathogenic bacteria are a leading cause of morbidity and mortality in children;with 1.5-2.5 million deaths estimated to occur annually among children aged less than 5 years and mostly in developing countries[15].This study was aimed to determine the prevalence of common enteropathogens including parasitic, viraland bacterialagents among kindergarten children suffering community gastroenteritisin Gaza.Outofthe 150 stool samples tested,60.7%were positive for enteropathogens.Comparing our findings with other studies conducted in Gaza strip and our region,it is in accordance,higher or sometimes lower than what was reported.In Gaza strip,Abu Elamreen et al.and Elmanama et al.found 51.5%and 66.7%positive stools for common enteropathogens in children aged less than 5 years with acute gastroenteritis and diarrhea[9,16].In a study conducted in Jordan by Youssef etal.,the prevalence of enteric pathogens in hospitalized children(aged 1-5 years)is in agreement with our findings(66.4%).However,Nimri and Meqdam in Jordan reported a higher prevalence than our study where they found 77.8%oftheirsamples had a potential enteric pathogen[1,17].In Saudi Arabia,El-Sheikh and El-Assouliin Jeddah reported a lower prevalence of enteropathogens(45.6%)than our findings in fecal samples collected from children(aged 0-5 years)suffering from acute diarrhea.In a recentstudy by Johargy et al.in Saudi Arabia,the authors reported enteropathogens in 39%of tested stool samples collected from pediatric patients aged less than 5 years[18,19].
The overallprevalence of enteropathogens in cases (diarrhea)and the asymptomatic control group (asymptomatic)was 88.5%and 11.1%,respectively. Comparing our findings with some published studies, there is consistency with some and discrepancy with others.For example,in Vietnam in a case-control study,Hien et al.identified enteric pathogens in 66% and 36%of cases and controls,respectively[20].Albert et al.in Dhaka,Bangladesh,reported 74.8%and 43.9%potentialenteric pathogen in caseswith diarrhea and controlswithoutdiarrhea in children aged less than 5 years,respectively[21].In Northwest Germany, Karsten etal.studied the incidence ofcommunity gastroenteritis of rural and urban population and found 35.8%of diarrheal samples(cases)were positive for enteropathogens and 8.7%were positive in the control group[10].These variations could be explained by the differences in the study population,endemicity of enteropathogens,socioeconomic situation,seasonal variations,laboratory techniques used and specific practices prevalentamong the differentpopulations.
In this study,59.2%of the examined children suffered from parasitic infection with one or more parasites.Several studies conducted in Gaza strip investigated the prevalence of parasitic infection among different age groups in different places and times around the year.Some of these studies are in accordance with our findings and others had higher orlower prevalence levels.Our findings(59.2%)were higher than thatreported by Yassin etal.and Shubair et al.in Gaza city(27.6%and 24.5%,respectively). However,in another study by AL-Zain etal.in Gaza strip,a higher prevalence than our findings was reported(72.9%).Abu Elsoud etal.in Egyptstudied the predictors of the intestinal parasitic infection among pre-school children living in the rural areas and found that 27%of them had intestinal parasitic infection[22-25].The most common enteric pathogen found was E.histolytica(28%).Other studies conducted in Gaza showed that E.histolytica was the most isolated pathogen from children[9,23,26].The prevalence rate of infection with G.lamblia was 26%.Many studies in the area reported lower or higher prevalence ofthis parasite depending upon the characteristics ofeach study.In 4 differentstudiesallconducted in Gaza strip, the prevalence of G.lamblia was 1.33%,8%,10.3% and 62%,respectively[23,27-29].These differences could be primarily explained by the differences in target population,time and duration of each study,seasonal variation,size of sample,and place of each study.In addition,it could be due to the influence of environmentalconditions,hygiene practice,levelof sanitation and laboratory techniques.
Rotavirus was isolated from 3.1%of cases.In some previous studies around the world,rotavirus was found asone ofthe mostfrequently detected enteropathogens, with high isolation rate as in Gaza,Tunisia,Saudi Arabia,Jordan,Europe and South America[1,10,18,30-34]. These differences could be explained by the difference of the age groups thatwere investigated in each study and also the laboratory methods used such as ELISA or PCR techniques.The targetpopulation in mostof these studies was infants and very young children who,according to the literature,have rotavirus as the commonestcausative agentof gastroenteritis.In fact, ourstudy focused on children aged 3-5 yearswhere less prevalence of rotavirus could be present.When we compared our findings to a study conducted in Gaza on similar age groups,almostthe same prevalence of rotavirus was detected[27].Moreover,in Bangladesh, Albertetal.studied 814 children with diarrhea.The children were up to 5 years of age.The isolation rate ofrotavirus was 4.2%,2.4%,and 1.2%from the 3-,4-and 5-year-old groups,respectively.These findings are in accordance with our findings in the three age groups(3,4 and 5 yearsold)[21].Ourstudy indicated that there was a tendency of decreasing rates of rotavirus infection in older children.This might partly be explained by the factthatolderchildren acquired protective immunity during previous,probably sub-clinical exposures to rotavirus,and as a resultbecome more resistantto infection with this agent[35].
Regarding the prevalence of bacterial pathogens, Shigella spp.and E.coli O157:H7 were isolated from 3.1%of symptomatic cases.Al Jarousha etal.studied bacterial enteropathogens associated with childhood diarrhea and found that1.6%and 2.3%of cases were affected by Shigella spp.and E.coliO157:H7,respectively[36].However,Abu El-Amreen etal.reported 4% of Shigella spp.and 0.24%of E.coli O157:H7, although Yousif et al.reported prevalence of 4.9%and 5.7%of Shigella spp.and E.coli O157:H7,respectively.These results are largely in agreement with our findings in the same age groups.
Finally,we noticed that the infection rate was decreased as the age increased.These findings are similar to other findings published in the country and our region where the highest prevalence of enteric pathogens was mostly detected in younger children[1,9,16,18,23].However,as a limitation of this study, we could not exclude all other causative agents such as anaerobic and/or fastidious growing bacteria and some viruses like norovirus which require specialprocedures and equipmentthat are notavailable in Gaza.
In conclusion,th is study demo nstrates a high prevalence ofparasitic enteropathogensand a relatively low prevalence of bacterialand viralenteropathogens among kindergarten children in Gaza.No difference in the prevalence of diarrhea or infection was detected between males and females.The 3-year-old group showed a significant higher positive rate of enteropathogens in comparison to the 4-and 5-year-old groups.
[1]Youssef M,Shurman A,Bougnoux M,et al.Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan. FEMS Immunol Med Microbiol 2000;28(3):257-263.
[2]Lorgelly K,JoshiM,Gomara I,etal.Infantgastroenteritis in the community:a costof illness study.Epidemiol Infect 2008;136(1):34-43.
[3]Navaneethan U,Giannella R A.Mechanisms of infectious diarrhea.Nat Clin Pract Gastroenterol Hepatol 2008; 5(11):637-647.
[4]Fr u¨hwirth M,Heininger U,Ehlken B,et al.International variation in disease burden of rotavirus gastroenteritis in children with community-and nosocomially acquired infection.Pediatr Infect Dis J 2001;20(8):784-791.
[5]OˊRyan M,Prado V,Pickering LK.A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis 2005;16(2):125-136.
[6]Parashar UD,Bresee JS,Glass RI.The global burden of diarrheal disease in children.Bull World Health Organ 2003;81(4):236.
[7]Elliott E J.Acute gastroenteritis in children.BMJ 2007 6;334(7583):35-40.
[8]WHO.Diarrhea:Why children are still dying and what can be done.(NLM classification:WS 312),Geneva.
[9]Abu Elamreen F,Abed A,Al sharif F.Detection and identification of bacterial enteropathogens by polymerase chain reaction and conventional techniques in childhood acute gastroenteritis in Gaza.Int J Infect Dis 2007;11(6): 501-507.
[10]Karsten C,Baumgarte S,Friedrich AW,et al.Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2 004.Eur J Clin Microbiol Infect Dis 2009;28(8):935-943.
[11]Chao HC,Chen CC,Chen SY,et al.Bacterial enteric infections in children:etiology,clinical manifestations and antimicrobial therapy.Expert Reu Anti Infect Ther 2006;4(4):629-638.
[12]Samie A,Guerrant R L,Barrett L,et al.Prevalence of Intestinal Parasitic and Bacterial Pathogens in Diarrhealand Non-diarrheal Human Stools from Vhembe District, South Africa.J Health Popul Nutr 2009;27(6):1-7.
[13]PCBS,ASpecialBulletin on the Palestinian Population as the World Population Reaches VIIBillion,the Palestinian Central Bureau of Statistics Ramalla,Palestine,2011.
[14]Huppertz HI,Busch D,Schmidt H,et al.Diarrhea in young children associated with Escherichia coli non-O157 organisms that produce Shiga-like toxin.J Pediatr 1996;128(3):341-346.
[15]Kosek M,Bern C,Guerrant R.The globalburden ofdiarrhealdisease as estimated from studies published between 1992 and 2000.Bull WHO 2003;81(3):197-204.
[16]Elmanama A,Abdelateef N.Antimicrobial Resistance of Enteric Pathogens Isolated from Acute Gastroenteritis Patients in Gaza strip,Palestine.IAJAA 2012;2(4):4.
[17]Nimri L F,Meqdam N.Enteropathogens associated with cases of gastroenteritis in a rural population in Jordan. Clin Microbiol Infect 2004;10(7):634-639.
[18]El-Sheikh SM,El-Assouli SM.Prevalence of Viral, Bacterial and Parasitic Enteropathogens among Young Children with Acute Diarrhea in Jeddah,Saudi Arabia. J Health Popul Nutr 2001;19(1):25-30.
[19]Johargy A,Ghazi H,Mummenah A.Frequency of viral, bacterial and parasitic enteropathogens among young children with acute diarrhea in Saudi Arabia.J Pak Med Assoc 2010;60(6):456-459.
[20]Hien BTH,Trang DT,Scheutz F,et al.Diarrhoeagenic Escherichia coli and other causes of childhood diarrhea: a case-control study in children living in a wastewateruse area in Hanoi,Vietnam.J Med Microbiol 2007; 56(Pt 8):1086-1096.
[21]Albert JM,Faruque AS,Faruque SM,et al.Case-Control Study of Enteropathogens Associated with Childhood Diarrhea in Dhaka,Bangladesh.J Clin Microbiol 1999; 37(11):3458-64.
[22]Yassin MM,Shubair ME,Al-Hindi AI,et al.Prevalence of Intestinalparasites among schoolchildren in Gaza city, Gaza Strip.J Egypt Soc Parasitol 1999;29(2):365-373.
[23]Shubair ME,Yassin MM,Al-Hindi AI,et al.Intestinal parasites in relation to hemoglobin level and nutritional status of school children in Gaza.J Egypt Soc Parasitol 2000;30(2):365-375.
[24]Alzain B,Al-Hindi A.Distribution of Strongyloides stercoralis and other intestinal parasites in household in Beitlah ia city Gaza strip,Palestine.Ann Al Qud s Med 2005;1(1):48-52.
[25]Abu El-soud F,Salama RA,Taha NS.Predictors of the Intestinal Parasitic Infection among Pre-schoolChildren in RuralLower,Egypt.EgyptJComm Med 2009;27(1):17-34.
[26]Alagha R,Teodorescu I.Prevalence of intestinalparasites in three localities in Gaza governorates-Palestine.Arch Public Health 2002;60:363-370.
[27]Abu-Elamreen FH,Abed A,Sharif FA.Viral,Bacterial and Parasitic Etiology of Pediatric Diarrhea in Gaza, Palestine.Med Princ Pract 2008;17(4):296-301.
[28]Astal Z.Epidemiological survey of the prevalence of parasites among children in Khan Younis governorate, Palestine.Parasitol Res 2004;94(6):449-451.
[29]Al-Hindi AI,El-KichaoiA.Occurrence of Gastrointestinal Parasites Among Pre-School Children,Gaza,Palestine. IUGJ 2008;16(1):125-130.
[30]Sdiri-Loulizi K,Gharbi-Khelifi H,de Rougemont A,etal. Acute Infantile Gastroenteritis Associated with Human Enteric Viruses in Tunisia.J Clin Microbiol 2008;46(4): 1349-1355.
[31]KheyamiAM,AreeshiMY,Dove W,etal.Characterization ofrotavirus strains detected among children and adults with acute gastroenteritis in Gizan,Saudi Arabia.Saudi Med J 2008;29(1):477-480.
[32]Forster J,Guarino A,Parez N,et al.Hospital-Based Surv eillance to Estimate th e Burden of Rotavirus Gastroenteritis Among European Children Younger Than 5 Years of Age.Pediatrics 2009;123(3):393-400.
[33]Jansen A,Stark K,Kunkel J,etal.Etiology of community acquired,acute gastroenteritis in hospitalized adult:prospective cohort study.BMC Infect Dis 2008;8:143-150.
[34]Ospino DU,Young G,Navarro O.Viralgastroenteritis and diversity ofrotavirus strains in Colombian children:a systematic review.J Infect Dev Ctries 2008;1;2(2):99-105.
[35]Bernstein DL.Rotavirus overview.Pediatr Infect Dis J 2009;28(3 Suppl):S50-3.
[36]Al Jarousha AM,El Jarou MA,El Qouqa IA.Bacterial Enteropathogens and Risk Factors Associated with Childhood Diarrhea.Indian J Pediatr 2010;78(2):165-70.
✉Corresponding author:Nahed Al Laham,PhD,Department of Laboratory Medicine,Faculty of Applied Medical Sciences,Al Azhar University-Gaza,P.O.Box 1277,Gaza,Palestine.Tel/Fax:+970 599 560533/+970 8 2823180,E-mail:dr.allaham@hotmail.com andn.lahamm@alazhar-gaza.edu.
Received 17 July 2013,Revised 24 September 2013,Accepted 24 July 2014,Epub 02 October 2014
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20130108
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Ventricular tachycardia ablation and substrate modification in ICD patients with electrical storm
- Induced pluripotentstem cells are induced pluripotentstem cell-like cells
- HLA antigens and anti-sperm antibody production in Iranian vasectomized men
- Unruptured pregnancy in a noncommunicating rudimentary horn at 37 weeks with a live fetus:a case report
- Surgicaloutcomes ofmini-open Wiltse approach and conventional open approach in patients with single-segment thoracolumbar fractures withoutneurologic injury
- Portalvein arterialization promotes liver regeneration after extended partialhepatectomy in a rat model
