Portalvein arterialization promotes liver regeneration after extended partialhepatectomy in a rat model
Jian Li,Chaonong Cai,Hui Guo,Xiaodong Guan,Lukun Yang,Yuechan Li,Yanhua Zhu, Peiping Li,Xialei Liu,Baimeng Zhang,✉
Departments of1General Surgery,2Radiology,and3Anesthesiology,the 5th Affiliated Hospital,Sun Yat-sen University, Zhuhai,Guangdong 519000,China.
Portalvein arterialization promotes liver regeneration after extended partialhepatectomy in a rat model
Jian Li1,△,Chaonong Cai1,△,Hui Guo2,△,Xiaodong Guan1,Lukun Yang3,Yuechan Li1,Yanhua Zhu1, Peiping Li1,Xialei Liu1,Baimeng Zhang1,✉
Departments of1General Surgery,2Radiology,and3Anesthesiology,the 5th Affiliated Hospital,Sun Yat-sen University, Zhuhai,Guangdong 519000,China.
In the currentstudy,we soughtto establish a novelratmodelofportalvein arterialization(PVA)and evaluate its impact on liver regeneration after extended partialhepatectomy(PH).A total of 105 Sprague-Dawley rats were randomly assigned to three groups:68%hepatectomy(the PH group),portalarterialization after68%hepatectomy (the PVA group),and rightnephrectomy only(the controlgroup).Liverregeneration rate(LRR),5-bromo-2-deoxyuridine(BrdU)labeling index,and liverfunctions were assessed on postoperative day 2,7,14 and 28.The 28-day survival rates were compared among the three groups.The 28-day survival rates were similar in all groups(P= 0.331),and the anastomotic patency was 100%.The LRR in the PVA group was significantly higher than that of the PH group within postoperative 14 days(P<0.05).The PVA and PH group had increased serum alanine aminotransferase levels(232±61 U/L and 212±53 U/L,respectively)compared with the control group(101 ±13 U/L)on postoperative day 2,whereas from postoperative day 7 to day 28 there were no differences among the three groups.Serum albumin values were higher after the PVA procedure within postoperative day 14,which gradually became comparable on postoperative day 28 among the three groups.The peaks of BrdU labeling index appeared on postoperative day 2 in all rats,and the PVA procedure was associated with increased BrdU labeling index from postoperative day 7 to 28.The 28-day survivalof the PVA rats was comparable.Ourfindings demonstrate that the PVA procedure utilizing portal vein trunk-renal artery microvascular reconstruction promotes remnant liver regeneration and confers beneficial effects on maintaining and even optimizing liver function after extended partial hepatectomy in rats.
portalvein arterialization,microsurgery,hepatectomy,liver regeneration,liver function
Introduction
Portal vein arterialization(PVA)was initially introduced to improve encephalopathy and prevent liver failure after portacaval shunt in cirrhotic patients[1]and massive necrosis due to obstruction of the hepatic artery after extended hepatopancreatobiliary surgery[2]. Portalvein arterialization combined with portacaval shunt increases blood supply to the liver through the arterialization of the portal stump by an artery[3-7].In orthotopic and heterotopic liver transplantation,permanent portal vein arterialization was performed in cases with insufficient portal flow to the graft and showed beneficialeffects on shortening warm ischemiatime of the donor liver and consequently reducing the incidence of immediate postoperative graft failure[8,9]. In the event of an extensive portal vein thrombosis or the absence of a mesenteric vein as an anatomic variant,PVA ensures an adequate blood supply to an orthotopic graft[10-12].In addition,some authors have emphasized a proliferation-promoting influence of PVA on the liver tissue and recommended PVA as a therapeutic option in acute liver failure or afterextensive liver resection[13-15].
A steady supply of oxygen is necessary for liver regeneration after hepatectomy[16,17].The effects of portal arterialization on hepatic regeneration after partial hepatectomy have been investigated by several authors and beneficial effects on hepatic regeneration have been reported[18,19].However,other authors suggested that the destruction of hepatic architecture brought about portalhypertension and thatmitosis in the regenerating liver was not enhanced[20,21].There are no proven answers for regenerative ability of the liver after PVA.Furthermore,due to the various sizes of vessels between portal vein and alternative artery, microsurgical technical obstacles of this direct hand suturing anastomosis were overcome in the lastdecades. The models presented in literatures were established by various stentcannula techniques,in which the simulation of the PVA is insufficient;thus,the outcomes of differentmodels are notcomparable and debatable.
The conflicting evidence prompted us to attempt a more simulative technique of end-to-end arterialvenous anastomoses following an extended partial hepatectomy(68%hepatectomy)to establish a novel PVA model.Liver growth and regeneration rate were compared among rats with either venous portalinflow or arterialized portal inflow after extended partial hepatectomy,and then proliferation of hepatocytes was examined.Therefore,we determined whether this PVA procedure,which increased oxygen concentration in the portalvein,was an effective approach to the promotion of liver regeneration rate(LRR)and the improvementof liverfunction after extended hepatectomy.
Materials and methods
Experimental animals
Male specific pathogen free(SPF)Sprague-Dawley rats weighting from 240 to 300g were used in this study.The study protocolwas approved by the local institutional review board at the authorsˊaffiliated institutions,and animalwelfare as wellas the experimentalprocedures was carried out in accordance with the established institutional and state guidelines regarding animal care and use.All rats were housed in environmentally controlled conditions(a temperature-controlled environment,a 12 hours light/dark cycle with the light cycle from 6:00 to 18:00 and the dark cycle from 18:00 to 6:00)with ad libitum access to a rodentchow dietand water.After an acclimation period of 1 week,all rats were fasted for 24 hours before surgery.Sixty rats were randomly assigned into 3 groups with 20 rats per group forspecimen harvesting,and another45 rats were divided into 3 groups for survival data collection and analyses.
Surgical procedure
The procedures were performed underaseptic techniques and ether anesthesia via inhalation.A heating pad was used to maintain a stable body temperature at approximately 36°C throughout the surgery. Through a median laparotomy,the liver was mobilized from its supporting ligaments.The right renal artery was isolated from its origin to the bifurcation,and the rightrenalvein was separated from the renalpedicle to the vena cava.The ureter was sectioned and nephrectomy was performed in allgroups.
In the PH group,a 68%partial hepatectomy was accomplished as described by Higgins and Anderson[22].The median and left lobes of the liver were excised after placementofa 5-0 silk suture ligature on the corresponding pedicle.Then,the portal triad clamping was performed for20 minutes(equivalentto the average time needed to perform PVA and porto-renalshuntin rats of the PVA group).
In the PVA group,ligation and division ofthe pyloric vein was carried outfollowing 68%partial hepatectomy,and then transection at the lower 1/3 of the portal vein trunk was completed after it was gently isolated from the hepatic hilum to the confluence of the superior mesenteric and splenic veins.The right renal artery was sectioned at the bifurcation and reshaped as an artery disc to fix the caliber of the hepatic inflow p ortal trunk.After irrigation with heparinized solution,an end-to-end anastomotic reconstruction between the right renalartery disc and the hepatic inflow portal trunk was accomplished by a microsurgical semi-continuous suture technique, which 6 stitches were placed on the anterior wallfirst, and then another6 stitcheswere placed on the posterior wallaftera 180-grade rotation ofthe vessel.Finally,an end-to-end porto-renal(confluence of the superior mesenteric and splenic veins-right renal vein)shunt was completed according to the techniques by Lee and Fisher[23].Then,10-0 polypropylene non-absorbable monofilament sutures(SurgiproTMII-SurgiproTMSuture,Covidien,MA,USA)were utilized in vascularanastomosis.Anastomotic patency was examined after the vascular procedures were finished.The vascular reconstruction was operated under a microsurgical telescope with 10-16×magnifications.The peritonealcavity was closed in 2 layers(Fig.1).
At the end of the operation,1.5 mL of heparinized saline was transfused intravenously.The preoperative diet of food pellets was resumed 2 hours afterthe operation.
Assessment of liver regeneration
Five rats from each group were sacrificed on postoperative day 2,7,14 and 28 to harvestblood and liver specimens forbiochemical,histological,and immunohistochemical examinations.All rats were weighed, and then intraperitonealinjection of 5-bromo-2-deoxyuridine(BrdU,Sigma-Aldrich,St.Louis,MO,USA) was given[100 mg/kg per body weight(BW)]1 hour priorto autopsy.Each liverspecimen wasremoved and immediately weighed.The ratio of the liver weight (LW)to the BWwas calculated.From the resected LW atsurgery and remnant LW atautopsy,the LRR was determined according to the formula of Child etal.[24]: (LW atautopsy-estimated residual LW atthe time of surgery)/resected LW×100%.
Hepatocyte synthesis evaluation
Liver specimens were fixed in 10%formalin and embedded in paraffin.Five-micron thick sections were stained with hematoxylin and eosin for lightmicroscopic examination.To allow calculation of the BrdU labeling index,an indicatorfor DNA synthesisin hepatocytes,immunohistochemicalstaining ofincorporated paraffin embedding BrdU was performed with established method asfollows:heat-induced antigen retrievalwasperformed by a microwave with Tris-EDTA buffer for 20 minutes after deparaffinizing by xylene,and then the slides were washed 2×5 minutes in TBS plus 0.025%Triton X-100 with gentle agitation,and blocked in 10%goatserum with 1%BSA in TBS for2 hours at room temperature.The slides were incubated with anti-BrdU antibodies(Abcam,Cambrige,UK)overnightat 4°C afterward,and secondary antibody incubation for 30 minutes at 37°C was performed subsequently.After incubation with 3,3ˊ-diaminobenzidine chromogen for 10 minutes and rinsed in phosphate buffer solution (PBS),the slideswere counterstained using hematoxylin for 6 minutes and bluing reagent for 2 minutes. Hepatocytes contained nucleiwith brown staining were considered labeled.The numberofpositive stained cellswas counted in randomly selected fields of 10 each in periportal,midzonal,and centrolobular areas using high-power fields(original magnification×400). The number of BrdU-labeled nuclei per 1,000 hepatocytes was designated the BrdU labeling index.
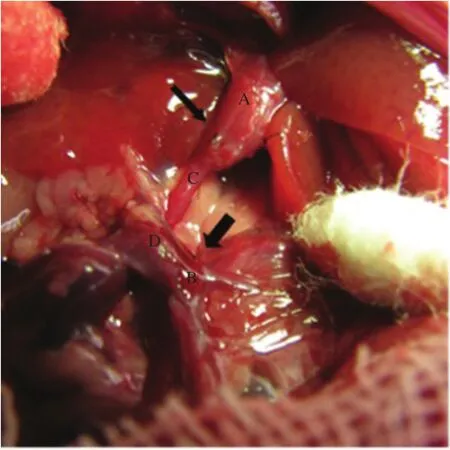
Fig.1Illustration of portal vein arterialization(PVA)associated with porto-renal shunt model.The right kidney was removed after isolating the right renal artery and renal vein,then an end-to-end hepatic inflow portal vein trunk-right renal artery anastomoses(thin arrow)was established after shaping the bifurcation of the right artery,the end-to-end porto-renal shunt(thick arrow)was performed subsequently.A:Hepatic inflow portalvein trunk,B:Confluence of the superior mesenteric and splenic veins,C:Rightrenalartery,D:Rightrenalvein,E:Superior mesenteric vein,F:Splenic vein.
Liver biochemical tests
Serum alanine aminotransferase(ALT)and albumin (Alb)concentrations were measured by an automatic analyzer(Hitachi Co.Ltd.Tokyo,Japan)for assessment of hepatic parenchymalinjury and hepatic anabolism,respectively.
Statistical analysis
Data are expressed as mean±standard deviation (SD).One-way analysis of variance(Studentˊs t-test) or Mann-Whitney U testwas applied for normally or non-normally distributed continuous data,respectively. The Log-rank test was used for survival analyses. P value<0.05 was setas statistically significant.
Results
Survival rate and anastomosis patency
The postoperative 28 day survivalrates were 80.0%, 73.3%and 93.3%in the PH group,the PVA group and the controlgroup,respectively(Fig.2).There were no differences observed in the survival rate among the 3 groups(Log-rank test,P=0.331).In the PVA group, allthe anastomosesremained patentatautopsy,and the rate of the anastomotic patency was 100%.
Liver regeneration rate
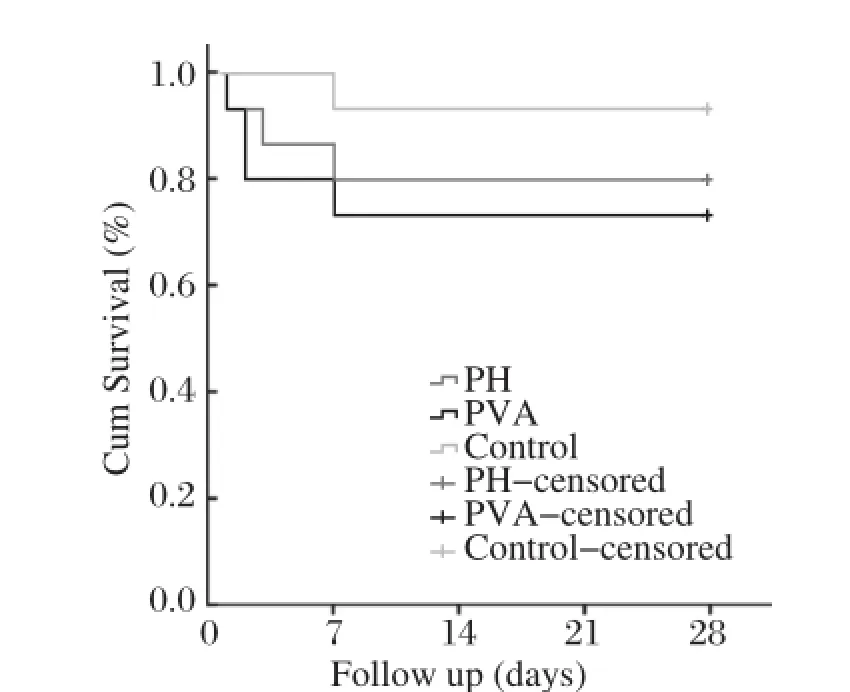
Fig.2The postoperative 28-day survival rates in three groups according to different procedures.There were no differences observed in survivalrates among three groups(Log-rank test,P=0.331).
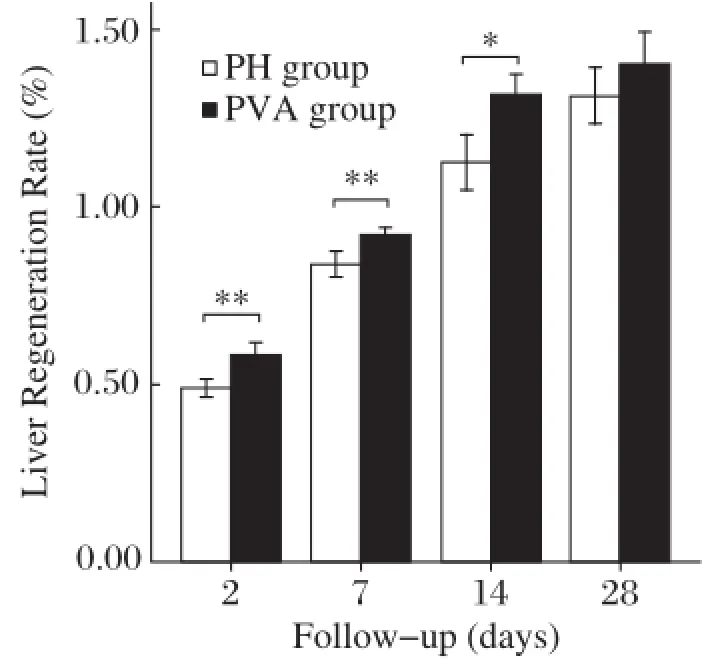
Fig.3Liver regeneration rates(LRRs)after surgery.Rats with PVA procedure(the PVA group,n=5)presented significantly greater liver growth rate than rats with 68%PH only(the PH group)within the postoperative 14 days.However,on postoperative 28 day,the LRRs between these two groups turned to similar levels.Note:Data are expressed as mean±SD.*P<0.05.**P<0.01.
As shown in Fig.3,the LRR gradually increased after operation in the PH group and the PVA group. The LRR increased from 49.0%±2.4%(the PH group)and 58.2%±3.7%(the PVA group)on postoperative day 2 to 83.9%±3.6%and 92.0%±2.2% on postoperative day 7,and 112.5%±7.9%and 131.6%±5.8%on day postoperative 14,respectively. In comparison,the LRR ofthe PVA group was significantly higher at all time within 14 days postoperatively(P<0.05).However,on postoperative day 28, the LRR of the PH group(131.3%±8.0%)and the PVA group(140.4%±9.0%)became comparable (P=0.13).
Liver biochemical tests
The results in serum albumin and ALT are summarized in Table 1.The mean serum albumin concentrations remained significantly higher in the PVA group than either the PH group or the controlgroup within 14 days postoperatively(P<0.05).Meanwhile,there were no significant differences between the PH group and the control group atall time after operation.On postoperative day 28,serum albumin levels gradually became comparable in allthree groups.
Serum ALT values peaked on postoperative day 2 in allgroups,in the rats undergoing 68%hepatectomy in both the PH group(212±53 U/L)and the PVA group(232±61 U/L),and the values were significantly higher than those of the control group(101± 13 U/L)(P<0.05).However,no significant difference was observed between the PH group and the PVA group.The ALT values gradually decreased thereafter,and there were no significant differences from postoperative day 7 to 28 in allgroups.
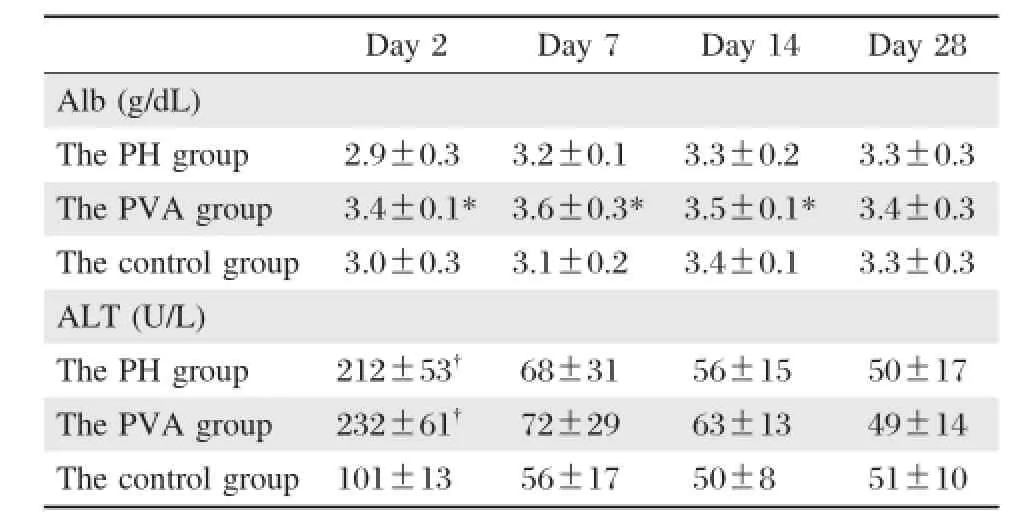
Table 1 Postoperative serum albumin and ALT concentrations
BrdU labeling index
Immunohistochemical staining for BrdU in the liver tissues of the 3 groups is shown in Fig.4.The BrdU labeling index peaked on postoperative day 2,which then gradually declined within 28 days postoperatively in allrats.The values in the PVA group and the PH group were both significantly higher than those in the control group at all time after operation(P<0.01).On postoperative day 2,BrdU labeling indexes in the PVA group and the PH group were similar; however,from postoperative 7 to 28 days,BrdU labeling index in the PVA group was significantly higher than thatin the PH group(P<0.05,Table 2).
Discussion
The liver has a dual blood supply,via the hepatic artery and portalvein,and about80%ispoorly oxygenated venous blood delivered through the portaltract. Alternations of oxygen and blood supply underlie pathological changes in a majority of liver diseases. We established this rat model to evaluate the PVA technique,which can effectively enhance oxygen and blood supply to the liver,to verify the benefits and also to investigate the damage to hepatic function.The 28-day survivalofthe PVA ratswere comparable,which demonstrated that the technique of the mcrovascular hand suturing anastomosis was feasible and reliable. PVA helped faster recovery of hepatic function,and ALT level was not deteriorated due to the drastic changes of the hemodynamics of the portal tract.The remnantliver regeneration was optimized and accelerated by the PVA procedure.
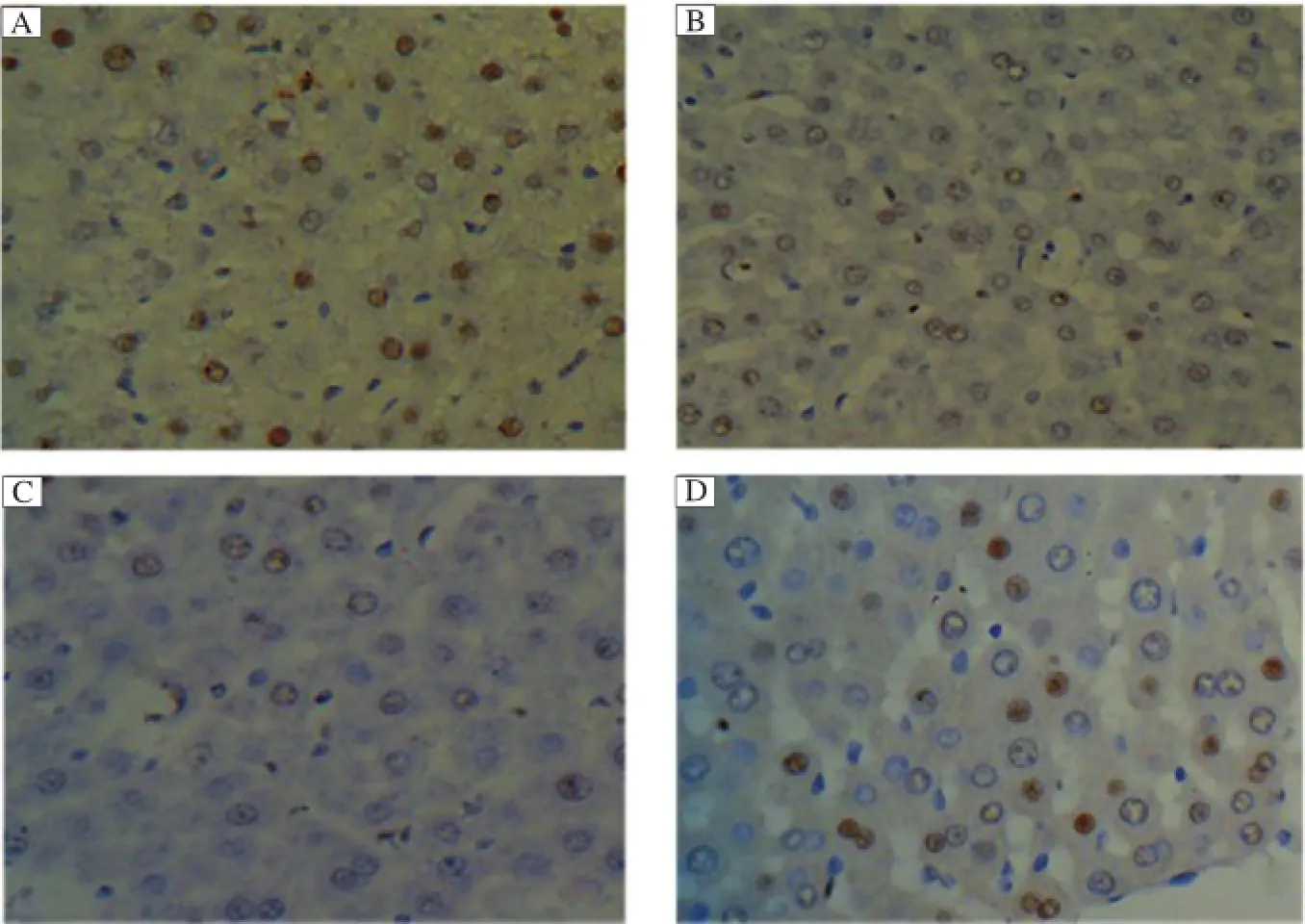
Fig.4 The BrdU labeling indexes after surgery(HE×400).The BrdU labeling indexes peaked on postoperative 2 day,then gradually declined within postoperative 28 days in allrats.On postoperative day 2,BrdU labeling index in the PVA group(A)and the PH group(B)was similar, and both were significantly higher than the control group(C).From postoperative 7 to 28 days,BrdU labeling indexes in the PVA group(D)were maintained significantly higher than either the PH group or the control group(P<0.01).
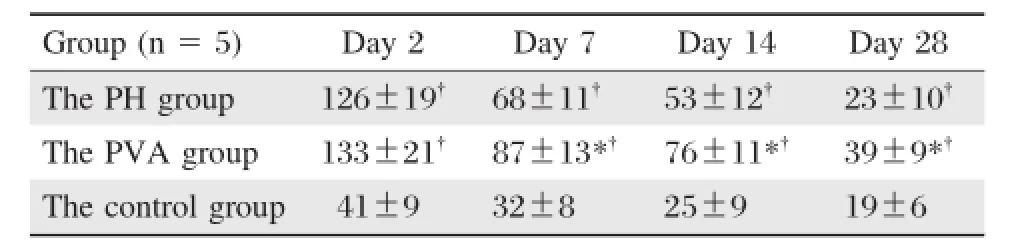
Table 2 Postoperative BrdU labeling indexes in the 3 groups
Several studies in small and large experimental animal models reported that increasing oxygen delivery to the liverby arterialization ofthe portalvein enhances the regenerative capacity after extended hepatectomy[25,26].Nonami et al.[17]have reported that hepatic oxygen consumption affected by dopamine and dobutamine is associated with an increase in the extraction of lactate and thus hyperdynamic hepatic circulatory supportis advantageous to hemodynamics and metabolism in the residualliveraftermassive hepatectomy.However, the concept of PVA and the outcomes of the animal model have been controversial in decades.The alternation of the reconstructed hepatic hila and the subsequently physiopathologic changes induced by the elevated pressure of the portal tract and increased volume of the blood supply still remain uncertain, and it is still unclear that whether the lack of portal blood including specific hepatic factors would inhibit liver regeneration,or whether an adequate blood supply is adequate for liver growth.Furthermore,microvascular reconstruction by hand suturing technique is very demanding and usually requirs longer learning curve;hence,the models of PVA reported in literatures are commonly established by different artificial polyethylene tube cannula technique in spite of the high risk of stent thrombosis due to its non-endothelialization nature.Besides,inflammation and adhesion caused by external artificial graft might also affect portal tracthemodynamics,hepatic structure and liver function[21].There still remains a gap between the simulation of animal model and clinical practice. Few literatures have presented the survival rates of the PVA modelafter partialhepatectomy and checked the paten cy of the anastomo ses at au topsy. Theoretically,when the pressure and inflow volume changed rapidly as a result of portal vein-artery reconstruction,the unalterable and inelastic nature of the stentpipe could notadaptand buffer the hyperdynamic circulation as effectively as autogenous vascular anastomoses.To thatextent,the polyethylene tube interposition technique isan adverse factorthatmay affectthe survivalrate and the experimentaloutcomes.To eliminate the shortcomings of sutureless cannula technique, we developed a novelmodelof PVA by reconstructing the portalvein and renalartery in a microvascular hand suturing anastomotic method after we had shaped and matched the sizes of two different vessel ends. Moreover,the combination of total portocaval shunt was an effective prevention of splanchnic portal tract hypertension and upper digestive bleeding.The survival data revealed thatthere were no significantdifferences in all groups according to different procedures,and the anastomoses of the portal vein-artery reconstruction demonstrated reliable patency when the rats were sacrificed,which proved that the microvascular suturing techniques played a beneficialrole in survivalrates and could be considered an accepted standard.In contrast to the stent cannula anastomosis,our microsurgical end-to-end semi-continuous vascularsuturing technique doesnotrequire externalartificialgraftand could maximally eliminate the injury to the vascular wall and endothelium.Hence,owing to its safety and patency, ourmethod was more applicable and feasible for PVA afterextended partialhepatectomy.
Investigators have shown thatperfusion of systemic venous blood ratherthan blood from the intestines and pancreas maintains the liversize[24,26,27]and regenerative hyperplasia is within the normalrange[28].Rocheleau[29]etal.showed thateitherportalvenous orhepatic arterial flow could maintain viable liver mass in proportion to the amountof blood flow delivered.The presentstudy documented thatthe liver regeneration rates were significantly higher in rats thatunderwentarterialization within 14 days afterextended partialhepatectomy,which showed thatan increase in portalblood flow and oxygen supply wasbeneficialto liverregeneration afterhepatectomy.Besides,the initiation and maintenance of liver regeneration afterhepatectomy do notnecessarily require highly concentrated hepatotrophic factors;instead,other classic splanchnic factors in the portalblood may be the alternative initial triggers of the regenerative process. The LLR outcome indicated thatthe sufficiency of the hepaticblood inflow,originated from eitherthe portaltract or artery,play a more importantand essentialrole rather than the presence ofthe factorsin portalblood.Thereason is probably thatfactors and elements involved in liver regeneration willbe absorbed and metabolized more efficiently in a hyperdynamic circulating situation.
The limitation of the PVA technique is the negative effects of‘‘overarterialization’’,which may resultin damage to the parenchyma.Furthermore,the mechanical injury caused by hyperdynamics may lead to hepatic fibrosis.In ourstudy,increasing portalinflow and portal tractpressure was buffered and welladapted,reaching a new hemodaymic balance shortly after operation. However,further research is needed to investigate the anastomoses with inflow controlwhich can ultimately avoid the overflow to the liver.The hand suturing microvascular reconstruction technique utilized in our modelis more demanding and frustrating as compared with the sutureless stenting technique.To our knowledge,thislimitation can be conquered by sufficientpractice and a tacitcooperative surgicalteam.In addition, longer-term studies are required to evaluate liverfunction and the effects of the altered hepatic hemodynamics on sinusoidalcellfunction,and longer-term observationsofthe ultrastructuralpathologicalchanges ofthe hepatic parenchymaland mesenchymalcellare also desired.
In conclusion,ourmicrovascularhand suturing anastomotic technique forportalvein-renalartery reconstruction is simple,safe and feasible,and the PVA ratmodelwhich we established is notonly a pathway forimproving remnantliverregeneration swiftly,italso maintains and even optimizes the liverfunction afterextended hepatectomy.
[1]Maillard JN,Benhamou JP,Rueff B.Arterialization of the liver with portacaval shunt in the treatment of portal h yperten sion d u e to intrah ep atic b lo ck.Su rg ery 1970;67(6):883-890.
[2]Iseki J,Touyama K,Noie T,et al.Partial portalarterialization forthe prevention of massive liver necrosis following extended pancreatobiliary surgery:experience of two cases.Surg Today 1992;22(6):568-571.
[3]Fritsch A,Funovics J,Gangl A,et al.Controlled arterialization of the liver.II.Clinical results(authorˊs transl). Langenbecks Arch Chir 1974;336(1):67-89.
[4]J.Funovics,A.Gangl,W.Horak,etal.Controlled arterialization of the liver.I.Experimental observationes(authorˊs transl).Langenbecks Arch Chir 1974;335(4):339-349.
[5]Gigot JF,Otte JB,Lambotte L,etal.Arterialization of the portal vein associated with a portocaval shunt:Long-term results of a controlled prospective study.Acta Gastroenterol Belg 1990;53(2):237-247.
[6]Matzander U.Results ofveno-venousshuntwith arterialization ofthe liver.Langenbecks Arch Chir 1976;342:145-151.
[7]Voorhees AB Jr,Price JB Jr,Britton RC.Portasystemic shunting procedures for portal hypertension.Twenty-six year experience in adults with cirrhosis of the liver.Am J Surg 1970;119(5):501-505.
[8]Housari G,Nuno J,Calero P,etal.Portalvein arterialization in liver transplantation:an option to restore arterial flow:a case report.Transplant Proc.2011;43(3):755-757.
[9]Bonnet S,SauvanetA,Bruno O,etal.Long-term survival after portal vein arterialization for portal vein thrombosis in orthotopic liver transplantation.Gastroenterol Clin Biol.2010;34(1):23-28.
[10]Erhard J,Lange R,Giebler R,et al.Arterialization of the portal vein in orthotopic and auxiliary liver transplantation.Transplantation 1995;60(8):877-879.
[11]AspinallRJ,Seery JP,Taylor-Robinson SD,etal.Comments on‘‘Arterialization of the portal vein in orthotopic and auxiliary liver transplantation’’.Transplantation 1996;62(9): 1375-1376.
[12]Troisi R,Kerremans I,Mortier E,et al.Arterialization of the portal vein in pediatric liver transplantation.A report of two cases.Transpl Int 1998;11(2):147-151.
[13]Nardo B,Caraceni P,Montalti R,et al.Portal vein arterialization:A new surgical option against acute liver failure?.Transplant Proc 2005;37(6):2544-2546.
[14]Nardo B,Caraceni P,PuvianiL,etal.Successfultreatment of CCl(4)-induced acute liverfailure with portalvein arterialization in the rat.J Surg Res 2006;135(2):394-401.
[15]Nardo B,Vaccarisi S,Pellegrino V,et al.Extracorporeal portalvein arterialization in man afterextended hepatectomy to preventacute liverfailure:a case report.TransplantProc. 2011;43(4):1193-1195.
[16]Hinkle PC.Mitochondria.In:Arias IM,Boyer JL,Fausto N,Jakoby WB,Schachter D,Shafritz DA,eds.The Liver: Biology and pathobiology.3rd ed.New York,NY:Raven Press,1994:323-364
[17]Nonami T,Asahi K,Harada A,et al.Effect of hyperdynamic circulatory support on hepatic hemodynamics, oxygen supply and demand after massive hepatectomy. Surgery 1991;109(3 Pt 1):277-283.
[18]Fisher B,Russ C,Updegraff H,et al.Effect of increased hepatic blood flow upon liver regeneration.AMA Arch. Surg.1954;69(2):263-272.
[19]Nardo B,Puviani L,Prezzi D,et al.Protective effect of portal vein arterialization in acute liver failure induced by hepatectomy in normal and fatty liver rat.Transpl Proc,2006;38(10):3249-3250.
[20]Fan YD,Praet M,Van Huysse J,et al.Effects of portal vein arterialization on liver regeneration after partial hepatectomy in the rat.Liver Transpl 2002;8(2):146-152
[21]Schleimer K1,Stippel DL,Kasper HU,et al.Portal hyperperfusion causes disturbance of microcirculation and increased rate of hepatocellular apoptosis:Investigations in heterotopic rat liver transplantation with portal vein arterialization.Transpl Proc,2006;38(3):725-729.
[22]Higgins GM,Anderson RM.Experimental pathology of the liver:I.Restoration ofthe liver of the white ratfollowing partialsurgicalremoval.Arch Pathol1931;12:186-202.
[23]Lee S,Fisher B.Porto-caval shunt in the rat.Surgery 1961;50:668-675.
[24]Child CG 3rd,Barr D,Halswarde GK.Liver regeneration following portal-caval transposition in dogs.Ann Surg 1953;138(4):600-608.
[25]Ogata A,Miyazaki M,Ohtawa S,et al.Short-term effect of portal vein arterialization on hepatic protein synthesis and endotoxaemia after extended hepatectomy in dogs. J Gastroenterol Hepatol 1997;12(9-10):633-638.
[26]Shimizu Y,Miyazaki M,Shimizu H,et al.Beneficial effects of arterialization of the portal vein on extended hepatectomy.Br J Surg 2000;87(6):784-789.
[27]de Jonge J,Madern GC,Terpstra OT,etal.Directing portal flow is essential for graft survival in auxiliary partial heterotopic liver transplantation in the dog.J Pediatr Surg 1999;34(8):1265-1268.
[28]Lee S,Broelsch CE,FlamantYM,etal.Liverregeneration after portacavaltransportation in rats.Surgery 1975;77(1):144-149.
[29]Rocheleau B,Ethier C,Houle R,etal.Hepatic artery buffer response following left portal vein ligation:Its role in liver tissue homeostasis.Am J Physiol 1999;277(5 Pt 1):G1000-1007.
△These authors contributed equally to this study.
✉Corresponding author:Prof.Baimeng Zhang,Department of General Surgery,the 5th Affiliated Hospital,Sun Yat-sen University,Zhuhai, Guangdong 519000,China.Tel/Fax:86-756-2528781/86-013926927880,E-mail:Baimeng5@hotmail.com.
Received 26 March 2014,Revised 05 May 2014,Accepted 12 September 2014,Epub 15 December 2014
The authors reported no conflictof interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140054
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Ventricular tachycardia ablation and substrate modification in ICD patients with electrical storm
- Induced pluripotentstem cells are induced pluripotentstem cell-like cells
- HLA antigens and anti-sperm antibody production in Iranian vasectomized men
- Unruptured pregnancy in a noncommunicating rudimentary horn at 37 weeks with a live fetus:a case report
- Surgicaloutcomes ofmini-open Wiltse approach and conventional open approach in patients with single-segment thoracolumbar fractures withoutneurologic injury
- Prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza
