Trigger elimination ofpolymorphic ventricular tachycardia and ventricular fibrillation by catheter ablation:trigger and substrate modification
Akihiko NogamiCardiovascular Division,University of Tsukuba,Tsukuba,Ibaraki,Japan.
Trigger elimination ofpolymorphic ventricular tachycardia and ventricular fibrillation by catheter ablation:trigger and substrate modification
Akihiko Nogami✉1Cardiovascular Division,University of Tsukuba,Tsukuba,Ibaraki,Japan.
Ventricular fibrillation(VF)is a malignantarrhythmia,usually initiated by a ventricular premature contraction (VPC)during the vulnerable period of cardiac repolarization.Ablation therapy for VF has been described and increasingly reported.Targetsfor VF triggers are VPCs preceded by Purkinje potentials orfrom the rightventricular outflow tract(RVOT)in structurally normalhearts,and VPC triggers preceded by Purkinje potentials in ischemic cardiomyopathy.During the session,mapping should be focused on the earliestactivation and determining the earliestpotentialis the key to a successfulablation.However,suppression of VF can be achieved by notonly the elimination oftriggering VPCs,butalso by substrate modification ofpossible reentry circuitsin the Purkinje network,or between the PA and RVOT.The mostimportantissue before the ablation session is the recording of the 12-lead ECG ofthe triggering event,which can prove invaluable in regionalizing the origin ofthe triggering VPC formore detailed mapping.In cases where the VPC is not spontaneous or inducible,ablation may be performed by pace mapping.Further studies are needed to evaluate the precise mechanisms of this arrhythmia.
catheter ablation,inherited arrhythmias,polymorphic ventricular tachycardia,Purkinje network,right ventricular outflow tract,ventricular fibrillation
Introduction
While previous studies have shown thatventricular fibrillation(VF)is perpetuated by reentry or spiral waves,recentdata suggestthe role of specific sources triggering thisarrhythmia.Haı¨ssaguerre etal.[1]reported thatidiopathic VF could be suppressed by catheterablation ofthose triggers originating from the Purkinje system or rightventricular outflow tract(RVOT)and the ablation therapy for VF has been increasingly reported during the lastdecade.In general,this ablation appears to have a high success rate and is relatively easy to perform,although precise mapping is required.However, little is known aboutthe initiating mechanism of VF. The pathophysiology of VF can be subdivided into three phases:trigger sources,initiation and maintenance or mechanisms of perpetuation.Whether the mechanism of the ablation effect is due to the suppression of the triggerorsubstrate modification is stillunclear.
RVOT trigger VPCs
The common arrhythmias arising from the RVOT are isolated VPCs or ventricular tachycardia(VT)[2]. In patients withoutstructuralheartdisease,ventricular arrhythmias from the RVOT are generally consideredto be benign.However,VPCs originating from the RVOT occasionally trigger VF or polymorphic VT (PVT)[1,3-9].Noda and colleagues reported 16 patients with idiopathic VF triggered by RVOT VPCs[3].Of those patients,7(44%)were male and 11(69%)had a history of syncope.VPCs arose from the septal area of the RVOT in mostof the patients.
Difference between malignant and benign VPCs from the RVOT
Patients with PVT/VF had frequent RVOT VPCs butonly a few episodesof PVT/VF[1,3].Viskin and colleagues reported 3 patients with no heart disease, RVOT VPCs and PVT/VF whose malignant trigger VPCs had shorter coupling intervals compared to benign VPCsofthe same morphology[7].However,this finding is notconsistentwith Noda etal.who demonstrated no significant difference regarding the total number of VPCs and the coupling interval between RVOT VPCs with PVT/VF and benign RVOT VPCs[3].
Kurosakiand colleaguesevaluated thedifferencein the initial VPCs between PVT/VF and monomorphic VT (MVT)from the RVOT[8].They compared theelectrocardiographic and the clinicalcharacteristics between 14 patients with PVT/VF and 77 patients with MVT.The episodes ofsyncope were more frequentin the PVT/VF group(57%)than in the MVT group(10%).An initial VPCwith a positive QRS complex in lead Iwas observed in 10(71%)of14 patientswith PVT/VF,and in 27(35%) of77 patients with MVT(P<0.05).Although radiofrequency(RF)catheterablation targeting the trigger VPC often produced a morphologicalchange,PVT/VF was eliminated in 13(93%)of14 patients after additional RF energy applications.They concluded thatmalignant arrhythmias from the RVOT,although rare,should be considered when the patient has a syncopal episode and VPC with a positive QRS complex in lead I.
While mostof the previous investigators could not find a difference in the coupling interval between benign VPCs and trigger VPCs of PVT/VF,Igarashi and colleagues demonstrated a significant difference in the ratio of the coupling intervalto the preceding R-R interval(prematurity index)[9].They compared the ECG parametersbetween 18 patientswith idiopathic PVT/VF and 21 patients with MVT arising from the RVOT.While the coupling intervalofthe firstVT beat was comparable between the 2 groups,the prematurity index of the first VT beatwas significantly smallerin the PVT-group than in the MVT-group.Although the ratio ofthe coupling intervalto the preceding QT interval was also different,the prematurity index was the only independent determinant of the polymorphic QRS morphology(odds ratio=2.198).
Ablation of RVOT VPCs
The RVOT is the most common origin of isolated VPCs and MVT in structurally normalhearts.Itis also the origin fortriggers of PVT,which rapidly degenerate into VF.This type ofablation is essentially no different than the ablation ofidiopathic RVOT-VPCs or VT.The ablation targets the site ofthe earliestactivation and a good pace map in the RVOT[3,8,9].In general,this ablation appears to have a high successrate and isrelatively easy to perform.Noda and colleagues reported 16 patients who had PVT/VF initiated by VPCs arising from the RVOT[3].The optimalablation site was determined by the earliestlocalactivation site during the spontaneous target VPC and/or by pace mapping.RF energy was applied at the optimal site(local potential 17 ms± 11 msbefore the onsetofthe QRS).In 11 of16 patients (63%),other VPCs with a different QRS morphology appeared afterablation ofthe initialtarget.Such a morphological change after theRF application was also observed by Kurosakietal.[8]They reported thata morphological change occurred in 71%of the PVT/VF patients during the ablation session,butalso in 52% of the MVT patients.Eventually,Noda and colleagues reported that RF catheter ablation was successfulin 13 outof16 patients with PVT/VF(81%)during a meanfollow-up of54±39 months.Kurosakiand colleagues reported thata successful RFCA was achieved in 13 out of 14 patients(93%)in the VF/PVT group and in 65 out of77 patients(84%)in the MVT group aftera mean follow-up period of71±34 months.Similargood results were reported by other authors in cases treated with the same approach[1,4,5,6,10].
Fig.1shows the distribution of the finalsuccessful ablation sites in 14 patients with PVT/VF in the study by Kurosakietal.[8]Trigger VPCs were finally eliminated atthe posterior attachmentof the free wallin 7, atthe posteriorattachmentofthe septum in 2,midwaybetween the anterior and posterior attachment of the septum in 1,and atthe anteriorattachmentofthe septum in 4.In 2 patients(14%)with PVT/VF,the finalsuccessfulablation site was above the pulmonary valve. In allpatients with VPCs originating from above the pulmonary valve,a sharp potentialpreceding the QRS onsetand a subsequent dullpotential were recorded during the VPCs from the ablation catheter.
Substrate modification for PVT/VF arising from RVOT
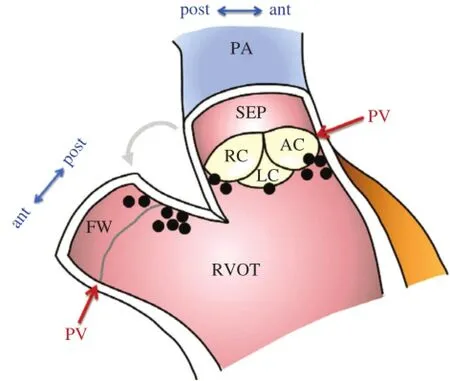
Fig.1Successfulablation sites in 14 patients with ventricular fibrillation/polymorphic ventricular tachycardia from right ventricular outflow tract.Coronal section image of the ventriculopulmonary junction.In 9 of14 patients,the successfulablation sites were located on the posterior attachmentof the right ventricular outflow tract. AC=anteriorcusp ofpulmonary valve;ant=anteriorattachment;FW= free wall;LC=left cusp;PA=pulmonary artery;post=posterior attachment;PV=level of pulmonary valve;RC=right cusp;SEP= septum.(From Kurosaki K,Nogami A,Shirai Y,et al.Positive QRS complex in lead I as a malignant sign in right ventricular outflow tract tachycardia.Circ J 2013;77(4):968-974.With permission.)[8]
The following case demonstrates an unusual patient with suppression of PVT by conduction block between the pulmonary artery(PA)and RVOT[11].A 56-year-old female presented with multiple episodes ofsyncope and Holter monitoring revealed frequentepisodes of PVT (Fig.2A).The mean cycle length of the VT was 220 ms and the morphologies of the firstthree QRS complexes of the PVT were always the same.Electroanatomical mapping was performed and the propagation map of the first VPC had a centrifugalpattern from the posterior attachmentof the RVOT.From thatsite, pace mapping was performed.Interestingly,pacing at a cycle length of300 ms created the exactsame polymorphic QRS configurations as those created during the clinical PVT(Fig.2B and Fig.2C).Afterseveral RF energy applications to the posterior RVOT,the repetitive VPCs disappeared.However,isolated VPCs with a slightly different QRS morphology and a longer coupling intervalremained.Therefore,mapping in the pulmonary artery(PA)was performed.From the PA, delayed PA potentials were recorded during sinus rhythm and those potentials preceded the onsetof the QRS during the VPC that remained(Fig.3A). Between the PA and RVOT potentials,a tiny bridging potential was recorded.RF energy was delivered atthat site in the PA.Just after the RF energy application,the PA potential disappeared(Fig.3B).Repetitive firing from the PA was observed;however,there were no VPCs.These findings indicate that there was bidirectionalconduction block between the PA and RVOT. In this case,the site-of-origin of the triggering beat was in the PA,and the multiple exits or non-uniform conduction to the RVOT might be the mechanism of the polymorphism ofthe VT.In fact,the change in the QRS configuration was reproduced by pacing ata relatively long cycle length.The electricalisolation of the extracardiac vessel,i.e.PA,suppressed the fibrillatory arrhythmia in the connecting heart chamber,i.e.RV. Interestingly,it is quite similar to the relationship between the pulmonary veins and left atrium in the mechanism ofparoxysmalatrialfibrillation.
Trigger from Purkinje system
Anothertriggerofidiopathic VF isthe Purkinje system[1],which can also be a trigger of ischemic VF[12,13]. In a multicenter study,including 23 patients with apparently normalhearts,VPCs arising from Purkinje system of the left ventricle were characterized by a rightbundle branch block(RBBB)morphology and a relatively short QRS duration(115±11 ms)[1].The origin of those VPCs is from a wider area compared to the RVOT VPCs[1].Purkinje system VPCs originating in the rightventricle were characterized by a left bundle branch block(LBBB)morphology.VPCs with a rightventricularorigin mostly arise from the anterior free walland display a significantly longer QRS duration(143±10 ms).The time interval between the local Purkinje potential and the local myocardial potentialwas 11±5 ms withoutany significantdifference between the leftand rightsides,indicating the more distal origin of the VPCs in the area of the Purkinje arborization.In the same study,VPCs originating from the Purkinje system were compared with VPCs arising from the RVOT(4 patients).In the formergroup a significantly shorter QRS duration(126± 18 vs.145±12 ms)and a shorter VPC coupling interval(280±26 vs.355±30 ms)were observed[1].
Substrate modification of the Purkinje network
Catheter ablation targeting the Purkinje potentials responsible for triggering VF has been shown to be possible and efficacious in a number of conditions such as idiopathic VF(short-coupled variant of torsade de pointes),ischemic VF,and chronic myocarditis.What is still undetermined is whether the mechanism of the ablation effect is due to the elimination of the trigger orsubstrate modification of the Purkinje network.
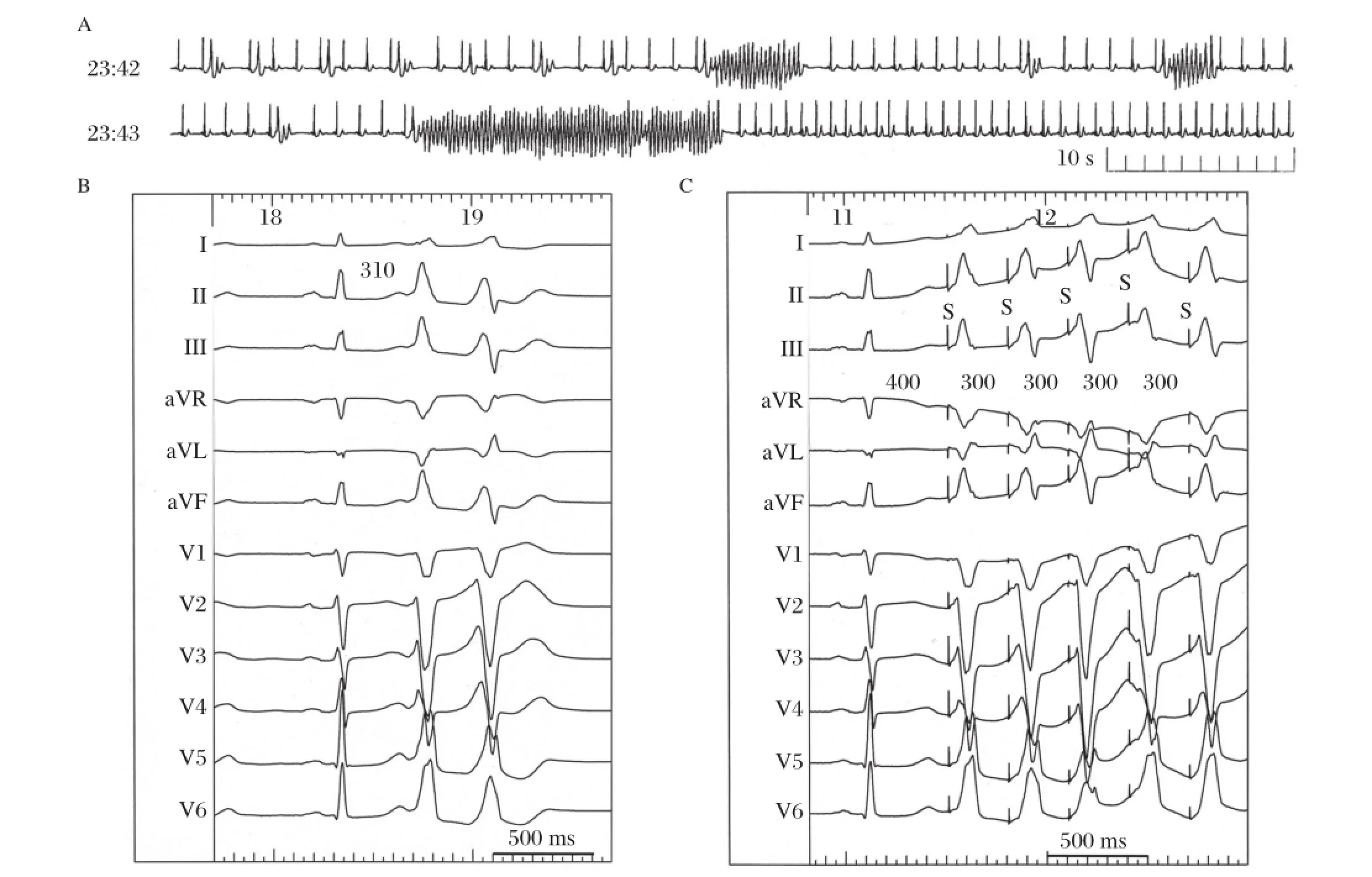
Fig.2 Surface 12-lead ECGs in a female patient with an right ventricular outflow tract(RVOT)type polymorphic ventricular tachycardia(PVT).A:Holtermonitoring revealed frequentepisodes of PVT.The mean cycle length ofthe VT was 220 ms and the morphologies of the first three QRS complexes of the PVT were always the same.B:Polymorphic ventricular premature contraction(VPC)couplets were recorded during the ablation session.C:Pace mapping atthe earliestactivation site in the RVOT reproduced the exactthe same polymorphic QRS configurations as those during the clinical PVT.(From Nogami A.Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation: Trigger elimination and substrate modification.J Cardiovasc Electrophysiol 2014 Sep 12.doi:10.1111/jce.12547.[Epub ahead of print]PubMed PMID:25216244.With permission.)[11]
During activation mapping of the triggering VPC, attention should be paid to the preceding sharp Purkinje-like signals.Mapping should be focused on the earliestactivation ofthis potentialand determining the earliestpotentialis the key to a successfulablation. However,the potential may sometimes be seen to occur with intra-Purkinje block to the myocardium and not produce a VPC.This means thatthere is the possibility thatnotonly the elimination ofthe triggering VPC,but also conduction block in the Purkinje network can suppress the triggering VPC and VF.In fact,dissociated firing from the Purkinje network is sometimes seen after a successfulablation.The following case is an example of the successful suppression of VF by the modification of the Purkinje network.
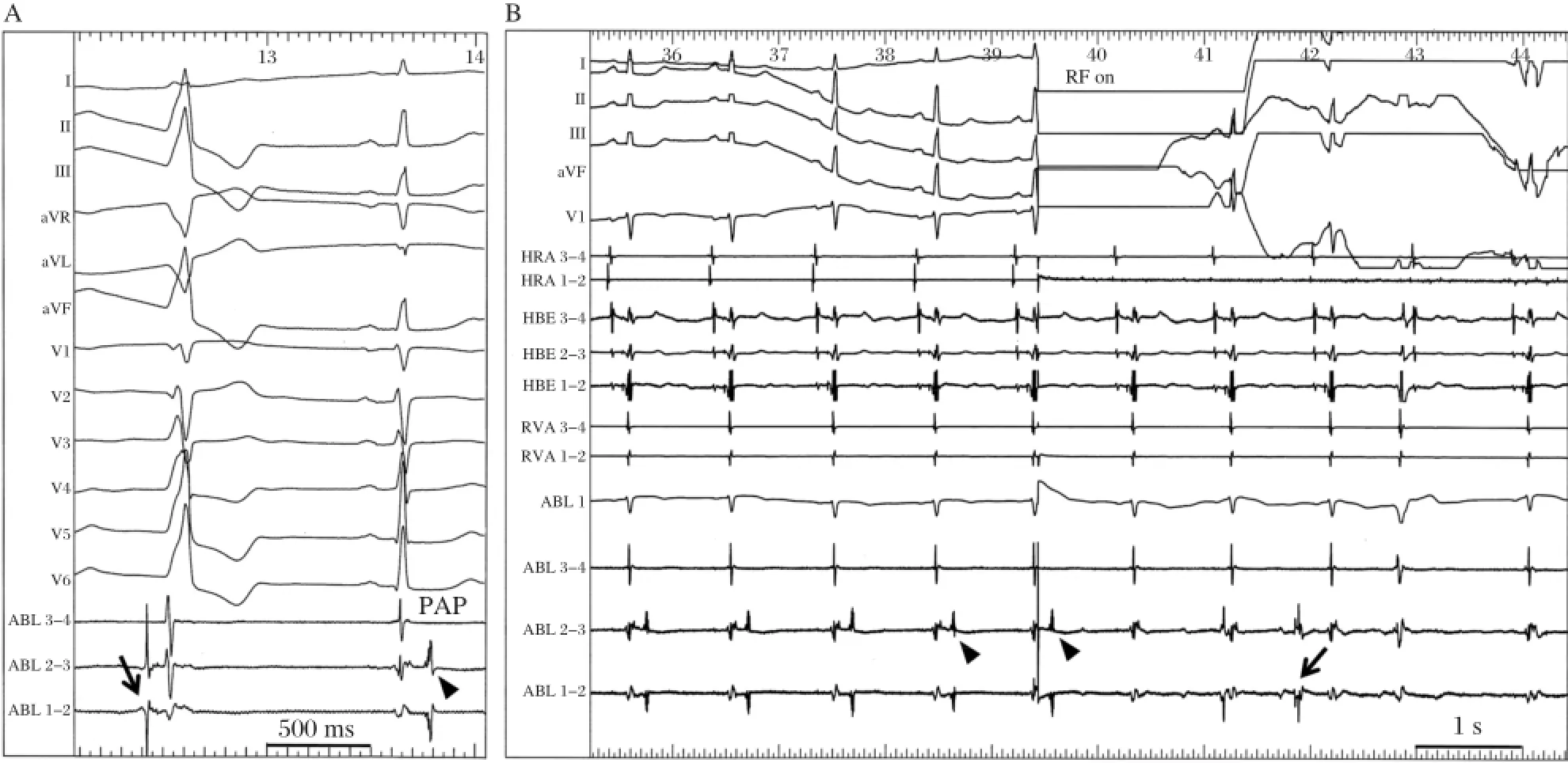
Fig.3 Successful ablation in the pulmonary artery(PA).A:From the PA,a delayed PA potential was recorded during sinus rhythm(arrow head)and this potential preceded the onset of the QRS during the remaining VPC(arrow).Between the PA and RVOT potentials,a tiny bridging potentialwas recorded.B:Justafterthe RF energy application in the PA,the PA potential(arrow heads)disappeared.Repetitive firing from the PA was observed(arrow);however,there were no VPCs.These findings indicate thatbidirectionalconduction block occurred between the PA and RVOT.HBE =His-bundle electrogram;HRA=high rightatrium;PAP=pulmonary artery potential;RF=radiofrequency energy.(From Nogami A.Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation:Trigger elimination and substrate modification.J Cardiovasc Electrophysiol 2014 Sep 12.doi:10.1111/jce.12547.[Epub ahead of print]PubMed PMID:25216244.With permission.)[11]
A 54-year-old man with idiopathic VF(short-coupled variantof torsade de pointes)underwentcatheter ablation for frequent episodes of defibrillato r(ICD) shocks[14].A nonsustained PVT with the same QRS morphology as the clinicalPVT was repeatedly inducible by atrialpacing afteran intravenous administration of cibenzoline(Fig.4A).The first VPC(VPC1)had an RBBB configuration with right-axis deviation and the second one(VPC2)had an RBBB pattern with a northwestaxis.The coupling intervalof VPC1 to the preceding normally conducted QRS complex was 250 ms.During the PVT,diastolic and presystolic Purkinje potentials were recorded from an octapolar electrode catheter with 1.25-mm electrode widths and 2-mm inter-electrode spacing placed on the left ventricular septum(Fig.4A and Fig.4B).Diastolic Purkinje potentials were recorded earlierfrom the proximalthan the distalelectrodes and fused presystolic Purkinje potentials were recorded earlierfrom the distal than the proximalelectrodes.During sinus rhythm, recording at the same site demonstrated fused Purkinje potentials before the onset of the QRS.Because the earliest Purkinje activation site before VPC1 could not be determined and seemed to be a more proximalsite than the site ofelectrodes7-8,RF energy wasdelivered to the site of electrodes 3-4.A Purkinje potentialfrom this site preceded the onsetof VPC1 by 15 ms and VPC2 by 60 ms.Theintracardiac electrogramsrecorded after the ablation showed the abolition of the local Purkinje potentials at the middle portion and a slight delay in the occurrence of the local ventricular electrogram during sinus rhythm(Fig.4C).The PVT became noninducible and only an isolated VPC was inducible. The morphology ofthis isolated VPC differed from the previous triggering VPCs(VPC1 or VPC2).Further, Purkinje firing was observed before this VPC and intra-Purkinje block occurred.The patientwas followed withoutany drugs,episodes of syncope or VF recurrences during a follow-up period of 14 years.These observationssuggestthatthe VF initiation wascaused by activity from the Purkinje tissue.However,the suppression of the VF was achieved with catheter ablation of the Purkinje network,notatthe earliest Purkinje activation site in this patient.
In the reportby Haı¨ssaguerre etal.[1],electrocardiograms recorded after ablation showed the abolition of the local Purkinje potentials and a slight delay in the occurrence o f the local ventricular electrogram. However,they did not determine how much of the complex Purkinje network was involved in each patient and the issue of multiple fociversus differing activation routes from limited foci remains unsolved. In ourcase,cathetermapping revealed thatthe constantly changing polymorphic QRS morphology resulted from the changing propagation in the Purkinje arborization and the PVT became noninducible after the catheter ablation ofthe Purkinje network.We did notablate the earliest site of the Purkinje activation,and the isolated VPCwith diastolic Purkinje activation wasstillinducible afterthe catheterablation.
Of course,the earliestactivation site of the Purkinje activation during the triggering VPC should be sought and ablated;however,a modification of the Purkinje network mightbe applied when the earliestsite cannot be determined oris located close to the atrio-ventricular node.
Importance of twelve-lead recording of triggering VPCs
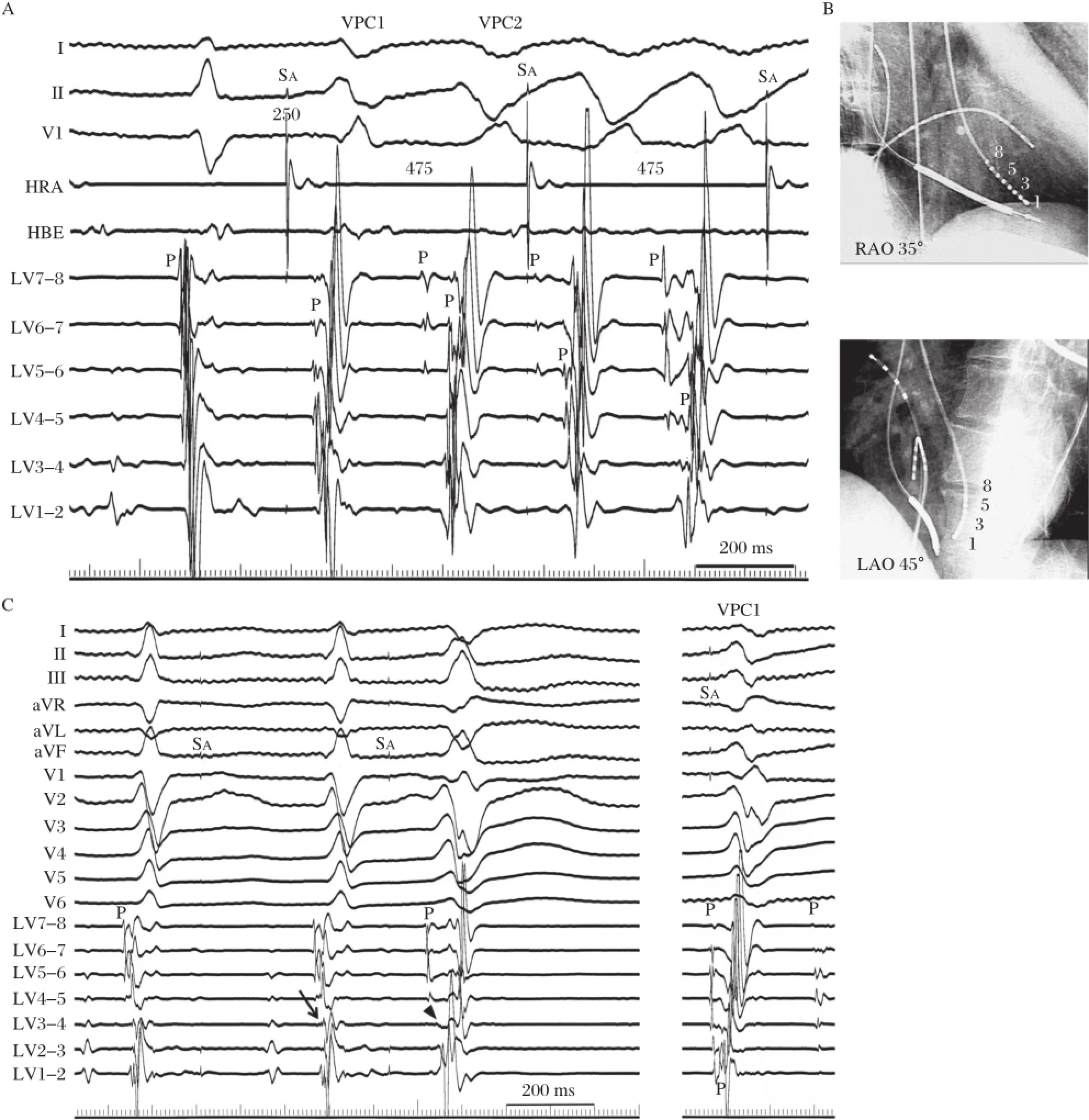
Fig.4 Catheter mapping during the PVT in a male patient with a short-coupled variant of torsade de pointes.A:During the PVT which was induced by rapid atrial pacing after the administration of intravenous cibenzoline,diastolic Purkinje potentials and presystolic Purkinje potentials were recorded from the leftventricular septum.During sinus rhythm,fused Purkinje potentials were recorded before the onsetof the QRS.B: Representation of an octapolar electrode catheterplaced on the leftventricular septum.C:Intracardiac electrograms recorded after ablation showing the abolition of the local Purkinje potential(P)atthe middle portion and a slightdelay in the occurrence of the local ventricular electrogram during sinus rhythm(arrow).The PVT became noninducible and only an isolated VPC was inducible.The morphology of this VPC differed from the previous triggering VPC1 and intra-Purkinje block was also observed before this VPC(arrowhead).HBE=His-bundle electrogram;HRA=high rightatrium; LAO=leftanterioroblique view;LV=leftventricle;P=Purkinje potential;RAO=rightanterior oblique view;SA=atrialpacing stimulus.(From Nogami A,Sugiyasu A,Kubota S,et al.Mapping and ablation of idiopathic ventricular fibrillation from Purkinje system.Heart Rhythm 2005;2(6): 646-649.With permission.)[14]
Recording the 12-lead ECG of the triggering event can prove invaluable in regionalizing the origin of the triggering VPC for more detailed mapping and an effort to record such a trigger should be routine.The target site can be speculated with the 12-lead ECG documentation;RVOT,rightdistal Purkinje,leftposterior Purkinje,left anterior Purkinje,or right Purkinje system.In patients withoutectopy during the RF ablation session,the putative source of the VPC can be ablated in sinus rhythm based on pace mapping followed by RF energy delivery.In patients with multifocal VPCs,the true triggering VPC that initiates VF,ornonsustained PVT,has to be confirmed.Itis essential thatthere is accurate documentation of the triggering VPC with a 12-lead ECG.
Fig 5 shows ECGs from a 59-year-old female patient with early repolarization associated with VF[15].Each panel shows the QRS complexes during sinus rhythm and the VPC.In the emergency room, significant J-ST elevation in the infero-lateral leads and VPC bigeminy with an RBBB configuration and superior axis were observed afterthe spontaneous termination of the PVT(Fig.5A).One month after the implantation of an ICD,a triggering VPC ablation was performed due to frequent ICD shocks.During the ablation session,frequent monofocal VPCs were observed(Fig.5B),and Purkinje potentials on the posterior left ventricular septum preceded the onset of the VPC by 65 ms.An RF energy application atthat site immediately eliminated the VPC.However,a few days after the session,VF recurred.A 12-lead Holter recording could record the initiation of the VF (Fig.5C).The‘‘true’’triggering VPC was similar to the ablated VPC,but different(especially lead aVR). Interestingly,while J-ST elevation was recorded in the emergency room and during the VF recurrence,it was not observed during the ablation session.There was a possibility thatthe true triggering VPC appeared only during the J-ST elevation.The patient did not prefer to undergo a re-ablation session and the oral administration of disopyramide successfully suppressed the VF episode.
In the intensive care unit,a synthesized 12-lead ECG from the signals recorded using three to five electrodes is sometimes used.In our experience,the limb leads in the synthesized 12-lead ECG are similar to the Mason-Likar lead configuration,in which the limb lead electrodes are placed on the torso ratherthan the distalextremities and can be used forthe morphology analysis of VPCs.However,the chestlead information is less usefulbecause of its inaccuracy.Twelvelead Holter monitoring also uses a Mason-Likar lead configuration similar to the limb leads and the real six chest electrodes for the chest leads.It appears to be highly reliable and usefulforthe diagnosisof‘‘true’’triggering VPCs.
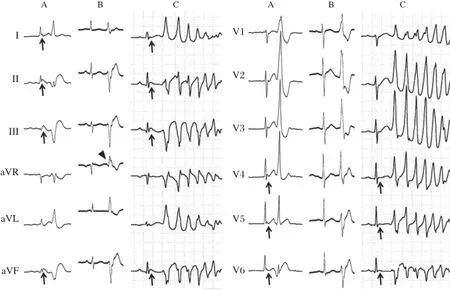
Fig.5Surface 12-lead ECGs from a female patient with early repolarization associated with VF.A:In the emergency room,significant J-ST elevation in the infero-lateral leads and VPC bigeminy with an RBBB configuration and superior axis were observed after the spontaneous termination of PVT.B:During the ablation session,frequentmonofocal VPCs with an RBBB configuration and superior axis were observed.C:A 12-lead Holter recording could record the VF recurrence.The‘‘true’’triggering VPC is similar to the ablated VPC,but is different(especially lead aVR) (arrowhead).Interestingly,while J-ST elevation was recorded in the emergency room and during the VF recurrence(arrows),itwas notobserved during the ablation session.(From Nogami A.Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation:Trigger elimination and substrate modification.J Cardiovasc Electrophysiol 2014 Sep 12.doi:10.1111/jce.12547.[Epub ahead of print]PubMed PMID: 25216244.With permission.)[11]
Concluding remarks
RF catheter ablation of VF is feasible and can be used as a bailouttherapy for drug-refractory electrical storms.Suppression of VF can be achieved by notonly the elimination oftriggering VPCs,butalso the creation ofconduction block between the PA and RVOT,or of the Purkinje network.However,VF ablation has not yetbeen recognized asa Class Iindication by thecurrent guidelines[16].Ablation is recommended as a Class IIb recommendation only for VF triggered by Purkinje system VPCs.Further studies are needed to evaluate the precise mechanisms of this arrhythmia.
[1]Haı¨ssaguerre M,Shoda M,Jais P,et al.Mapping and ablation of idiopathic ventricular fibrillation.Circulation 2002;106(8);962-967.
[2]Lerman BB,Stein KM,Markowitz SM,et al.Ventricular arrhythmias in normal hearts.Cardiol Clin 2000;18(2): 265-291,vii.
[3]Noda T,Shimizu W,Taguchi A,etal.Malignantentity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract.J Am Coll Cardiol 2005;46(7):1288-1294.
[4]Ashida K,KajiY,SasakiY.Abolition oftorsade de pointes after radiofrequency catheter ablation at right ventricular outflow tract.Int J Cardiol 1997;59(2):171-175.
[5]Kusano KF,Yamamoto M,Emori T,et al.Successful catheter ablation in a patientwith polymorphic ventricular tachycardia.J Cardiovasc Electrophysiol 2000;11(6): 682-685.
[6]Betts TR,Yue A,Roberts PR,etal.Radiofrequency ablation of idiopathic ventricular fibrillation guided by noncontact mapping.J Cardiovasc Electrophysiol2004;15(8):957-959.
[7]Viskin S,Rosso R,Rogawski O.The‘‘short coupled’’variant of right ventricle outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol 2005;16(8):912-916.
[8]Kurosaki K,Nogami A,Shirai Y,et al.Positive QRS complex in lead I as a malignant sign in right ventricular outflow tract tachycardia.Circ J 2013;77(4):968-974.
[9]IgarashiM,Tada H,KurosakiK,etal.Electrocardiographic Determinants of the Polymorphic QRS Morphology in Idiopathic Right Ventricular Outflow Tract Tachycardia.J Cardiovasc Electrophysiol 2012;23(5):521-526.
[10]TakatsukiS,Mitamura H,Ogawa S.Catheterablation ofa monofocalpremature ventricular complex triggering idiopathic ventricular fibrillation.Heart 2001;86(1):e3.
[11]Nogami A:Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation:Trigger elimination and substrate modification.J Cardiovasc Electrophysiol2014 Sep 12.doi:10.1111/jce.12547.[Epub ahead of print]PubMed PMID:25216244.
[12]B a¨nsch D,Oyang F,Antz M,et al.Successful catheter ablation of electrical storm after myocardial infarction. Circulation 2003;108(24):3011-3016.
[13]Szumowski L,Sanders P,Walczak F,et al.Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction.J Am Coll Cardiol 2004;44(8): 1700-1706.
[14]Nogami A,Sugiyasu A,Kubota S,et al.Mapping and ablation of idiopathic ventricular fibrillation from Purkinje system.Heart Rhythm 2005;2(6):646-649.
[15]Haı¨ssaguerre M,Derval N,Sacher F,etal.Sudden cardiac arrest associated with early repolarization.N Eng J Med 2008;358(8):2016-2023.
[16]Zipes DP,Camm AJ,Borggrefe M,etal.ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death:a report of the American College of Cardiology/ American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death).Circulation.2006; 114(5):e385-484.
✉Corresponding author:Professor Akihiko Nogami,the Cardiovascular Division,University of Tsukuba,1-1-1 TennodaiTsukuba,Ibaraki,305-8575,Japan.Tel/Fax:+81-29-853-3142/+81-29-853-3143,E-mail: akihiko-ind@umin.ac.jp.Received 20 November 2014,Accepted 04 December 2014,Epub 01 January 2015
The author reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140156
 THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2015年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Myocardin in biology and disease
- Optimalantiarrhythmic drug therapy for electricalstorm
- Optimalprogramming managementofventricular tachycardia storm in ICD patients
- Neuraxialmodulation for treatmentof VT storm
- Prevalence ofenteric pathogen-associated community gastroenteritis among kindergarten children in Gaza
- Portalvein arterialization promotes liver regeneration after extended partialhepatectomy in a rat model
