四元盐水体系热力学性质和相平衡的预测*
翦立新,吴晓娅,谭雨齐,陈子芳,尹 霞
(湖南大学 化学传感与计量学国家重点实验室, 湖南 长沙 410082)

1 模型方法
为准确描述电解质溶液的热力学性质,Clegg和Pitzer等人[3]提出了一个用于计算任意多元电解质溶液由稀到浓的热力学性质的模型(简称PSC模型),该模型关于体系中各物种活度系数的计算公式较为复杂,基本形式如下:

A=a+bT,(A=Bca,WW,ca,UW,ca,VW,ca,Wijk,
QW,ijk,Uijk)
在计算三元体系及多元体系相图之前,需要知道体系中各固相的平衡常数.对任何给定的盐Mv+Xv-·nH2O,其溶解平衡可表示为:
Mv+Xv-·nH2O=v+M++v-X-+nH2O
达到平衡时,平衡常数K的表达式为:
lnK=v+lnaM++v-lnaX-+nlnaH2O
aM+,aX-和aH2O分别为M+,X-和水的活度.利用二元模型参数和二元体系的溶解度数据可计算出不同温度下的平衡常数K,并将其与温度关联成以下形式:
lnK=A+B/T+CT+DlnT
结合所获得的二元及三元模型参数以及不同温度下的平衡常数K,就可以计算三元和四元体系的溶解度.本文在模型计算中所用的程序均为作者用C语言自行编写.
2 三元体系溶解度的计算
2.1 三元体系溶解度的计算
KCl-H2O体系的PSC模型参数采用Holmes等人[4]所提供的298.15 K和423.15 K时的渗透系数,KNO3-H2O体系的模型参数拟合于Zeng等人[5]及Barry[6]所给的298.15 K和425.5 K的水活度数据.对于MgCl2-H2O体系,本文用Rard等人[7]提供的298.15 K的水活度和Fanghänel等人[8]所给的373.45 K的水活度拟合二元参数,Mg(NO3)2-H2O体系的二元参数拟合于298.15 K[9]和333.15 K[10]的水活度数据,NaCl-H2O体系的二元参数拟合于文献[11]所提供的298.15 K和348.15 K的水活度,NaNO3-H2O体系的二元参数采用Pearce等人[12]提供的298.15 K的水活度和Bobmann等人[13]提供的424.96 K的数据.所有二元参数与温度的关系式列于表1.

表1 二元PSC模型参数

表2 四元体系中固相的ln K与温度T (K)的关系

100 w

100 w
2.2 三元盐水体系的热力学计算
根据本组前期研究[2]可知,仅用PSC模型的二元参数难以准确计算三元体系的相图.为获得三元盐水体系的准确信息,用相关体系的溶解度数据分别拟合三元相互作用参数,所用实验数据来源及参数与温度的关系式见表3.结合表1~3中的二元及三元模型参数分别计算KCl-MgCl2-H2O,KNO3-Mg(NO3)2-H2O,KCl-KNO3-H2O,MgCl2-Mg(NO3)2-H2O,KNO3-NaNO3-H2O,MgCl2-NaCl-H2O,Mg(NO3)2-NaNO3-H2O,KCl-NaCl-H2O和NaCl-NaNO3-H2O体系的相图,并与实验值对比,结果见图2~10.
对于KCl-KNO3-H2O体系,预测了温度范围在273.15 K~364.15 K的共饱和线(图4(a)中的点划线),并与文献值[17](图4(a)中的符号)进行比较,发现二者完全吻合.同时计算了不同温度下共饱和点组成所对应的饱和溶液的水活度,并按式(4)换算成饱和蒸汽压,与实验值[17]进行对比(见图4(b)),结果发现计算值与实验值基本一致.
aw(T)=p(T)/p*(T)
式中p(T)和p*(T)分别为温度T时盐溶液和纯水的饱和蒸汽压.
另外将预测的KNO3-NaNO3-H2O和NaCl-NaNO3-H2O体系在363.15 K时饱和溶液的水活度与实验值[18]对比,结果分别见图6(b)和图10(b)),预测值与实验值基本吻合,且误差在实验值所给误差范围内.

图3 KNO3-Mg(NO3)2-H2O体系溶解度计算值与实验值[14]比较


图4 KCl-KNO3-H2O体系热力学计算值与实验值[14, 17, 18]比较

图5 MgCl2-Mg(NO3)2-H2O体系溶解度的计算值与实验值[16]比较
3 四元盐水体系溶解度的预测



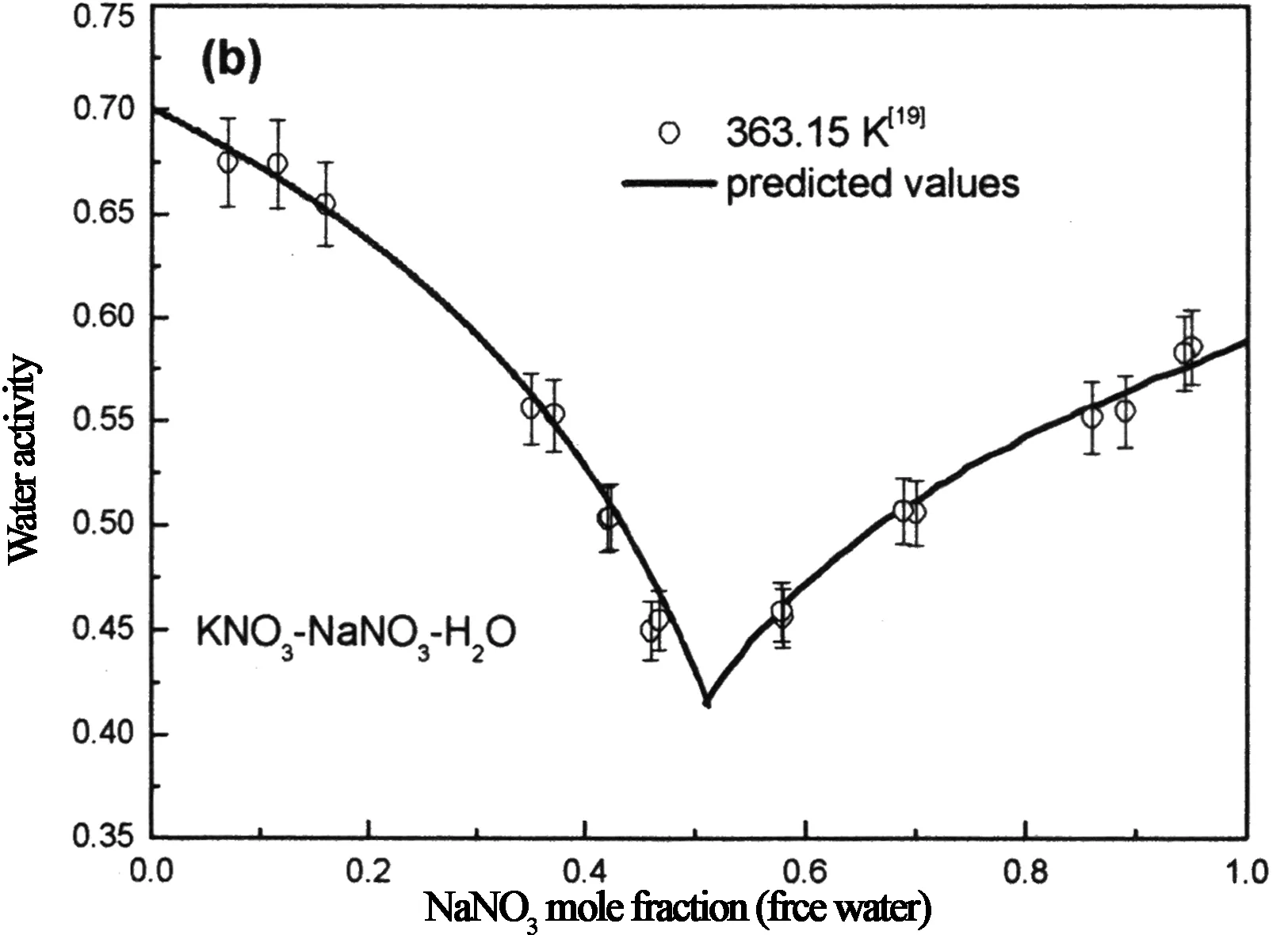
图6 KNO3-NaNO3-H2O体系热力学计算值与实验值[14, 19]比较

图7 MgCl2-NaCl-H2O体系溶解度的计算值与实验值[14]比较

图8 Mg(NO3)2-NaNO3-H2O体系溶解度的计算值与实验值[16]比较

图9 KCl-NaCl-H2O体系溶解度的计算值与实验值[14]比较
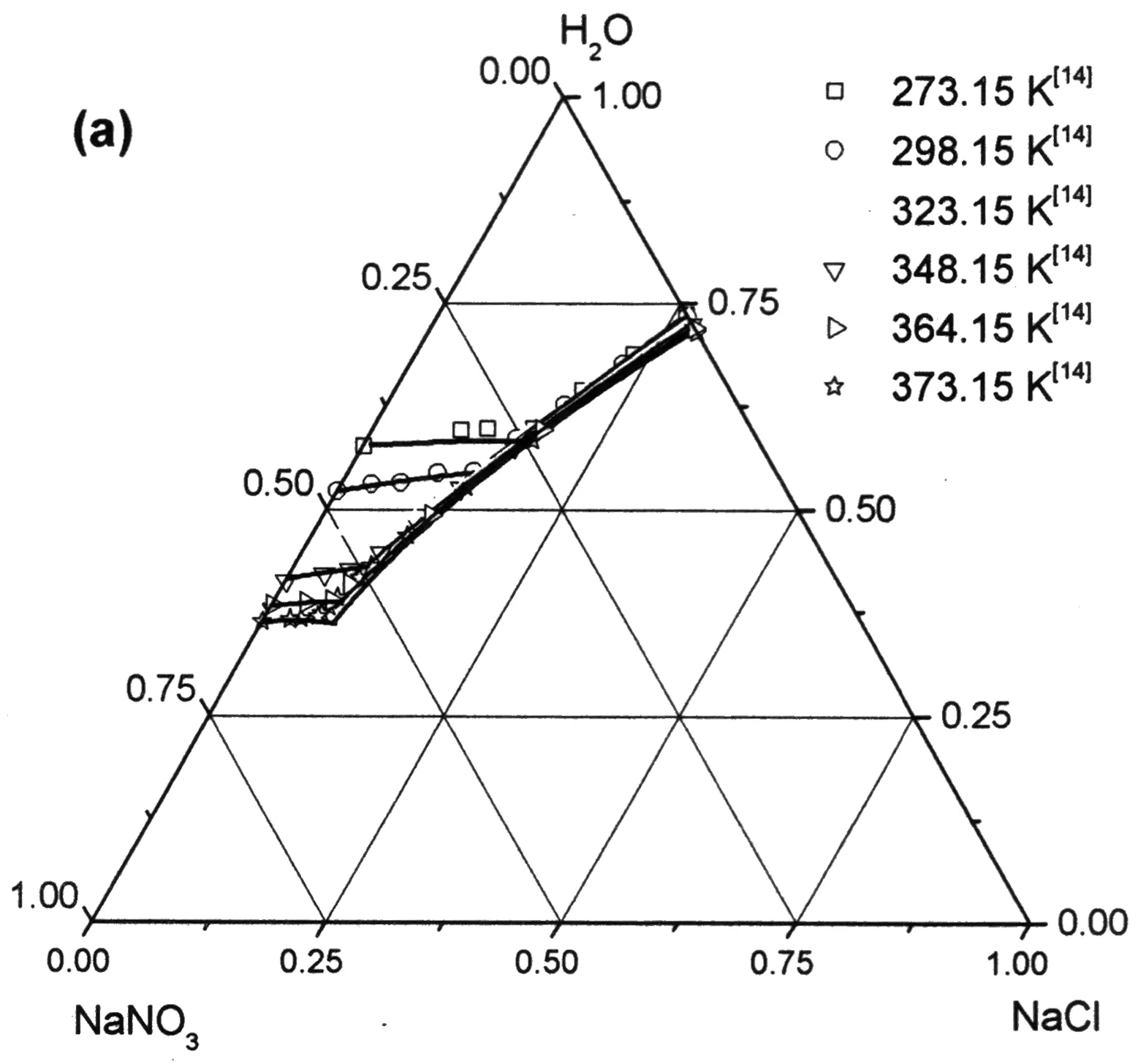

NaNO3 mole fraction (free water)

表3 三元PSC模型参数
J(Mg2+)

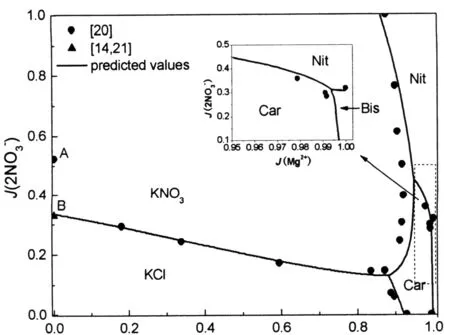
图12 K+,Na+∥体系干盐图的预测值与实验值[22]比较

图13 Mg2+,Na+∥体系干盐图的预测值与实验值[16]比较

图14 298.15 K时K+,Mg2+,Na+∥体系干盐图的预测值与实验值[25]比较
4 结 论
表4 K+,Na+∥体系的饱和蒸汽压预测值与实验值[23]比较
所用的二元模型参数拟合于水活度或渗透系数实验值,三元模型参数通过拟合三元体系的溶解度实验数据得到.
计算结果充分说明应用PSC模型,借助有限的二元及三元盐水体系的实验数据,可较为准确地预测复杂盐水体系的热力学性质,为工程应用提供可靠的理论指导.
[1]WANG W L, ZENG D W, YIN X,etal. Prediction and measurement of gypsum solubility in the systems CaSO4+ HMSO4+ H2SO4+ H2O (HM = Cu, Zn, Ni, Mn) at 298.15 K[J]. Industrial and Engineering Chemistry Research, 2012, 51: 5124-5134.
[2]YIN X, CHEN Q Y, ZENG D W,etal. Phase diagram of the system KNO3+ LiNO3+ Mg(NO3)2+ H2O[J]. Calphad, 2011, 35: 463-472.
[3]CLEGG S L, PITZER K S, BRIMBLECOMBE P. Thermodynamics of multicomponent, miscible, ionic solutions. Mixtures including unsymmetrical electrolytes[J]. J Phys Chem, 1992, 96(23): 9470-9479.
[4]HOLMES H F, MESME R E. Thermodynamic properties of aqueous solutions of the alkall metal chlorides to 250 ℃[J]. J Phys Chem, 1983, 87: 1242-1255.
[5]ZENG D W, WU Z D, YAO Y,etal. Isopiestic determination of water activity on the system LiNO3+ KNO3+ H2O[J]. J Sol Chem, 2010, 39: 1360-1376.
[6]BARRY J C, RICHTER J, STICH E. Vapour pressures and ionic activity coefficients in the system KNO3+ H2O from dilute solutions to fused salts at 425 K, 452 K and 492 K[J]. Ber Bunsenges Phys Chem, 1988, 92: 1118-1122.
[7]RARD J A, MILLER D G. Isopiestic determination of the osmotic and activity coefficients of aqueous magnesium chloride solutions at 25 ℃[J]. J Chem Eng Data, 1981, 26(1): 38-43.
[8]FANGHANEL T, GRJOTHEIM K. Thermodynamics of aqueous reciprocal salt systems. Ⅲ. Isopiestic determination of osmotic and activity coefficients of aqueous MgCl2, MgBr2, KCl and KBr at 100.3 ℃[J]. Acta Chemica Scandinavica, 1990, 44: 892-895.
[9]RARD J A, WIJESINGHE A M, WOLERY T J. Review of the thermodynamic properties of Mg(NO3)2(aq) and their representation with standard and extended ion-interaction (Pitzer) models at 298.15 K[J]. J Chem Eng Data, 2004, 49: 1127-1140.
[10]EWING W W, KLINGER E, BRANDNER J D. Studies on the vapor pressure-temperature relations and on the heats of hydration, solution and dilution of the binary system magnesium nitrate-water[J]. J Am Chem Soc, 1934, 56: 1053-1057.
[11]GIBBARD JR H F, SCATCHARD G, ROUSSEAU R A,etal. Liquid-vapor equilibrium of aqueous sodium chloride, from 298 to 373 K and from 1 to 6 mol·kg-1, and related properties[J]. J Chem Eng Data, 1974, 19: 281-288.
[12]PEARCE J N, HOPSON H. The vapor pressures of aqueous solutions of sodium nitrate and potassium thiocyanate[J]. J Phys Chem, 1937, 41: 535-538.
[13]BOBMANN E, RICHTER J, STARK A. experimental results and aspects of analytical treatment of vapour pressure measurements in hydrated melts at elevated temperature[J]. Ber Bunsenges Phys Chem, 1993, 97: 240-245.
[14]LINKE W F, SEIDELL A. Solubilities: inorganic and metal-organic compounds[M]. 4th Ed. Washington DC: American Chemical Society, 1965.
[15]FANGHANEL T H, KRAVCHUK K, VOIGT W,etal. Solid-liquid-phase equilibria in the system KCl-MgCl2-H2O at elevated temperatures. Ⅰ. The binary system MgCl2-H2O at 130-250 ℃[J]. Allg Chem, 1987, 547: 21-26.
[16]SIEVERTS A, MULLER H Z. Reciprocal system MgCl2, Na2(NO3)2, H2O[J]. Anorg Allg Chem, 1930, 189: 241-257.

[18]APELBLAT A , KORIN E. Temperature dependence of vapor pressures over saturated aqueous solutions at invariant of the NaCl + KCl + H2O, NaCl + NaNO3+ H2O, KCl + KBr + H2O, KCl + KI + H2O, KCl + KNO3+ H2O, and KCl + K2SO4+ H2O systems[J]. J Chem Eng Data, 2009, 54(5): 1619-1624.
[19]CARROLL S, CRAIG L, WOLERY T. Deliquescence of NaCl-NaNO3, KNO3- NaNO3, and NaCl-KNO3salt mixtures from 90 to 120 ℃[J]. J Geochim Trans, 2005, 6(2): 19-30.


[21]宋彭生, 罗志农. 三元水盐体系25 ℃溶解度的预测[J]. 化学通报, 1983, 12: 13-19.
SONG Peng-sheng, LUO Zhi-nong. Prediction of solubiltiy of the ternary salt-water system at 25 ℃[J]. Chemistry(Huaxue Tongbao), 1983, 12: 13-19. (In Chinese)
[22]REINDERS W Z. The reciprocal system KCl + NaNO3= KNO3+ NaCl and the preparation of nitrate[J]. Anorg Allgem Chem, 1915, 93: 202-212.
[23]APELBLAT A, KORIN E. Temperature dependence of vapor pressures over saturated aqueous solutions at invariant points of the NaCl + KNO3+ H2O, NaCl + Na2CO3+ H2O, and NaCl + Na2SO4+ H2O systems[J]. J Chem Eng Data, 2011, 56: 988-994.
[24]黄雪莉, 朱丽娟, 梁涛, 等. 298.16 K Na+, K+, Mg2+//Cl-, NO3--H2O五元体系的相平衡研究[J]. 化学学报, 2007, 65: 798-802.



