剥脱综合征与LOXL1基因多态性相关性的meta分析*
唐 聪, 季青山, 钟敬祥△, 钟景贤
(1暨南大学第一附属医院眼科,广东 广州 510632; 2江门新会新希望眼科医院,广东 江门 529100)
剥脱综合征(exfoliation syndrome,XFS)是一种累及全身结缔组织的异常细胞外基质生成的病变,与老年细胞代谢过程异常有关[1]。全球60岁以上人群中有20%~30%的人群受到该病影响,这种特征性的组织改变可以诱发广泛的眼内并发症,包括青光眼、色素播散、晶体半脱位、瞳孔散大不全、血-房水屏障功能障碍、虹膜后粘连和角膜内皮失代偿等[2]。XFS具有较高的白内障和青光眼的发生率,常与严重的慢性继发性开角型青光眼有关,引起剥脱综合征性青光眼(exfoliation glaucoma,XFG),目前被认为是开角型青光眼的主要原因之一,并在全世界范围的开角型青光眼中占 25%。它同原发性开角型青光眼比较,具有眼压高、难控制、视野进行性损害迅速且严重、对药物治疗效果差的特点[3]。
XFS/XFG的发生同几个染色体位点有密切关系,类赖氨酰氧化酶1(lysyl oxidase-like 1,LOXL1)基因多态性分析发现与 XFS/XFG 显著相关[4-7]。最初报告LOXL1是一种新的人类氨基酸序列同源基因,2007年首先报道发现它同XFS有相关性[8],并且从这时起,有很多研究文献报道在不同种群中LOXL1基因多态性同XFS的相关性。研究种群主要包括高加索人、非洲人和亚洲人(包括中国和日本),研究结果提示各个种群间差异较大[9-39]。例如:在以往已经发表的关于LOXL1基因多态性和XFG的相关性meta分析提示,在白种人群中,LOXL1(G等位基因rs1048661)和LOXL1(T等位基因rs3825942)同XFS/XFG呈正相关,而这2个等位基因在日本人群中对于XFS/XFG的发生呈明显负相关[28]。为了更全面更准确评价两者之间的关系,我们查阅大量独立的在不同种群之间的研究结果,应用meta分析的方法,研究在不同种群中LOXL1基因多态性(rs1048661、rs2165241和rs3825942)与XFS/XFG发病的相关性。
材 料 和 方 法
1 材料
2名研究者各自独立地利用PubMed和Embase搜索符合选择标准的英文文献,搜索的关键词是“LOXL1或者lysyl oxidase-like 1”和“glaucoma、exfoliation或者pseudo exfoliation”,截止日期到2013年5月。研究文献纳入标准:(1)文献主要内容是LOXL1基因多态性(rs1048661、rs2165241和rs3825942)与XFS/XFG发病相关性研究;(2)研究方式是病例对照研究和队列研究;(3)具体研究方案包括样本量大小、等位基因分布或者基因型频率;(4)当出现多个相同或重复的研究数据时,采用最近发表的和样本量大的文献;(5)当不同文章作者报道的是同样的研究结果时,视为各自独立研究。排除标准:(1)家系研究;(2)其它不相关基因的多态性研究。
2名研究者在所有纳入标准和排除标准上达成共识。如果2人对检索到的文献内容有不同意见,由第3位研究者来判断是否符合纳入标准。每一篇文献的内容包括:第1作者姓名、文献发表年份、被研究人群的种族、样本量大小、等位基因和基因型频率。
2 统计学方法
2.1采用STATA统计软件进行异质性检验 根据Q-test及I2值判断异质性,若P<0.10和I2>50%说明纳入研究存在异质性,选择随机效应模式进行meta分析,反之,说明纳入研究一致性好,应选择固定效应模式进行meta分析。
2.2绘制森林图 LOXL1基因多态性(rs1048661、rs2165241和rs3825942)与XFS/XFG发病相关性评估用优势比(odds ratio,OR)和95% 可信区间(confidence interval,CI),总体效应统计学处理用Z检验,以P<0.05为差异有统计学意义。
2.3敏感性分析 通过依次剔除相关研究,观察各个研究对总体效应影响来判断meta分析结果是否稳定可靠。
2.4发表偏倚评估 通过森林图及Begg’s定量检验发表偏倚。
结 果
1 文献检索结果
经PubMed和EMBASE检索,共检出相关文献159篇,符合纳入标准的文献31篇,含33个病例对照研究(图1)。来源于高加索人种21个、亚洲人10个和非洲人2个。详细资料和特征见表1。
2 Meta分析结果
2.1异质性检验 总体异质性分析,3组单核甘酸多态性分析研究均存在明显的异质性(P<0.01,I2>90%)。进一步亚组分析发现,除在亚洲人群rs1048661(P<0.01,I2=94%) 和高加索人群rs3825942(P<0.01,I2=66%)研究存在明显异质性,余未见明显异质性。
2.2森林图 如图2所示,总效应测定结果显示rs1048661多态性与XFS/XFG发病无明显相关(GvsT: OR=0.91, 95% CI=0.62~1.35,P>0.05)。进一步根据种群进行亚组分析,结果显示在高加索和非洲人群中rs1048661多态性与XFS/XFG发病呈正相关(GvsT: OR= 2.19, 95% CI=1.96~2.45,P<0.01; OR= 23.42, 95% CI=4.48~122.52,P<0.01),而在亚洲人群中呈负相关(GvsT: OR= 0.06, 95% CI=0.02~0.18,P<0.01)。
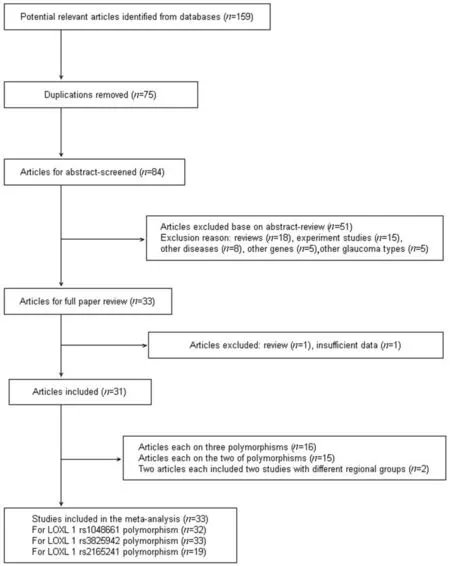
Figure 1. Flow diagram of the study selection for the meta-analysis.
如图3所示,总效应测定结果显示rs3825942多态性与XFS/XFG发病呈明显正相关(GvsA: OR=9.21, 95% CI=5.12~16.54,P<0.01)。亚组分析显示在高加索和亚洲人群中rs3825942多态性与XFS/XFG发病呈正相关(OR= 8.80, 95% CI=6.05~12.79,P<0.01及OR= 14.92, 95% CI=9.15~24.34,P<0.01),而在非洲人群呈负相关(OR= 0.09, 95% CI=0.06~0.15,P<0.01)。
图4所示,总效应测定结果显示rs2165241多态性与XFS/XFG发病呈正相关(TvsC: OR=1.94, 95%CI=1.44~2.61,P<0.01)。亚组分析显示在高加索人群中rs2165241多态性与XFS/XFG眼发病呈正相关(OR= 3.41, 95% CI=3.11~3.73,P<0.01),而在亚洲人群中呈负相关(OR= 0.15, 95% CI=0.09~0.25,P<0.01)。
2.3敏感性分析结果 单个研究未影响总体效应分析,说明本meta分析结果稳定可靠。
2.4发表偏倚评估 rs1048661 和 rs3825942研究存在明显发表偏倚(t=3.2,P<0.01;t=2.6,P<0.05)。而rs2165241无明显发表偏倚,见图5A~C。
讨 论
XFS 不论从患病率还是从发病机制方面全世界已经在积极地进行研究分析,当前研究XFS发病机制的重点主要集中在LOXL1基因3个单核苷酸多态性位点(rs2165241、rs1048661和rs3825942)的研究,但是结论不尽相同。本研究通过meta分析的方法,全面定量地综合了关于LOXL1基因这3个位点多态性与XFS/XFG的相关性研究结果,得出LOXL1基因3个位点多态性在不同种群中与XFS/XFG的发病关系存在明显差异。在高加索人群中,rs1048661 G和rs2165241 T增加XFS/XFG发病风险,在亚洲人群中上述2个位点却对XFS/XFG发病起着保护作用。与此同时rs3825942 G增加了高加索和亚洲人群XFS/XFG发病风险,而对非洲人群具有保护作用。
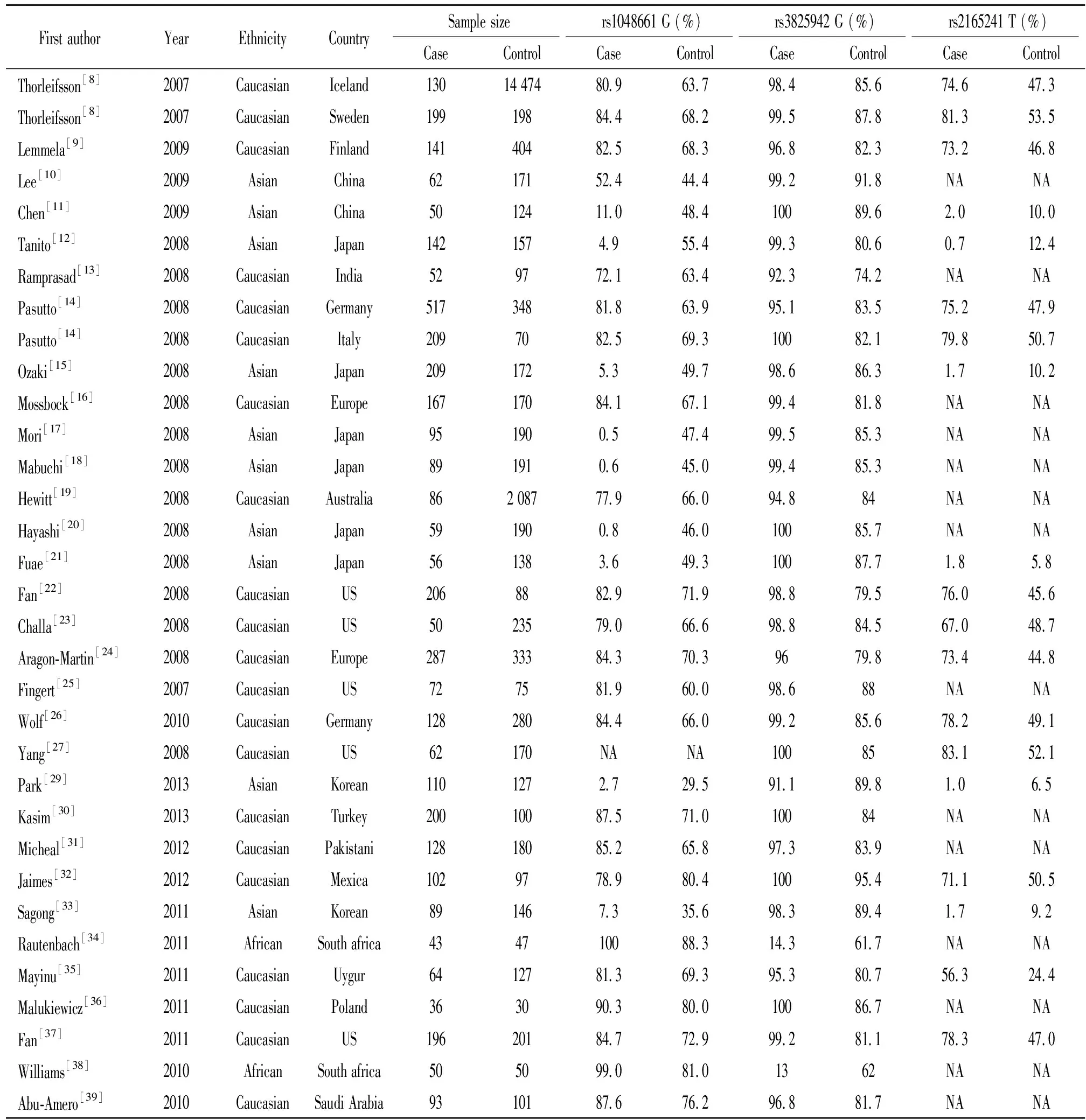
表1 关于LOXL1基因多态性和剥脱综合征纳入meta分析的文献基本情况
LOXL1是赖氨酸氧化酶家族成员之一,赖氨酸氧化酶属于含铜的细胞外酶,其功能是通过赖氨酸的氧化脱氨或羟侧链作用催化结缔组织中胶原蛋白和弹性蛋白的共价交联,参与弹性组织形成,通过诱导胶原交联和产生弹性分子来维持细胞外基质稳定。LOXL1 广泛分布在许多组织,如人类的皮肤、心脏、胰脏、肺脏、肾、肝和骨骼肌中。近来已发现 LOXL1 mRNA 和蛋白质在所有眼组织中都有表达,如角膜、虹膜、晶状体、睫状体、视网膜、视神经,但LOXL1 在虹膜表达最高,这也是在 XFS 受累最早并且最重的组织。多项研究证实这3个位点的等位基因频率、基因型、基因单倍体型和复合基因型在不同人群中有所不同,且LOXL1基因多态性是导致 XFS/XFG遗传易感性的因素[9-41]。
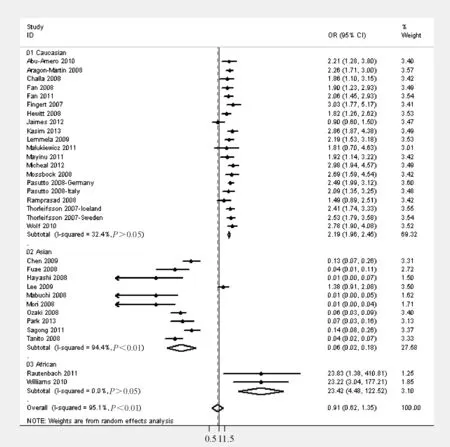
Figure 2. Forest plot from the meta-analysis of rs1048661 and risk of XFS/XFG in allelic risk model (G vs T) for different ethnicities.
然而LOXL1基因多态性在XFS/XFG发病机制中起着什么样作用,目前仍不十分清楚。本meta分析研究结果发现rs1048661和rs2165241多态性对不同种群中XFS/XFG发病产生相反影响,说明LOXL1基因单核苷酸突变并非单独与XFS/XFG发病相关,可能存在基因遗传性表达差异和年龄、性别、环境相互作用的影响,这有待进一步实验研究证实。
最近有许多研究发现基因多态性和临床疾病相关[42-43]。本次meta分析要尽可能全面地收集所有相关研究文献进行系统评价,研究内容主要是已经发表的相关英文文献,对于一些未发表的或者非英语语种文献没有进行收集,这对研究结果可能会产生偏倚,其次,XFS/XFG是一种与年龄有关的复杂疾病,它的起病是包括遗传因素和环境因素等多因素综合作用的结果,本次meta分析没有涉及基因和环境相互作用对XFS/XFG的影响,对该病的发病机制需更进一步研究;再次,本次研究的种群主要是高加索人、非洲人和亚洲人,没有涉及到其他种群的发病特点。在研究过程中,我们对原始文献进行严格的筛选,尽可能减少了各种可能存在的偏倚,而且敏感性分析结果支持原有结论,说明该结论具有较强的科学性和说服力。

Figure 3. Forest plot from the meta-analysis of rs3825942 and risk of XFS/XFG in allelic risk model (G vs A) for different ethnicities.
[参 考 文 献]
[1] Naumann GO, Schlötzer-Schrehardt U, Küchle M.Pseudoexfoliation syndrome for the comprehensive ophthalmologist: intraocular and systemic manifestations[J]. Ophthalmology, 1998,105(6):951-968.
[2] Ritch R. Exfoliation syndrome: the most common identifiable cause of open-angle glaucoma[J]. J Glaucoma,1994,3(2):176-177.
[3] Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome[J]. Surv Ophthalmol,2001,45(4):265-315.
[4] Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome[J]. Am J Ophthalmol, 2006,141(5):921-937.e2.
[5] Sein J, Galor A, Sheth A, et al. Exfoliation syndrome: New genetic and pathophysiologic insights[J]. Curr Opin Ophthalmol, 2013,24(2):167-174.
[6] Kenyon K, Modi WS, Contente S, et al. A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24-q25[J]. J Biol Chem,1993,268(25):18435-18437.

Figure 4. Forest plot from the meta-analysis of rs2165241 and risk of XFS/XFG in allelic risk model (T vs C) for different ethnicities.

Figure 5. Begg’s funnel plot for publication bias test.Each circle represents a separate study for the indicated association. A: rs1048661; B: rs3825942; C: rs2165241.
[7] Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase[J]. J Biol Chem,1995,270(13):7176-7182.
[8] Thorleifsson G, Magnusson KP, Sulem P, et al. Common sequence variants in theLOXL1 gene confer susceptibility to exfoliation glaucoma[J]. Science,2007,317(5843):1397-1400.
[9] Lemmela S, Forsman E, Onkamo P, et al. Association ofLOXL1 gene with Finnish exfoliation syndrome patients[J]. J Hum Genet,2009,54(5):289-297.
[10] Lee KY, Ho SL, Thalamuthu A, et al. Association ofLOXL1 polymorphisms with pseudoexfoliation in the Chinese[J]. Mol Vis,2009,15:1120-1126.
[11] Chen L, Jia L, Wang N, et al. Evaluation ofLOXL1 polymorphisms in exfoliation syndrome in a Chinese population[J]. Mol Vis, 2009,15:2349-2357.
[12] Tanito M, Minami M, Akahori M, et al.LOXL1 variants in elderly Japanese patients with exfoliation syndrome/glaucoma, primary open-angle glaucoma, normal tension glaucoma, and cataract[J]. Mol Vis, 2008,14:1898-1905.
[13] Ramprasad VL, George R, Soumittra N, et al. Association of non-synonymous single nucleotide polymorphisms in theLOXL1 gene with pseudoexfoliation syndrome in India[J]. Mol Vis,2008,14:318-322.
[14] Pasutto F, Krumbiegel M, Mardin CY, et al. Association ofLOXL1 common sequence variants in German and Italian patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma[J]. Invest Ophthalmol Vis Sci,2008,49(4):1459-1463.
[15] Ozaki M, Lee KY, Vithana EN, et al. Association ofLOXL1 gene polymorphisms with pseudoexfoliation in the Japanese[J]. Invest Ophthalmol Vis Sci,2008,49(9):3976-3980.
[16] Mossbock G, Renner W, Faschinger C, et al. Lysyl oxidase-like protein 1 (LOXL1) gene polymorphisms and exfoliation glaucoma in a Central European population[J]. Mol Vis,2008,14:857-861.
[17] Mori K, Imai K, Matsuda A, et al.LOXL1 genetic polymorphisms are associated with exfoliation glaucoma in the Japanese population[J]. Mol Vis,2008,14:1037-1040.
[18] Mabuchi F, Sakurada Y, Kashiwagi K, et al. Lysyl oxidase-like 1 gene polymorphisms in Japanese patients with primary open angle glaucoma and exfoliation syndrome[J]. Mol Vis,2008,14:1303-1308.
[19] Hewitt AW, Sharma S, Burdon KP, et al. AncestralLOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people[J]. Hum Mol Genet,2008,17(5):710-716.
[20] Hayashi H, Gotoh N, Ueda Y, et al. Lysyl oxidase-like 1 polymorphisms and exfoliation syndrome in the Japanese population[J]. Am J Ophthalmol,2008,145(3):582-585.e2.
[21] Fuse N, Miyazawa A, Nakazawa T, et al. Evaluation ofLOXL1 polymorphisms in eyes with exfoliation glaucoma in Japanese[J]. Mol Vis,2008,14:1338-1343.
[22] Fan BJ, Pasquale L, Grosskreutz CL, et al. DNA sequence variants in theLOXL1 gene are associated with pseudoexfoliation glaucoma in a U.S. clinic-based population with broad ethnic diversity[J]. BMC Med Genet,2008,9:5.
[23] Challa P, Schmidt S, Liu Y, et al. Analysis ofLOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma[J]. Mol Vis,2008,14:146-149.
[24] Aragon-Martin JA, Ritch R, Liebmann J, et al. Evaluation ofLOXL1 gene polymorphisms in exfoliation syndrome and exfoliation glaucoma[J]. Mol Vis,2008,14:533-541.
[25] Fingert JH, Alward WL, Kwon YH, et al.LOXL1 mutations are associated with exfoliation syndrome in patients from the midwestern United States[J]. Am J Ophthalmol,2007,144(6):974-975.
[26] Wolf C, Gramer E, Muller-Myhsok B, et al. Lysyl oxidase-like 1 gene polymorphisms in German patients with normal tension glaucoma, pigmentary glaucoma and exfoliation glaucoma[J]. J Glaucoma,2010,19(2):136-141.
[27] Yang X, Zabriskie NA, Hau VS, et al. Genetic association ofLOXL1 gene variants and exfoliation glaucoma in a Utah cohort[J]. Cell cycle,2008,7(4):521-524.
[28] Chen H, Chen LJ, Zhang M, et al. Ethnicity-based subgroup meta-analysis of the association ofLOXL1 polymorphisms with glaucoma[J]. Mol Vis,2010,16:167-177.
[29] Park do Y, Won HH, Cho HK, et al. Evaluation of lysyl oxidase-like 1 gene polymorphisms in pseudoexfoliation syndrome in a Korean population[J]. Mol Vis,2013,19:448-453.
[30] Kasim B, Irkec M, Alikasifoglu M, et al. Association ofLOXL1 gene polymorphisms with exfoliation syndrome/glaucoma and primary open angle glaucoma in a Turkish population[J]. Mol Vis, 2013,19:114-120.
[31] Micheal S, Khan MI, Akhtar F,et al. Role of lysyl oxidase-like 1 gene polymorphisms in Pakistani patients with pseudoexfoliative glaucoma[J]. Mol Vis,2012,18:1040-1044.
[32] Jaimes M, Rivera-Parra D, Miranda-Duarte A, et al. Prevalence of high-risk alleles in theLOXL1 gene and its association with pseudoexfoliation syndrome and exfoliation glaucoma in a Latin American population[J]. Ophthalmic Genet,2012,33(1):12-17.
[33] Sagong M, Gu BY, Cha SC. Association of lysyl oxidase-like 1 gene polymorphisms with exfoliation syndrome in Koreans[J]. Mol Vis,2011,17:2808-2817.
[34] Rautenbach RM, Bardien S, Harvey J,et al. An investigation intoLOXL1 variants in black South African individuals with exfoliation syndrome[J]. Arch Ophthalmol,2011,129(2):206-210.
[35] Mayinu, Chen X. Evaluation ofLOXL1 polymorphisms in exfoliation syndrome in the Uygur population[J]. Mol Vis,2011,17:1734-1744.
[36] Malukiewicz G, Lesiewska-Junk H, Linkowska K, et al. Analysis ofLOXL1 single nucleotide polymorphisms in Po-lish population with pseudoexfoliation syndrome[J]. Acta Ophthalmol,2011,89(1):e64-e66.
[37] Fan BJ, Pasquale LR, Rhee D, et al.LOXL1 promoter haplotypes are associated with exfoliation syndrome in a U.S. Caucasian population[J]. Invest Ophthalmol Vis Sci,2011,52(5):2372-2378.
[38] Williams SE, Whigham BT, Liu Y, et al. MajorLOXL1 risk allele is reversed in exfoliation glaucoma in a black South African population[J]. Mol Vis,2010,16:705-712.
[39] Abu-Amero KK, Osman EA, Dewedar AS, et al. Analysis ofLOXL1 polymorphisms in a Saudi Arabian population with pseudoexfoliation glaucoma[J]. Mol Vis,2010,16:2805-2810.
[40] Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family[J]. Prog Nucleic Acid Res Mol Biol,2001,70:1-32.
[41] Schlotzer-Schrehardt U, Hammer CM, Krysta AW, et al.LOXL1 deficiency in the lamina cribrosa as candidate susceptibility factor for a pseudoexfoliation-specific risk of glaucoma[J]. Ophthalmology,2012,119(9):1832-1843.
[42] 李淑华,胡巢凤,刘特长.热性惊厥儿童白细胞介素-1β(-511)基因多态性分析[J].中国病理生理杂志,2006,22(6):1168-1170.
[43] 曾志荣,陈 斌,李初俊,等.IL-1B-511单核苷酸多态性与我国胃癌高发区胃黏膜萎缩关系的研究[J].中国病理生理杂志,2007,23(11):2226-2228.

