Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
Wei-Cai Fu, Yan Jiang, Lin Zhang
Department of Ophthalmology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
Wei-Cai Fu, Yan Jiang, Lin Zhang*
Department of Ophthalmology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
Objective: To explore effect of retinal stem cells (RSCs) combined with copolymer-1 (COP-1) immunotherapy on optic nerve damage in glaucoma rat model. Methods: A total of 40 SD rats were selected for glaucoma model and were randomly divided into 4 groups to observe protective effects of RSCs transplantation combined with COP-1. Results: Brain-derived neurotrophic factor (BDNF) and insulin like growth factor-1 (IGF-1) were either positive in retina of RSCs transplanted or COP-1 immunological treated rat. Positive rate of BDNF and IGF-1 and expression of mRNA and protein were significantly higher in RSCs transplantation combined with COP-1 immunotherapy treated rats compared with the other 3 groups, in which amount of apoptotic RGCs was lowest. Conclusions: RSCs transplantation combined with COP-1 immunotherapy can promote the secretion of BDNF and IGF-1. They protect RGCs in glaucoma rats in coordination, significantly reduce the number of apoptosis RGCs so as to alleviate the optic nerve damage. It ponits a new research direction for treatment of glaucoma.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
Retinal stem cells
Polymer -1
Immunotherapy
Glaucoma model
Rat
1. Introduction
Intraocular pressure of glaucoma patients stays intermittent or continuous high, and continuous high intraocular pressure can cause damage of eyeball tissue or visual function to finally cause vision loss even blindness with modality of less than 1%, however, it has become one of three blinding diseases universally[1,2]. Most researches consider that damage of retinal ganglion cells (RGCs) is the main reason for blindness of glaucoma patients, but recently there is no effective method to prevent progressive apoptosis of RGCs[3,4]. Thus, in recent years researchers start to apply retinal stem cells transplantation to treat this disease[5]. This study aims to analyze effects of RSCs combined with copolymer-1 (COP-1) on glaucoma rat model.
2. Materials and methods
2.1. Experiment materials
A total of 50 SD clean grade rats aged 8-10 weeks and weighted 180-220 g which were purchased from Institute of Laboratory Animal, Chinese Academy of Medical Sciences were selected. Animal qualified number was 01-3001. The rats were kept in clean animal house of experimental animal institute (Certification NO: SYXK11-00-0014) under environment temperature 20-23 ℃ and humidity 40%-80%. Male and female were separated and fed with water and food freely.
2.2. Establishment of glaucoma model
A total of 532 laser diodes were used to photocoagulatecorneal limbus vascular network, temporal corneal limbus, superior temporal sclera superficial vein and inferior temporal sclera superficial vein of 40 rats. The rest 10 rats were not given any special treatment. Intraocular pressure, conjunctiva, cornea and aqueous water of rats were observed 21 days after photocoagulation[6,7].
2.3. Retina stem cell culture
Five SD rats from control group were selected and removed eye balls under general anesthesia, and other tissue of eyeball was removed under microscope only with marginal part of ciliary body tissue kept. The tissue was cut into pieces and digested by 0.25% trypsin plus 0.02% EDTA solution. After 7 minutes medium with 10% FBS was used to stop digestion and the sample was centrifuged for two times by 1 000 r/min. The cells were added with medium and inoculated in culture bottles after discarding supernatant. The cells were cultured under 37 ℃ and 5% CO2. Immunofluorescent staining was used to identify RSCs and primary cells were kept for experiment[8,9].
2.4. Treatment of different groups
A total of 40 glaucoma model rats were numbered and randomly divided into A, B, C and D groups. Phosphate buffer solution (PBS) were injected in group A and C, COP-1 were injected in group B and D on d1. PBS were injected in group A and B, COP-1 were injected in group C and D on d7.
2.5. Indexes observation
Expression of brain-derived neurotrophic factor (BDNF) and insulin like growth factor-1 (IGF-1) was tested in groups 2-3 weeks after treatment by immunofluorescent assay. Polymerase chain reaction (PCR) was used to compare the mRNA expression difference[10]. Western blot was used to compare expression of BNDF and IGF-1[11]. Terminaldeoxynucleoitidyl transferase mediated nick end labeling was used to observe RGCs apoptosis of rat retina tissue frozen section[12,13].
2.6. Statistical analysis
All data in our study were analyzed by SPSS13.0. Enumeration data was analyzed by Chi-square test and measurement data was analyzed byttest. The test level was set as α=0.05. The difference was considered as statistically significant when P<0.05.
3. Results
3.1. Immunofluorescent staining results
BDNF and IGF-1 showed positive expression with red color in B, C and D groups and mainly concentrated at nerve fiber layer and retinal ganglion cell layer, as shown in Figure 1.

Figure 1. Expression of BDNF and IGF-1 under confocal microscope in group D.
3.2. mRNA expression difference
Expression of BDNF and IGF-1 was significantly higher in group D compared with the other three groups. Expression of BDNF and IGF-1 in group A was significantly lower than the other three groups (P<0.05), there was no significant difference between group B and group C (P>0.05), as shown in Table 1.
Table 1 BDNF and IGF-1 mRNA expression
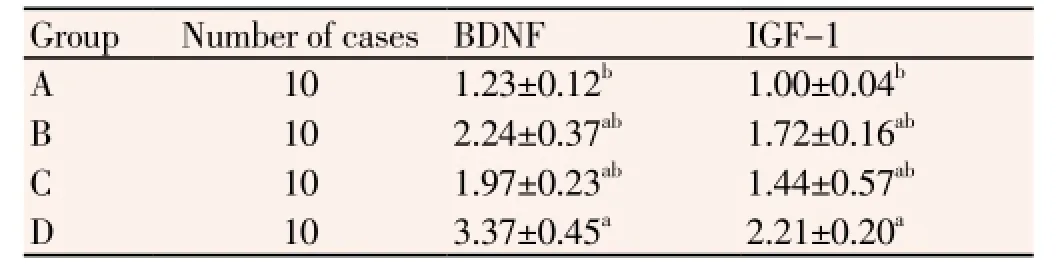
Table 1 BDNF and IGF-1 mRNA expression
Note:aCompared with group A, P<0.05;bCompared with group D, P<0.05.
?
3.3. Protein expression
Western blot analysis showed that BDNF and IGF-1 protein expression in group D was significantly higher compared with the other 3 groups, BDNF and IGF-1 protein expression in group A was significantly lower compared with the otherthree groups (P<0.05), there was no statistical difference between group B and group C (P>0.05), as shown in Table 2 and Figure 2.

Figure 2. Protein expression of BDNF and IGF-1.
Table 2 Protein expression of BDNF and IGF-1 in 4 groups

Table 2 Protein expression of BDNF and IGF-1 in 4 groups
Note:aCompared with group A, P<0.05;bCompared with group A, P<0.05.
?
3.4. Analysis of RGCs apoptosis
The average value of apoptosis cells in single vision was 11.2 in group A, 9.0 in group B, 9.5 in group C and 6.2 in group D. The amount of apoptotic RGCs was significantly lower in group D than the other 3 groups, The amount of apoptotic RGCs was significantly higher in group A than the other 3 groups (P<0.05), the amount had no statistical difference between group B and group C (P>0.05).
4. Discussion
In recent years, scholars have been looking for better methods to treat glaucoma from optimal nerve regeneration, RSCs transplantation and induction of RSCs directional differentiation. Stem cells are potential in self-renewal and differentiation. Researchers have verified that nerve stem cells can merge into rat tissue to promote formation of mature brain cells[10,11]. However there is less research about RSCs merging in rat retina. Besides, Niyadurupolaet al[12-15] found that immune system showed significant effects on the development of glaucoma, and artificial COP-1 can protect the immune reaction to stop secondary damage of RGCs. To explore RSCs transplantation combined with COP-1 immune therapy on treating glaucoma we selected 50 SD rats for research.
Researchers have found that BDNF and IGF-1 showed positive expression in rat retina after RSCs transplantation or COP-1 immunotherapy, however, positive expression rate, mRNA and protein expression of BDNF and IGF-1 in rats which accepted RSCs transplantation combined with COP-1 immunotherapy was significantly higher than the other 3 groups, amount of apoptotic RGCs was also lowest, showing that nerve nutrition and growth factor recovered effectively in this group to promote the calcium ion concentration, decrease partial pressure of oxygen, and inhibit free oxygen radicals and release of harmful neurotransmitter to further stop RGCs apoptosis and decrease intraocular pressure[16-20], the mechanism is as follows: BDNF can be active in rats for a long term and release nutrition factor to supply nutrition and protection for glaucoma rats; As a self-reactive antigen, injection of COP-1 can activate local immune reaction and microglial cells to remove pieces of dead cells and glutamic acid. Besides, Werkmeisteret al[21-30] found that RSCs transplantation combined with COP-1 could provide protection for central nerve cells and promote production of IGF-1, it’s also critical in regulation of nerve formation.
In conclusion, RSCs transplantation combined with COP-1 immunotherapy can promote the secretion of BDNF and IGF-1, collaboratively protect RGCs in glaucoma rats, which significantly reduces the number of apoptosis RGCs so as to alleviate the optic nerve damage to open a new research direction for treatment of glaucoma.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Kalesnykas G, Oglesby EN, Zack DJ, Cone FE, Steinhart MR, Tian J, et al. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Investigative Ophthalmol & Visual Sci 2012; 53(7): 3847-3857.
[2] Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Ann Rev Neurosci 2012; 35: 153-179.
[3] Chidlow G, Ebneter A, Wood JPM, Casson RJ. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol 2011; 121(6): 737-751.
[4] Howell GR, Soto I, Ryan M, Graham LC, Smith RS, John SW. et al. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J Neuroinflamm 2013; 10(1): 1-8.
[5] Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Investigative Ophthalmol & Visual Sci 2011; 52(1): 434-441.
[6] Liu M, Duggan J, Salt TE, Cordeiro MF. Dendritic changes in visual pathways in glaucoma and other neurodegenerative conditions. Experimental Eye Res 2011; 92(4): 244-250.
[7] Minton AZ, Phatak NR, Stankowska DL, He S, Ma HY, Mueller BH, et al. Endothelin B receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PloS One 2012; 7(8): e43199.
[8] Almasieh M, Zhou Y, Kelly ME, Casanova C, Di Polo A. Structural and functional neuroprotection in glaucoma: role of galantaminemediated activation of muscarinic acetylcholine receptors. Cell Death & Dis 2010; 1(2): e27.
[9] Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Inv 2012; 122(4): 1246.
[10] Crabb JW, Yuan X, Dvoriantchikova G, Ivanov D, Crabb JS, Shestopalov VI. Preliminary quantitative proteomic characterization of glaucomatous rat retinal ganglion cells. Exp Eye Res 2010; 91(1): 107-110.
[11] Porciatti V, Nagaraju M. Head-up tilt lowers IOP and improves RGC dysfunction in glaucomatous DBA/2J mice. Exp Eye Res 2010; 90(3): 452-460.
[12] Niyadurupola N, Sidaway P, Ma N, Rhodes JD, Broadway DC, Sanderson J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Inv Ophthalmol & Visual Sci 2013; 54(3): 2163-2170.
[13] Quigley HA, Cone FE, Gelman SE, Son JL, Pease ME. Lack of neuroprotection against experimental glaucoma in c-Jun N-terminal kinase 3 knockout mice. Exp Eye Res 2011; 92(4): 299-305.
[14] Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proceed Nat Academy Sci 2010; 107(11): 5196-5201.
[15] Hood DC, Raza AS. Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma. Biomed Optics Express 2011; 2(5): 1097.
[16] Nguyen JV, Soto I, Kim KY, Bushong EA, Oglesby E, Valiente-Soriano FJ, et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proceed Nat Acad Sci 2011; 108(3): 1176-1181.
[17] Wang D Y, Ray A, Rodgers K, Ergorul C, Hyman BT, Huang W, et al. Global gene expression changes in rat retinal ganglion cells in experimental glaucoma. Inv Ophthalmol & Visual Sci 2010; 51(8): 4084-4095.
[18] Ju WK, Kim KY, Duong-Polk KX, Lindsey JD, Ellisman MH, Weinreb RN. Increased optic atrophy type 1 expression protects retinal ganglion cells in a mouse model of glaucoma. Mol Vision 2010; 16: 1331.
[19] Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012; 119(5): 979-986.
[20] Ding QJ, Cook AC, Dumitrescu AV, Kuehn MH. Lack of immunoglobulins does not prevent C1q binding to RGC and does not alter the progression of experimental glaucoma. Inv Ophthalmol & Visual Sci 2012; 53(10): 6370-6377.
[21] Werkmeister RM, Cherecheanu AP, Garhofer G, Schmidl D, Schmetterer L. Imaging of retinal ganglion cells in glaucoma: pitfalls and challenges. Cell Tissue Res 2013: 1-8.
[22] Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol 2011; 519(4): 599-620.
[23] Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci 2013; 33(44): 17444-17457.
[24] Fu CT, Sretavan DW. Ectopic Vesicular glutamate release at the optic nerve head and axon loss in mouse experimental glaucoma. J Neurosci 2012; 32(45): 15859-15876.
[25] Saragovi HU, Sarunic MV, Dergham P, Nedev H, Galan A, Young M, et al. A balance of neuroprotective versus neurotoxic mechanisms in experimental and human glaucoma. Inv Ophtalmol Visual Sci 2011; 52(6): 3108.
[26] Fernandes KA, Harder JM, Fornarola LB, Freeman RS, Clark AF, Pang IH, et al. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis 2012; 46(2): 393-401.
[27] Nakano N, Hangai M, Nakanishi H, Mori S, Nukada M, Kotera Y, et al. Macular ganglion cell layer imaging in preperimetric glaucoma with speckle noise-reduced spectral domain optical coherence tomography. Ophthalmology 2011; 118(12): 2414-2426.
[28] Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp Eye Res 2010; 91(2): 127-134.
[29] Tezel G, Thornton IL, Tong MG, Luo C, Yang X, Cai J, et al. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma. Inv Ophthalmol & Visual Sci 2012; 53(13): 8222-8231.
[30] Crish SD, Calkins DJ. Neurodegeneration in glaucoma: progression and calcium-dependent intracellular mechanisms. Neuroscience 2011; 176: 1-11.
ment heading
10.1016/S1995-7645(14)60047-X
*Corresponding author: Lin Zhang, Chief Physician, Professor, Department of Ophthalmology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
E-mail: 28906390 @ qq.com
Foundation project: It is supported by Shanghai Science and Technology Commission Project Fund (No 11ZR14121300).
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
