Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
Qian Hu, Jiong Chen, Chao Jiang, Heng-Fang Liu
Emergency Department, Fifth Affiliated Hospital of Zhengzhou University, Henan 450003, China
Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
Qian Hu, Jiong Chen, Chao Jiang, Heng-Fang Liu*
Emergency Department, Fifth Affiliated Hospital of Zhengzhou University, Henan 450003, China
Objective: To explore protective effect of rosiglitazone on myocardial ischemia reperfusion injury. Methods: A total of 48 male SD rats were randomly divided into control group (A), I/ R group(B),high dose of rosiglitazone (C), low dose of rosiglitazone (D). Plasm concentration of creatine kinase (CK), CK-MB, hsCRP, Superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), nitric oxide (NO) and endothelin (ET) were measured 1 h later after I/R. 24 h after I/R hearts were harvested to observe pathological and ultrastructural changes. Immunohistochemistry and western blotting was used to test CD40 expression in myocardial tissue. Area of myocardial infarction were tested, arrhythmia rate during I/R was recorded.
Results: Plasm concentration of creatine kinase (CK), CK-MB, hsCRP, NO, MDA and ET were decreased in group C, D compared with group B. Plasm concentration of T-SOD and GSHPx was increased significantly in group C, D compared with group B. Compared with group B, pathological and ultrastructural changes in group C, D were slightly. Myocardial infarction area and arrhythmia rate were lower in group C, D compare with group B. Conclusions: Rosiglitazone can protect myocardium from I/R injury by enhancing T-SOD and GSH-Px concentration, inhibit inflammatory reaction, improve endothelial function, reduce oxidative stress and calcium overload.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
Rosiglitazone
1. Introduction
Myocardial ischemia/reperfusion injury often occurs after thrombolysis, percutaneous transluminal coronary angioplasty, and coronary artery bypass surgery for acute myocardial infarction. The underlying pathophysiological mechanism is that the expression of cytokines, in particular cell surface adhesion molecules induces the aggregation and adherence of inflammatory cells, resulting in immunemediated injury to histiocytes[1,2], which greatly influences the therapeutic effects and prognosis in patients with myocardial infarction. Peroxisome proliferator-activated receptor gamma (PPAR-γ) is mainly expressed in adipose tissue and immune system and closely related to adipocyte differentiation, insulin resistance and organism immunity[3-5]. Rosiglitazone can regulate insulin gene transcription, which is involved in glucose production, transport and utilization, by activating PPAR-γ and it can also alleviate myocardial ischemia/reperfusion injury by inhibiting inflammatory reaction, improving endothelial function and reducing oxidative stress[6-8]. Whether rosiglitazone exhibits protective effects on myocardial ischemia/reperfusion injury remains poorly understood. This study investigated this problem, hoping to provide new thoughts for clinical treatment of myocardial ischemia/ reperfusion injury and achieve favorable economic and social benefits.
2. Materials and methods
2.1. Animals and reagents
Male Japanese big-ear rabbits, weighing (2.50±0.25) kg, were provided by Laboratory Animal Center, Zhengzhou University, China.
Rosiglitazone was produced by Shanghai No.1 Biochemicaland Pharmaceutical Co., Ltd., China (batch No. 06050211). It was used together with physiological saline in the clinic and should be sterilized before use. Glucose injection was produced by Ji’nan Limin Pharmaceutical Co., Ltd., China (batch No. 0512547) and sodium chloride injection by Shenyang Zhiying Pharmaceutical Factory, China (batch No. 06080204). Superoxide dismutase (SOD), malondialdehyde (MDA), nitric oxide (NO), glutathione peroxidase (GSHPx), and endothelin (ET) kits were purchased from Nanjing Jiancheng Bioengineering Institute, China.
2.2. Instruments
The following instruments were used in this study: 7170A full-automatic biochemical analyzer (Hitachi, Japan), 721 visible spectrophotometer (Shanghai Precision & Scientific Instrument Co., Ltd., China), UV300 ultraviolet-visible spectrophotometer, K15-C low-temperature ultracentrifuge (Beijing Medical Centrifuge Factor, China), MDFU40865 low temperature and ultra low temperature freezer, HX-200 animal respirator, SHH.7121.600 electrothermal constanttemperature water tank (Beijing Ever Light Medical Equipment Co., Ltd., China), MC-ASCENT enzyme reader, electronic balance, DYY-6C electrophoresis apparatus, CH20 optical microscope (Olympus, Shinjuku-ku, Tokyo, Japan), CIAS-1000 image analyzer, RF-535 fluorescence detector, LC-9A high performance liquid chromatograph (Shimadzu, Japan), C-35A microscope (Olympus), and IX-70 inverted fluorescence microscope.
2.3. Methods
2.3.1. Establishment of acute myocardial ischemia/reperfusion injury models in rabbits
Following anesthesia by 20% urethane (1 g/kg), chest hair was sheared, and an incision was made along the midsternal line and started over the clavicle and till the xiphoid process of the sternum. The second, third and fourth ribs were interrupted at the left sternal border and the pericardium was cut open to expose the heart. A 2/0 silk suture was introduced into the root region of the circumflex branch of left coronary artery for later ligation
2.3.2. Grouping and drug administration
Forty-eight rabbits were randomly divided into four groups. In the sham operation group, the suture was introduced into the circumflex branch of the left coronary artery and twisted, and ligation was not performed; before surgery, rabbits were raised with common pellet feeds for 3 d. In the ischemia/ reperfusion group, 30-minute ischemia followed by 60 min reperfusion was performed, and before surgery, rabbits were also raised with common pellet feeds for 3 d. Based on the procedure in the ischemia/reperfusion group, 3 mg and 0.5 mg rosiglitazone was orally administered in the rosiglitazone high-dose and low-dose groups, respectively. On the day of surgery, the circumflex branch of the left coronary artery was ligated in the latter three groups. When an obvious ST-segment elevation, a sign of successful ligation, was observed in the electrocardiogram, 1-hour reperfusion was performed. Then blood was taken from the common carotid artery through the use of heparin anticoagulation tube for detection of biochemical indices.
2.3.3. Index detection
Plasma plasma cardiac troponin Ⅰ (CTnⅠ), creatinine kinase-MB (CK-MB) and C-reactive protein (hsCRP) levels were detected in the blood immediately taken from the common carotid artery.
After 10-minute centrifugation at 2 500 r/min, the supernatant of the common carotid artery blood was collected for detection of plasma total superoxide dismutase (T-SOD), GSH-Px, MDA, NO and ET levels.
2.3.4. Sample preparation
Following washes with fixing solution, myocardial tissue on the left ventricular anterior wall was dissected into several strips, fixed in the fixing solution, rinsed with 0.09 mmol/L KH2PO4for 15 min, and preserved in KH2PO4. Strip-shaped tissue was hydrated in a graded series of ethanol, cleared by xylene, and embedded by paraffin. Then part of tissue was used for immunohistochemical detection and the remainder was sliced, stained with hematoxylin-eosin, and observed under the optical microscope.
2.3.5. Immunohistochemistry
Paraffin sections were dehydrated, dewaxed with xylene and dehydrated alcohol, retrieved by citric acid antigen, enveloped, hybridized, developed and mounted by gum.
2.4. Statistical analysis
All data were statistically processed using SPSS 17.0 software. Measurement data were expressed as mean±SD. Q-test was used for difference comparison between two groups and one-way analysis of variance for difference comparison among multiple groups. A level of P<0.05 indicates significant difference and P<0.05 highly significant difference. Pathomorphological data were analyzed by contrast description.
3. Results
3.1. Effects of rosiglitazone on plasma CTnⅠ, CK-MB and hsCRP levels in rabbits with myocardial ischemia/reperfusion injury
Compared with sham operation group, plasma CTnⅠ, CKMB and hsCRP levels in the ischemia/reperfusion group were significantly increased. Plasma CTnⅠ, CK-MB and hsCRP levels in the rosiglitazone high-dose group and rosiglitazone low-dose group were significantly lower thanin the ischemia/reperfusion group (Table 1).

Table 1 Plasma CTnⅠ, CK-MB and hsCRP levels in each group.
3.2. Effects of rosiglitazone on plasma NO, ET and GSH-Px levels in rabbits with myocardial ischemia/reperfusion injuryCompared with sham operation group, plasma NO and GSH-Px levels were significantly decreased, while plasma ET level was significantly increased in the ischemia/ reperfusion group. Compared with ischemia/reperfusion group, plasma NO and GSH-Px levels were significantly increased and plasma ET level was significantly decreased in the rosiglitazone high and low dose groups. There were no significant differences in plasma NO, ET and GSH-Px levels between rosiglitazone high and low dose groups (Table 2).
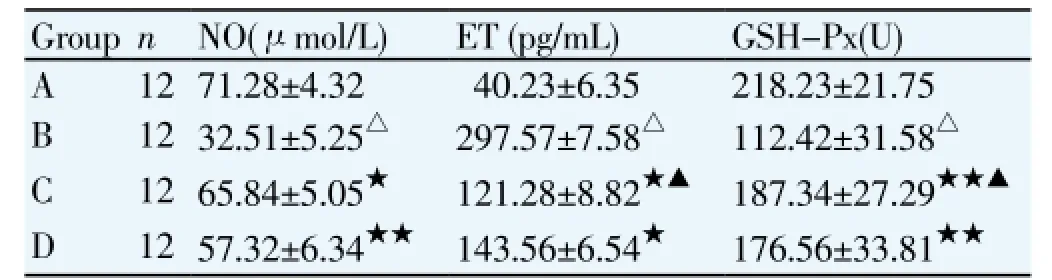
Table 2 Plasma NO, ET and (GSH-Px levels in each group.
3.3. Effects of T-SOD and MDA levels in rabbits with myocardial ischemia/reperfusion injury
Compared with sham operation group, plasma MDA level was significantly increased and plasma T-SOD level was significantly decreased in the ischemia/reperfusion group. Compared with ischemia/reperfusion group, plasma MDA level was significantly decreased and plasma T-SOD level was significantly increased in the rosiglitazone high and low dose groups (Table 3).
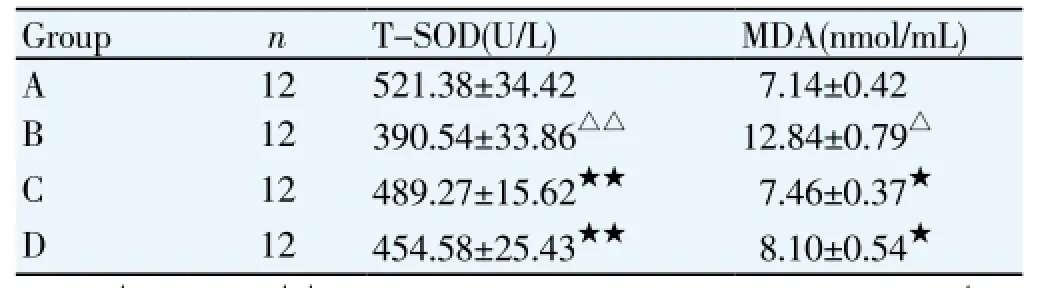
Table 3 Plasma MDA and ET levels in each group.
3.4. Optical microscope observation of pathological structureThrough the optical microscopy, in the sham operation group, myocardial fibers were orderly arranged, and no invasion of inflammatory cells in the myocardial interstitium was observed; in the rosiglitazone high and low dose groups, nuclei of myocardial cells were uniform in size and distribution, but myocardial fibers exhibited slight swelling, and fibrocytes and inflammatory cells invaded occasionally; in the ischemia/reperfusion group, myocardial fibers were swollen and disordered, a large number of inflammatory cells invaded the myocardial interstitium, and obvious local necrosis was even observed.

Figure 1. Optical microscope observation of pathological structure.Group A: full of muscle fiber, the nuclear shape is normal.Group B: extensive necrosis, muscle fiber fracture, severe muscle cell nucleus dissolved, disappear, neutrophils large seepage.Group C: myocardial cell nuclei are uniform and mild myocardial fibers swelling, occasional fibroblast cells and inflammatory cells infiltration. Group D: myocardial cell nuclei are uniform and mild myocardial fibers swelling, occasional fibroblast cells and inflammatory cells infiltration.
Sham operation group: intact muscle fiber and normal nuclear morphology.
Ischemia/reperfusion group: wide necrosis, muscle fiber was greatly broken, a large number of myocyte nuclei dissolved or disappeared, and many neutrophil granulocytes effused.
Rosiglitazone high and low dose groups: myocyte nuclei were uniform in size, myocardial fiber was slightly swollen, and fibroblasts and inflammatory cells invaded occasionally (Figure 1).
4. Discussion
Acute myocardial infarction is recently a leading healthor life-threatening disease, and myocardial ischemia/ reperfusion can cause irreversible damage to, even death or apoptosis of, injured myocardial cells, which further aggravates myocardial damage and increases mortality[9,10]. There is evidence that various inflammatory factors, including neutrophil, monocyte, mastocyte, macrophage, tumor necrosis factor-α, interleukin-1, -6, and -8 participate in myocardial ischemia/reperfusion[11-13]. Protein-protein interaction between PPAR-γ and nuclear factor κB (NF-κB) effectively inhibits homogenous ciselement binding in the promoter region of NF-κB and inflammatory factor genes[14-16].
PPAR-γ is expressed in many immunocytes and PPAR-γ ligand 15d-PGJ2 regulates various immunological responses. PPAR-γ even in a very low concentration of 15d-PGJ2 can inhibit lipopolysaccharide-induced or NF-κB-, STAT1- and activator protein 1 (AP-1)-mediated transcription[17,18]. Both 15d-PGJ2 and rosiglitazone can inhibit the production of PPAR-γ, thereby inhibiting interleukin-2 gene expression and PHA-induced human T cell proliferation, and finally hampering homogenous cisacting element binding in the promoter region between activated T cells and interleukin-2[19-21]. In addition, PPAR-γ also regulates the differentiation of some immunocytes, including T cells, mononuclear macrophages and nature killer cells. PPAR-γ ligand inhibits the production of interferon-γ in natural killer cells and T cells by PPAR γ-dependent and non-dependent approach. Gene chip technique showed that PPARγ expression was 5-8 times greater in type 2 T cells than in type 1 T cells[22-24]. After addition of interleukin-4 and interferon-γ antibodies, type 2 immunocytes can induce PPAR-γ expression in natural killer cells. The activated PPAR-γ can successfully inhibit the expression of inflammatory factors, including tumor necrosis factor-α, and interleukin-1, -2 and -6 in monocytes, exhibiting anti-inflammatory effects. An effective control of interleulin-2 gene expression in the early stage of lymphocyte differentiation is very important for activation of lymphocytes. The activated PPAR-γ can effectively inhibit interleukin-2 gene expression and prevent the early activation of T lymphocytes, thereby exerting antiinflammatory effects[25,26].
Results from this study showed that pretreatment with PPAR-γ agonist rosiglitazone can significantly increase plasma SOD, GSH-Px and NO levels and decrease plasma cTNI, CK-MB, hsCRP, MDA and ET levels, significantly decrease the incidence of arrhythmia, reduce myocardial infarct volume, protect cardiocyte mitochondrial function, and maintain intact mitochondrial ultrastructure in a rat model of myocardial ischemia/reperfusion injury.
Based on the present results, we can infer that rosiglitazone pretreatment can protect myocardium and alleviate ischemia/ reperfusion injury. However, the precise mechanism remains unclear. Rosiglitazone, as a PPAR-γ agonist, influences the formation and activation of inflammatory cells or inflammatory factors and simultaneously inhibits the expression of monocyte chemoattractant protein-1, intercellular adhesion molecule-1 and inducible nitric oxide synthase by regulating PPAR-γ expression[27,28]. In addition, Khandoudiet al[30] found that thiazolidinediones can inhibit c-Jun amino-terminal kinase phosphorylation and activator protein-1 DNA binding activity, and thereby alleviate myocardial ischemia/ reperfusion injury and improve cardiac function.
Taken together, rosiglitazone can be used to treat myocardial ischemia/reperfusion injury by inhibiting inflammatory reaction, improving endothelial function, and reducing reactive oxygen species. Nevertheless, the precise mechanism will be further studied.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Huang JV, Greyson CR, Schwartz GG. PPAR-γ as a therapeutic target in cardiovascular disease: Evidence and uncertainty. J Lipid Res 2012; 53(9): 1738-1754. Epub 2012 Jun 8.
[2] Touyz RM, Schiffrin EL. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vascul Pharmacol 2006; 45(1): 19-28. Epub 2006 Jun 16.
[3] Yew T, Toh SA, Millar JS. Selective peroxisome proliferatoractivated receptor-γ modulation to reduce cardiovascular risk in patients with insulin resistance. Recent Pat Cardiovasc Drug Discov 2012; 7(1): 33-41.
[4] Robinson E, Grieve DJ. Significance of peroxisome proliferatoractivated receptors in the cardiovascular system in health and disease. Pharmacol Ther 2009; 122(3): 246-263.
[5] Balakumar P, Rose M, Ganti SS. PPAR dual agonists: are they opening Pandora’s Box? Pharmacol Res 2007; 56(2): 91-98. Epub 2007 Mar 14.
[6] Wilding JP. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes Metab 2012; 14(11): 973-982.
[7] Barbieri M, Filippo CD, Esposito A. Effects of PPARs agonists on cardiac metabolism in littermate and cardiomyocyte-specific PPAR-cγ- knockout (CM-PGKO) mice. PLoS One 2012; 7(4): e35999.
[8] Duan SZ, Ivashchenko CY, Russell MW. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res 2009; 97(4): 372-379.
[9] Reifel-Miller A, Otto K, Hawkins E. A peroxisome proliferatoractivated receptor alpha/gamma dual agonist with a unique in vitro profile and potent glucose and lipid effects in rodent models of type 2 diabetes and dyslipidemia. Mol Endocrinol 2011; 19(6): 1593-1605.
[10] Touyz RM, Schiffrin EL. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vascul Pharmacol 2006; 45(1): 19-28.
[11] van Bilsen M, van Nieuwenhoven FA. PPARs as therapeutic targets in cardiovascular disease. Expert Opin Ther Targets 2010; 14(10): 1029-1045.
[12] Kataoka Y, Yagi N, Kokubu N, Kasahara Y, Abe M, Otsuka Y. Effect of pretreatment with pioglitazone on reperfusion injury in diabetic patients with acute myocardial infarction circulation. Circ J 2011; 75(8): 1968-1974.
[13] Matetzky S, Freimark D, Chouraqui P. The distinction between coronary and myocardial reperfusion after thrombolytic therapy by clinical markers of reperfusion. J Am Coll Cardiol 2011; 32(5): 1326-1330.
[14] Garini A, Astorri E, Bonifazi C. Non-invasive evaluation of coronary reperfusion. Analysis of the ST segment before and after thrombolysis in acute myocardial infarct. Minerva Cardioangiol 2011; 45(9): 407-414.
[15] Quintana M, Kahan T, Hjemdahl P. Pharmacological prevention of reperfusion injury in acute myocardial infarction. A potential role for adenosine as a therapeutic agent. Am J Cardiovasc Drugs 2004; 4(3): 159-167.
[16] Ogasawara D, Shite J, Shinke T, Watanabe S, Otake H, Tanino Y, et al. Pioglitazone reduces the necrotic-core component in coronaryplaque in association with enhanced plasma adiponectin in patientswith type 2 diabetes mellitus. Circ J 2009; 73: 343-351.
[17] Ishii H, Ichimiya S, Kanashiro M. Effects of receipt of chronic statin therapy before the onset of acute myocardial infarction: a retrospective study in patients undergoing primary percutaneous coronary intervention. Clin Ther 2009; 28(11): 1812-1819.
[18] Haider AW, Andreotti F, Hackett DR. Early spontaneous intermittent myocardial reperfusion during acute myocardial infarction is associated with augmented thrombogenic activity and less myocardial damage. J Am Coll Cardiol 2012; 26(3): 662-667.
[19] Nakayama T, Komiyama N, Yokoyama M. Pioglitazone induces regression of coronary atherosclerotic plaques in patients with type 2 diabetes mellitus or impaired glucose tolerance: a randomized prospective study using intravascular ultrasound. Int J Cardiol 2010; 138(2): 157-165.
[20] Yang HB, Zhao XY, Zhang JY. Pioglitazone induces regression and stabilization of coronary atherosclerotic plaques in patients with impaired glucose tolerance. Diabet Med 2012; 29(3): 359-365.
[21] Clementi F, Di Luozzo M, Mango R. Regression and shift in composition of coronary atherosclerotic plaques by pioglitazone: insight from an intravascular ultrasound analysis. J Cardiovasc Med (Hagerstown) 2009; 10(3): 231-237.
[22] Kawamori R. Evidences demonstrating the effects of antiatherosclerotic actions of pioglitazone--special emphasis on PROactive Study and PERISCOPE Study. Nihon Rinsho 2010; 68(2): 235-241.
[23] Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 2009; 32(3): 187-202.
[24] Yue TL, Chen J, Bao WK. In vivo myocardial protection from ischemia/ reperfusion injury by the peroxisome proliferatoractivated receptor-[gamma] agonist rosiglitazone. Circulation 2011; 104(21): 2588-2594.
[25] Cheng G, Palanisamy AP, Evans ZP. Cerulenin blockade of fatty acid synthase reverses hepatic steatosis in ob/ob mice. PLoS One 2013; 8(9):112-113.
[26] Wayman NS, Hattori Y, Mcdonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, et al. Ligands of the peroxisome proliferator -activated receptors reduce myocardial infarct size. FASEB J 2002; 16(9): 1027-1040.
[27] Morrison A, Yan X, Tong C, Li J. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am J Physiol Heart Circ Physiol 2011; 301(3): H895-H902.
[28] Zingarelli B, Hake PW, Mangeshkar P. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factorkappaB, heat shock factor 1, and Akt. Shock 2012; 28(5): 554-563.
[29] Khandoudi N , Delerive P, Berrebi B, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A. Rosiglitazone ,aperoxisome proliferator-activated receptor - gamma , inhibits the Jun NH(2)-terminal kinase/ activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes 2009; 51(5): 1507-1514.
ment heading
10.1016/S1995-7645(14)60036-5
*Corresponding author: Heng-Fang Liu, Ph.D., Chief Physician, Emergency Department, Fifth Affiliated Hospital of Zhengzhou University, Henan 450003, China.
Foundation project: It is supported by Henan Province Natural Science Foundation (283 v2110).
Ischemia reperfusion injury
Rabbit
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats
- Correlation of expression of STAT3, VEGF and differentiation of Th17 cells in psoriasis vulgaris of guinea pig
- Effect of anesthesia on cognitive status and MMP-2 expression in rats
- Ultrasonic diagnosis and vasoactive substances examination in patients with cirrhosis
- Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines
- Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
