Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats
Zhen-Chang Wang, Shan Yang, Jing-Jing Huang, Song-Lin Chen, Quan-Qiang Li, Yuan Li
1Liver Disease Center, First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, Naning 530023, China
2Guangxi University of Traditional Chinese Medicine, Nanning 530007, China
Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats
Zhen-Chang Wang1*, Shan Yang2, Jing-Jing Huang1, Song-Lin Chen1, Quan-Qiang Li2, Yuan Li1*
1Liver Disease Center, First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, Naning 530023, China
2Guangxi University of Traditional Chinese Medicine, Nanning 530007, China
Objective: To investigate the impact of bone marrow mesenchymal stem cells on Smad expression of hepatic fibrosis rats. Methods: A total of 48 adult female SD rats were randomly divided into three groups, normal control group (n=10), observation group (n=19) with liver fibrosis model rats injected with BMSCs cells; model group (n=19), with liver fibrosis model rats injected with physiological saline. Serum index, TGF-β1 and Smad expression were detected.
Results: Type Ⅲ procollagen, Ⅳ collagen, hyaluronic acid, laminin levels of observation group were significantly lower than those of model group (P<0.05). The content and expression of TGF-β1 in serum and liver tissue of observation group were significantly lower than those of model group(P<0.05). Compared with normal control group, the Smad3, Smad4 mRNA and protein expression of model group were significantly increased, the Smad7 mRNA and protein expression were significantly reduced (P<0.05). Compared with model group, Smad3, Smad4 mRNA and protein expression of observation group were significantly reduced, and Smad7 mRNA expression were significantly increased (P<0.05). Conclusions: BMSCs can regulate Smad expression to some extent, and reduce the degree of liver fibrosis.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
Bone mesenchymal stem cells
1. Introduction
Bone mesenchymal stem cell (BMSCs) is able to differentiate into endoderm hepatic cells which can resist liver fibrosis and protect liver cells. Transplantation of exogenous BMSCs can treat hepatic inheritance metabolic diseases to some extent. Liver fibrosis is the intermediate by which a variety of chronic liver diseases develop into cirrhosis. It has been reported that in recent years that BMSCs transplantation has a good effect on the treatment of liver fibrosis. It can solve donor organ shortage for bio-artificial liver transplantation and hepatocyte transplantation, and also has a great significance in controlling the liver fibrosis[1-3].
However, a variety of factors in the surrounding environment will influence and determine the BMSCs differentiation direction and lead to the unstable therapeutic effects. In this paper, we studied the impact of BMSCs on the Smad expression levels of liver fibrosis rats and explored their relationship with collagen deposition in the live, to analyze the possible molecular mechanisms of liver fibrosis in rats from the molecular level.
2. Materials and methods
2.1. Animal model and grouping
Totally 50 adult female clean grade SD rats were selected, 8 to 10 weeks old, weighting 80-300 g. All were purchased from Shanghai SLAC Laboratory Animal Co. Rats took food and water freely in experimental period. Ten rats were used as normal control group, the rest 40 rats as liver fibrosis model group. The model was established by CCl4sc method. 300 mL/L CCl4paraffin oil solution was injected intraperitoneally by 3 mL/kg, 2 times/w for 8 weeks, and two rats died during the experiment. A total of 38 CCl4treated SD rats were randomly divided into the observation group (n=19): 0.2 mL suspension with BMSCs cell suspension (containing 5×106cells) were given by tail vein injection atthe first 6, 8 w of the modeling; the model group 19 (n=19): equivalent normal saline were given by tail vein injection at the first 6, 8 w of the modeling; All SD rats were sacrificed after 10 w modeling of the liver fibrosis and the liver tissue samples were obtained for detection.
2.2. Reagents and instruments
Lymphocyte separation medium (Shanghai Hua Jing Reagent Company), L-DMEM medium (Gibco, USA). Smad3, Smad7 and β-actin antibody (Santa Cruz, USA), PCR primers (Sangon, Shanghai), SYBR Green Mix and reverse transcription kit (TOYOBO Corporation, Japan), TGFβ1 immunohistochemistry kit, corollary diluent (1:100) and DAB chromogenic agent (all purchased from Wuhan Boster Biological Engineering Company), fetal bovine serum (Tianjin Hao Yang companies). JEM-1220 transmission electron microscope, YLN-2000 gel imaging analysis system, Immunohistochemistry kit: TGFlB, smad2, 3 (batch BAl396); Smad7 (batch BAl399); corollary diluent (1:100) (batch SAl022); DAB chromogenic reagent were all purchased from Wuhan Boster Biological Engineering Co.
2.3. BMSCs isolation and culture
Young male SD rats were collected, marrow suspension were isolated from long bone of limbs after 10% chloral hydrate anesthesia and routine disinfection. Interface layer cells were by absorbed density gradient centrifugation, and washed in L-DMEM culture medium twice. Cells were collected and inoculated with 1×105/cm2in 25 cm2flasks, and added with L-DMEM medium which contained 100 U/ mL streptomycin, 100 U/mL penicillin and 10% fetal bovine serum. They were cultured in 37℃, 5% CO2incubator. Attachment culture method was used in BMSCs isolation and purification. The cells were proliferated.
2.4. Methods
2.4.1. Tissues sample collection
Blood was extracted by extirpating eyealls, and then rats were sacrificed. The blood was centrifuged, and storage in the refrigerator. One left lobe and one right lobe of liver were obtained, and fixed in 10% neutral formalin. Routine paraffin section and HE staining were performed then the tissue was observed under the light microscopic.
2.4.2. Serum index detection
Type Ⅲ procollagen, Ⅳ collagen, hyaluronic acid, laminin were detected by radioimmunoassay strictly according to the instruction of kits.
2.4.3. TGF-β1 detection
Serum TGF-β1 levels was measured by double antibody sandwich method. Immunohistochemistry (SP method) was used to observe liver TGF-β1 distribution. Color extent and range were analyzed by Olympus Bx50 image analyzer. Ten good high power fields of each specimen were selected, and brown colored particles were positive staining cells. The color degree was 1 (weak), 2 (medium), 3 (strong), and the color range was in accordance with the percentage of highpower field, 1(<25%), 2 (25%-50%), 3(50%-75%), 4(>75%). The color rendering index = color degree×color range.
2.4.4. Smad detection
PCR detection: Liver tissue total RNA was extrected by TRIzol instructions. Transcription the mRNA was reversed to cDNA for quantitative PCR reaction. Smad3 upstream primer: 5’-CCAGTGCTACCTCCAGTGTr-3‘, downstream: 5’-CT-GGTGGTCGCTAGnTrCTC -3’; Smad7 upstream: 5‘-GGCTITCAGATFCCCAACTrC-3’, downstream 5’-CGCCATCCACTTC-CCTFGT-3‘; internal reference β -actin upstream: 5’-CGTTGACATCCGTA-AAGACCTC-3‘, downstream: 5’-rAGGAGCCAGGGCAGTAATCT-3‘. Western blot detection method was used to extract total protein of liver tissue, then rabbit anti-mouse TGF-β1 antibody (1:200) primary antibody and HRP-conjugated goat antirabbit secondary antibody (1:500) binding reaction was carried out after electrophoresis and transmembrane. They were incubated avoid light at room temperature for 1 h. The membrane was washed to detect β-actin expression as an internal reference. Fluorescence system scan was used and the gray integral value of Smad3, Smad7 protein bands was recorded. The ratio of samples integral value and internal reference integral value was used for statistical analysis.
2.5. Statistical analysis
All data were analyzed with SPSS 16.0 software. Data were expressed as mean±SD values, the difference between two groups were compared with t test. P<0.05 was considered as statistical significant difference.
3. Results
3.1. Type Ⅲ procollagen, Ⅳ collagen, hyaluronic acid and laminin
Type Ⅲ procollagen, Ⅳ collagen, hyaluronic acid and laminin in the observation group and model group were significantly higher than the normal control group (P<0.05). Type Ⅲ procollagen, Ⅳ collagen, hyaluronic acid and laminin of the observation group were significantly lower than the model group (P<0.05) (Table 1).
3.2. TGF-β1 level
The contents and the expression of TGF-β1 levels in serum and liver tissue of the observation group and model group were significantly higher than the normal control group (P<0.05); The contents and the expression of TGF-β 1 levels in serum and liver tissue of the observation group were significantly lower than the model group (P<0.05) (Table 2).
3.3. Smad3, Smad4 mRNA expression
Compared with the normal control group, the Smad3, Smad4 mRNA expression of the observation group and model group were significantly higher than the normal control group (P<0.05). Compared with the model group, the Smad3, Smad4 mRNA expression of the observation group were significantly reduced, the Smad7 mRNA expression was significantlyincreased (P<0.05) (Table 3).
3.4. Smad3, Smad4 protein expression
Compared with the normal control group, the Smad3, Smad4 protein expression of the observation group and model group were significantly higher than the normal control group, while the Smad7 protein expression was significantly decreased (P<0.05); Compared with the model group, the observation group Smad3, Smad4 protein expression was significantly reduced, Smad7 protein expression was significantly increased (P<0.05) (Table 4).
4. Discussion
Liver fibrosis is caused by the imbalance of synthesis and degradation of extracellular matrix and the overdeposition of extracellular matrix, which is the main pathological processes from various chronic liver disease to cirrhosis. BMSCs are a class of stem cells from mesoderm with multipotential differentiation potential. Study confirmed that BMSCs can differentiate into chondrocytes, osteoblasts, myocardial cells, fat cells and other mesoderm-derived tissue cells, but also differentiate into hepatocyte-like cells and neuron-like cells from the endoderm and ectoderm[4-7]. Taiet al[8] transplanted human BMSCs into allyl alcohol induced liver injury rats, and obsered human hepatocytespecific alpha-fetoprotein, AGPR, CK18, CK19 and ALB expression by immunohistochemistry. It showed human BMSCs could be induced into hepatocytes in appropriate microenvironment. We observed the effect of BMSCs on the Smad expression of liver fibrosis rats, and explored the mechanism of BMSCs liver fibrosis reversal effect at the molecular level in this study.
It has been reported that the TGFβ-Smad signaling pathway can regulate the damage repair,tumor growth and metastasis and other biological behavior is also the main message pathway of liver fibrosis[9]. TGF-β1 plays an important role in the process of liver fibrosis, while Smads protein as a key post-receptor messenger protein that mediates TGF-β1 signal from the cell membrane to nucleus. Smad protein family is involved in the regulation cell transformation, proliferation, synthesis, secretion and apoptosis, which can be divided into three categories in accordance with the different functions: the receptor activated SMADs is the target molecules of TGF-β1 receptor complex downstream effects; Common type Smad, such as Smad4, can combined with receptor-activated Smad and plays a role in the TGF-β1 signal transduction as a transit molecules; inhibitory Smad, mainly includes Smad7, can inhibit the activation of receptor-activated SMADs and block the biological effects of TGF-β1[10-13]. Studies suggest that the adjustment of key Smads molecules level expression have an effective anti-fibrotic effect which can be used as the assessment index[14].
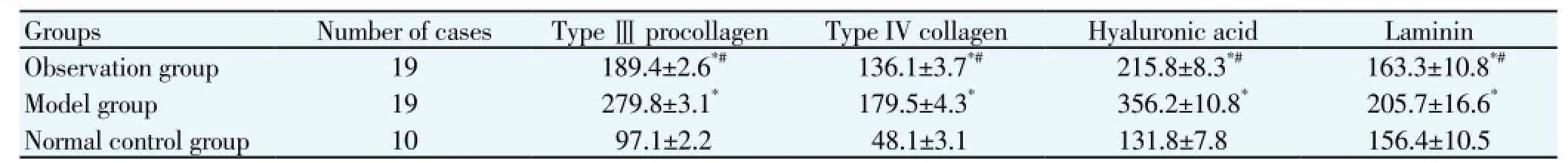
Table 1 Comparison of hepatic serological markers detection of rats in each group (ng/mL).

Table 2 Contents and the expression of TGF-β1 levels in serum and liver tissue of two groups.
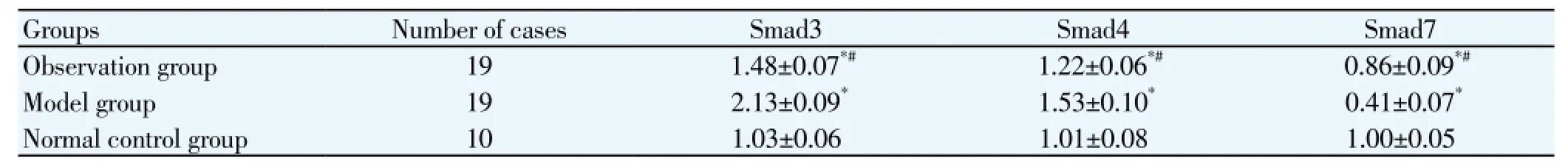
Table 3 Comparison of Smad3, Smad4 mRNA expression of two groups
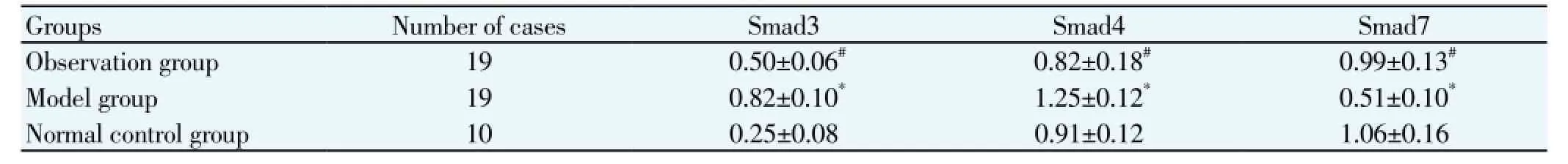
Table 4 Comparison of Smad3, Smad4 protein expression of two groups.
Most reports showed that BMSCs can reduce the liver fibrosis. Lianget al[15] found that after 4 weeks of BMSCs transplantation for the treatment of CCl4-induced hepatic fibrosis in rats, the degree of liver fibrosis can be significantly reduced. Zhanget al[16] found that the level of fibrosis in the hepatic lobule was significantly decreased after BMSCs transplantation. Zhao[17] found that the serum hyaluronic acid, serum type IV collagen and serum type III procollagen levels of the mesenchymal stem cells treatment group were significantly lower than the model group. This study detected the liver serological markers of rat and found that the liver tissue structure has been significantly improved after BMSCs transplantation. The number of false lobe was significantly reduced and the fibrous septa turned thinner. Ⅲ procollagen, Ⅳ collagen, hyaluronic acid, laminin level of the observation group were significantly higher than the normal control group. Although the observation group Ⅲprocollagen, Ⅳ collagen, hyaluronic acid, laminin level is still significantly higher than the normal control group, the collagen fibers in the observation group was significantly reduced compared with the model group, the hyaluronic acid, laminin levels were also significantly reduced. It showed that BMSCs can block the excessive production and deposition of hepatic collagen or other extracellular matrix,mitigate and control liver fibrosis, also have the degradation effect on the formed extracellular matrix.
The results also show that Smad3, Smad4 mRNA and protein expression levels of the model group were significantly increased, the difference was significant compared with the normal control group(P<0.05), which showed the Smad3, Smad4 expression levels were increased significantly during rat experimental hepatic fibrosis formation. The Smad3, Smad4 mRNA and protein expression levels of the observation group were significantly lower than the model group, which showed that BMSCs can inhibit the Smad3, Smad4 expression to some extent. Xinget al[18] considered that Smad3-mediated most of the pro-fibrotic effects of TGFβ. Transgenic rat Liver type Ⅰ collagen reduced with the decrease of liver endogenous Smad4 expression, which showed the inhibition of Smad3, Smad4 expression has a potential anti-fibrotic effect. Smad7 is an antagonistic protein which is able to tightly bind with Tβ RIV, so the binding of Smad2 or Smad3 with TβRIV were blocked by Smad7, and the Smad2 or Smad3 phosphorylation were inhibited[19]. By this way, Smad7 can complete the inhibition of TGF-β signal transduction. Subeqet al[20] injected the Smad7cDNA transfection adenovirus vector to fibrosis rats and found that α-smooth muscle actin and liver collagen expression both decreased. In this study, the Smad7 mRNA and protein expression of the observation group were significantly higher than the model group, which showed that BMSCs can increase the expression of Smad7 and reduce fibrosis from another point of view.
In summary, injection of BMSCs into the vail vein for liver fibrosis rat can significantly improve liver histological status. BMSCs can regulate the expression of Smad to some extent, and provide a new method chronic liver fibrosis and other fibrotic diseases.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Xu J, Chen G. The progress of between bone marrow mesenchymal stem cells reverse liver fibrosis. World Chin J Digestol 2010; 18(22): 2291-2295.
[2] Huang YL, Zhou CG, Chen Y, Zhang Y, Zhang QY. The research progress of TGF/Smad signaling pathways andliver fibrosis. Pract Med J 2011; 27(10): 1883-1884.
[3] Zhou W, Chen PF, Wu XL, Jiang R, Xu YH. Bone marrow mesenchymal stem cells contribute to liver fibrosis. Chin J Hepatol 2011; 19(9): 706-708.
[4] Zhang YL, Yao L. Transforming growth factor beta Smad signaling pathways and liver fibrosis. Chin J Integr Trad Western Med Liver Dis 2011; 21(6): 383-385.
[5] Piryaei A, Valojerdi M R, Shahsavani M. Differentiation of bone marrow derived mesenchymal stem cells into hepatocytelike cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev 2011; 7(1): 103-118.
[6] Xiong ZF, Xing CZ, Hu H, Wang YC, Ren HR, Zheng TY, et al. Chinese mugwort extract Smad3, Smad7 expression of immune liver fibrosis rats. Lishizhen Med Materia Medica Res 2013; 24(3): 627-628.
[7] Gao Y, Chen GM, Liang ZY, Xie YQ, Yang SZ. Impact of Qigong Kangxian prescription on Smad2/3 and Smad7 in hepatic fibrosis rat. Chin J Exp Formulas Chin Med 2013; 19(14): 229-232.
[8] Tai QW, Zhang JH, Zhao JM, Wen H. Effect of bone marrow mesenchymal stem cells on liver fibrosis of rats. Chin J Exp Surg 2012; 29(10): 1947-1949.
[9] Zheng SJ, Xing XY, Wu JS, Wang SM, Zhang Y, Hai YP, et al. ShRNA targeted to rat Smad3 decreases the profibrosis role of TGF β1 in HSC - T6 cells. Chin J Clin Hepatol 2012; 28(4): 289-295.
[10] Hu YL, Kong L. Role of TGFβ1 and CTGF signaling pathway in experimental hepatic fibrosis. Basic Med Sci Clin 2012; 32(1): 66-70.
[11] Li ML, Deng MH. Progress in research of gene treatment for liver cirrhosis with bone marrow mesenchymal stem cells. Arch General Surg 2011; 5(3): 252-255.
[12] Shang W, Jiang CY. Liver fibrosis of TGF-beta 1/Smad signaling pathways. Chin J Clin Phys 2013; 7(7): 3112-3114.
[13] Xu S, Bao JF, Zhou M, Zhang YS. The experimental relationship study of liver fibrosis different syndrome types and TGF-beta 1/ Smad gene protein expression. J Chin Med 2010; 28(1): 23-28.
[14] Zheng CS. The influence on TGF-β1, Smad2, Smad4 mRNA signal pathway in rabbit with liver hepatic fibrosis treated by Jiaweiyinshao powder. J Chin Med 2012; 27(9): 2462-2464.
[15] Liang ZY, Qin SY, Jiang HH, Wang DX, Su SB, Chen CH. Investigation of bone marrow mesenchymal stem cells on apoptosis of hepatic fibrosis rats of hepatic stellate cells and the expression of Caspase-3. Chongqing Med 2010; 39(20): 2721-2723.
[16] Zhang W, Chen XY, He QY, Wang S, Li CY, Duan FL, et al. Efficacy study of different transplanting ways of bone marrow mesenchymal stem cells in treatment of liver fibrosis. J Pract Med 2013; 29(6): 889-891.
[17] Zhao JX, Shang HT, Guo HY, Lu WT, Bi JJ. Experimental studies of BMSCs on treatment of hepatic fibrosis induced by carbon tetrachloride in rats. Pract J Clin Med 2011; 15(7): 13-15.
[18] Xing XY, Chen P, Zheng SJ, Duan ZP. The roles of SMAD3 in hepatic fibrosis. Chin Hepatol 2010; 15(2): 130-133.
[19] Lee WJ, Park SE, Rah DK. Effects of hepatocyte growth factor on collagen synthesis and matrix metalloproteinase production in keloids. J Korean Med Sci 2011; 26(8): 1081-1086.
[20] Subeq YM, Ke CY, Lin NT, Lee CJ, Chiu YH, Hsu BG. Valsartan decreases TGFβ1 production and protects against chlorhexidine digluconate induced liver peritoneal fibrosis in rats. Cytokine 2011; 53(2): 223-230.
ment heading
10.1016/S1995-7645(14)60048-1
*Corresponding author: Zhen-Chang Wang, Tutor Postgraduate Students, Chief Physician, M.M., Liver Disease Center, First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, Naning 530023, China.
Tel: 13877108005
E-Mail: wangzhenchang924@163.com
Yuan Li, M.D., Guangxi University of Traditional Chinese Medicine, Nanning 530007, China.
Tel: 18878771682
Foundation project: It is supported by Guangxi Scientific and Technological Project (No 11107009-3-1) and Guangxi Natural Science Fund Projects (No 2010 GXNSFA013211).
Hepatic fibrosis rats
Smadv
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Correlation of expression of STAT3, VEGF and differentiation of Th17 cells in psoriasis vulgaris of guinea pig
- Effect of anesthesia on cognitive status and MMP-2 expression in rats
- Ultrasonic diagnosis and vasoactive substances examination in patients with cirrhosis
- Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines
- Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
- Expression of PI3-K, PKB and GSK-3β in the skeletal muscle tissue of gestational diabetes mellitus
