Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines
Xiao-Qi Gu, Yong-Mei Li, Jing Guo, Li-Hua Zhang, Dong Li, Xiao-Dong Gai
1Oral Medical School of North China University, Jilin 132011, China
2Medical School of North China University, Jilin 132011, China
Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines
Xiao-Qi Gu1, Yong-Mei Li1, Jing Guo1, Li-Hua Zhang1, Dong Li1, Xiao-Dong Gai2*
1Oral Medical School of North China University, Jilin 132011, China
2Medical School of North China University, Jilin 132011, China
Objective: To investigate the repairing effect of low intensity pulsed ultrasound (LIPUS) on the Beagle canines periodontal bone defect. Methods: A total of 12 Beagle dogs with periodontal bone defect model were randomly divided into control group, LIPUS group, guided tissue regeneration (GTR) group and LIPUS+GTR group, with three in each. After completion of the models, no other proceeding was performed in control group; LIPUS group adopt direct exposure to radiation line LIPUS processing 1 week after modeling; GTR group adopted treatment with GTR, following the CTR standard operation reference; LIPUS+GTR group was treated with LIPUS joint GTR. Temperature change before treatment and histopathological change of periodontal tissue after repair was observed. Results: There was no significant difference in temperature changes of periodontal tissue between groups (P>0.05). The amount and maturity of LIPUS+GTR group were superior to other groups; new cementum, dental periodontal bones of GTR group were superior to the control group but less than LIPUS group; new collagen and maturity of the control group is not high relatively. Conclusions: LIPUS can accelerate the calcium salt deposition and new bone maturation, thus it can serve as promoting periodontal tissue repair, and shortening the periodontal tissue repair time.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
LIPUS
1. Introduction
Periodontal disease is a chronic inflammatory periodontal disease with high incidence, periodontal disease often causes dental periodontal bone defects, support tissue and periodontal attachment are damaged, eventually lead to tooth loss[1-3]. Current clinical treatments of periodontal bone defect mainly include: bone graft, guided tissue regeneration (GTR) and guided bone regeneration, etc[4]. Early stage of periodontal tissue regeneration technique mainly create regenerative environment, increase the cells at defective places, enhance cell proliferation, differentiation and regeneration[5-7]. But when periodontal tissue defect area is large, clinical treatment effect would be poor due to lack of tissue regeneration[8]. LIPUS is a safe noninvasive treatment, it can accelerate bone and soft tissue repairment. Studies have shown that[9], LIPUS has a promoting effect for dental periodontal bone defect treatment. Based on LIPUS potential biological effect and the theory of periodontal tissue regeneration, the authors establish a Beagle canines periodontal ipads defect model, observation LIPUS joint BGR to defect tissue repair effect, provide reference for clinical LIPUS auxiliary treatment of periodontal disease.
2. Materials and methods
2.1. Experimental animals
A total of 12 Beagle male dogs, aged 1-1.5 years old, were purchased from Animal Experiment Center of Jilin University. They were healthy, weighting 10 to 15 kg. Complete jaw teeth on both sides were chosen for experimental tooth, with normal periodontal and periodontalbones tissue, no hyperemia or swelling gums. Experiments on animals process strictly followed the Regulations on Administration of Experimental Animals.
2.2. Instrument and reagent
Polytetrafluoro ethylene (ePTFE) ultrasonic acoustic membrane, LIPUS therapeutic apparatus were provided by the Ultrasonic Medical Engineering Research Center; DM6801A thermocouple temperature meter was provided by Shenzhen Victory Instrument Plant. S-3000N scanning electron microscope was HITACHI company (Japan). Sleep new II injection and diazepam injection, kang pat medical glue, medical ultrasonic coupling agent were provided by the Southwest Pharmaceutical co., Ltd.
2.3. Modeling
Experimental animals with general anesthesia underwent the buccal teeth gingival sulcus incision along the first premolar. The mucoperiosteal flap was turned over to a third range of tip, 5 mm×5 mm bone defect was ground at 4 mm under glaze surface to cementum surface. Periodontal membrane was scraped. Histological markers was made on the edge of bone defect at the tip; location markers was made on the tip surface at 10 mm distance from defects. Fixing ePTFE film adhesion was implanted on the surface of bone defects, and no ePTFE membrane in blank control group. Mucosal flap suture was reset. Postoperatively penicillin sodium 800 000 U was injected in muscle for days, stitches was taken out after a week when the modeling was set up, High viscosity of sugar pap was given for 2 months.
2.4. Methods
A total of 12 Beagle dogs with periodontal bone defect model were randomly divided into control group, LIPUS group, GTR group and LIPUS+GTR group, with three in each. After completion of the models, control group had no other processing; LIPUS group adopt direct exposure to radiation line LIPUS processing 1 week after modeling, probe position reference was tagged along the line placement, LIPUS parameters: ISATA 60 mw/cm2; Frequency of 1.5 MHz; Pulse width 200 mu s; Repetition rate 1.0 KHz; The processing time was 20 min/d; GTR group adopted treatment with GTR, following the CTR standard operation reference; LIPUS+GTR group was treated with LIPUS joint GTR.
2.5. Index observation
Thermal bimetallic temperature meter was used to measure LIPUS surface temperature before processing, average the defect values was measured at 3 positions including the near, middle and far, then the histopathology change of periodontal tissue after repair was observed.
2.6. Statistical analysis
Data was analyzed using SPSS12.0, and expressed as mean± sd.ttest was adopted, P<0.05 was considered as statistically significant.
3. Results
3.1. Temperature change
After treatment temperature change of control group was (0.07±0.73) ℃, LIPUS group was (0.77±1.08) ℃,GTR group was (-0.31±1.18)℃,LIPUS+GTR group was (0.43±0.72). There was no significant differences in temperature change between 4 groups after treatment (P>0.05).
3.2. Defect tissue change
Mucosal flap adhesion was visible in the control group, defect area had been filled by the organization, with soft connective tissue attaching to the surface; in LIPUS group, GTR group and LIPUS+GTR group, after mucosal flap opened, ePTFE membrane was observed with combination to new tissue with loose connection, and it was easy to remove. Defect parts had been filled by regeneration tissue, which was softer than normal bone tissue (Figure 1).
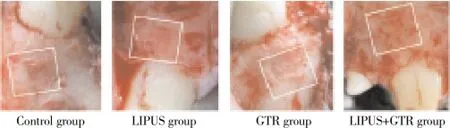
Figure 1. Defect tissue observation of four groups after treatment.
3.2. Histopathology observation of periodontal tissue after treatment
Histopathology of Beagle periodontal tissue was normally structure clear, mature collagen structure, mature lamellar bone, Haversian canal, and plate lamellar bone with Masson dyeing in red and blue were visible in periodontal bones, as shown in Figure 2; Control group was observed with group new bone in bone defects area, with majority of cartilage collagen fiber, red and blue Masson staining, part of the new tooth bone grows inward along the defect rim, as shown in Figure 3; in LIPUS group, new cementum growing along the defects was visible on the surface, periodontal membrane was rich in blood vessels, new osteoblasts along the bone edge and mature collagen structure were visible, Masssondyeing wa red in majority as shown in Figure 4; in GTR group, new growth of cementum growing along the defects was visible, arranged in terms of cementum cell style. the periodontal membrane was rich in blood vessels, osteoblast proliferation is significant, maturity of new bone was not high, and red and blue in Massson dyeing, as shown in Figure 5; in LIPUS+GTR group, bone tissue in the group or strips in the bone defects, with part of active osteoblast in pleomorphism, active proliferation, mature collagen structure, and obvious Massson dyeing in red were observed, as shown in Figure 6.
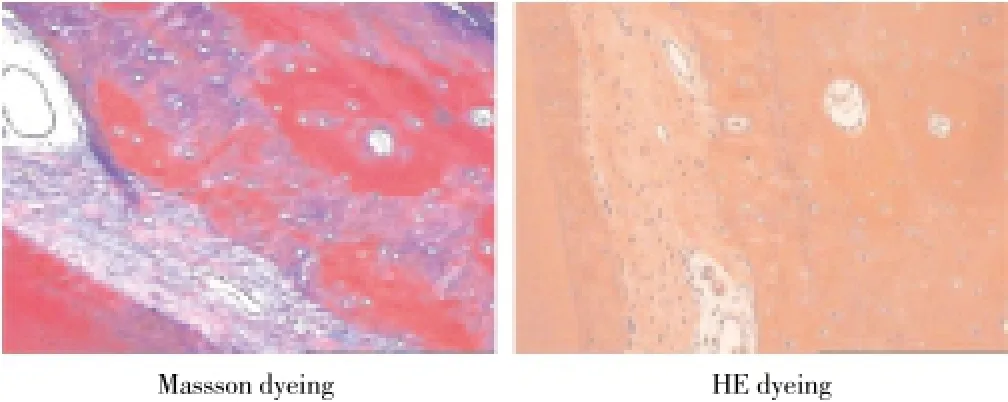
Figure 2. Histopathological slice of normal canine teeth in Beagle dog (×100).
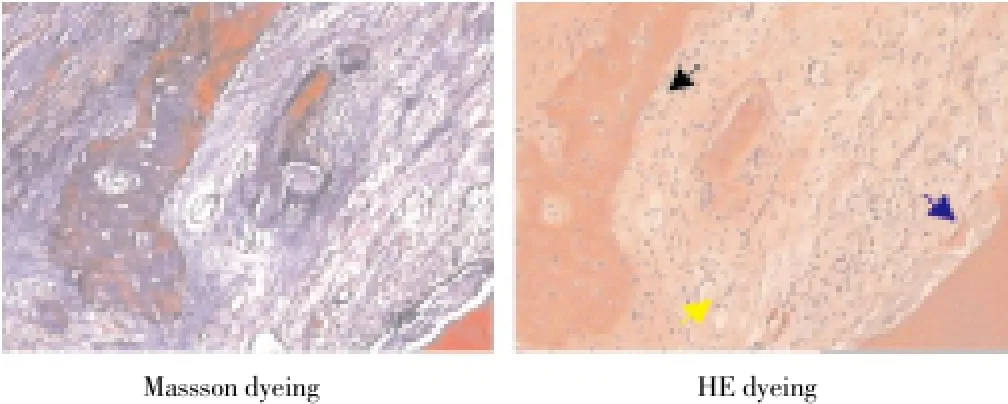
Figure 3. Histopathological slice of control group of canine teeth (× 100).
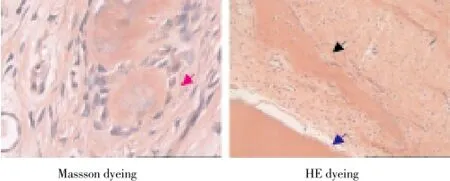
Figure 4. Histopathological slice of LIPUS group of canine teeth (× 100).
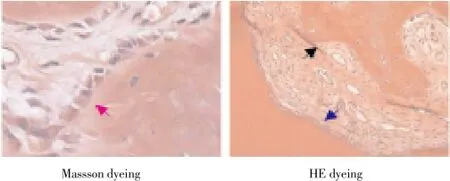
Figure 5. Histopathological slice of GTR group of canine teeth (× 100).
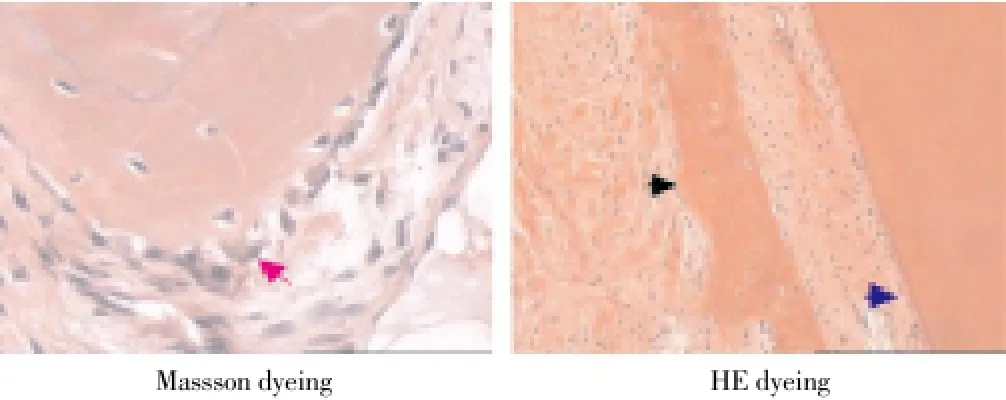
Figure 6. Histopathological slice of LIPUS+GTR group of canine teeth (×100).
4. Discussion
Factors such as congenital malformation, trauma, or periodontal disease often lead to tooth periodontal bone defects, and thus to teeth support tissue destruction and tooth loss. Periodontal tissue of dogs has similar structure as humans have, so it is often used in periodontal animal experiments[10-13]. A third pointed in dental root was chosen for experiment in modeling the tooth periodontal bone mass defect model, facilitating maintenance of tissue regeneration spaces. LIPUS is a kind of pulse ultrasonic with strength less than 100 mW/cm2, with low frequency and intensity, when processing the tissues it produces less heat without invasion, thus can promote periodontal tissue repair. Research has shown that LIPUS can aptically improve metal protease, collagen enzyme activity through the tissues in favor of the tissue repairmen[14-16].
In this group of data, temperature change is no statistical difference in four groups before and after processing with LIPUS (P>0.05), eliminating LIPUS’s possible thermal effect in processing tissue, intimating it is safe, noninvasive and suitable for periodontal area treatment[11,12]. LIPUS can induce osteoblast differentiation, stimulate proliferation of cells and extracellular matrix, speed up the calcium salt deposition[17], and can strengthen alkaline phosphatase activity in bone cells, promote synthesis of collagen[18]in a great significant way for restoration. GTR alone need nearly six months to establish normal periodontal tissue structures, because of its slow speed in tissue repair interfered by unfavorable factors of body[19]. LIPUS can promote callus formation, callus vascularization and mineralization degree, increase the quantity and quality of new bone. Studies have shown that[20-22], connective tissue is the highest degree in reconstruction of periodontal defects after 4 weeks. In this study, the control group also shows significant connective tissue regeneration, osteoid is as showing with blue Masson staining, collagen and Havers system are low in maturity. Masson staining shows that the obvious newborn bone in LIPUS group and LIPUS+GTR group, a significant rise in collagen maturity than the control group, abundantcapillaries and cell components. Mature Havers system is formed in new bones; GTR group also presents periodontal bone regeneration, but collagen maturity is lower than that of LIPUS group and LIPUS+GTR group, indicating that LIPUS can promote periodontal defect repairmen and alveolar bone maturation.
We could draw the final conclusion from this experiment that LIPUS can accelerate the calcium salt deposition and new bone maturation, thus it can serve as promoting periodontal tissue repair, and shortening the periodontal tissue repair time.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Wu P, Song JL, Feng G, Dong N, Zhao CL, Wang ZB. Repair effect of low intensity pulsed ultrasound on the Beagle canines periodontal bone defect. J West China Oral Med J 2010; 28(5): 522-525.
[2] He P, Song JL, Gao X, Deng F, Zhao CL, Wang ZB, et al. Auxiliary effect of low intensity pulsed ultrasound on the root bifurcation lesionsⅡof Beagle dog. China’ Tissue Engin Res Clin Reh 2011; 15(7): 1219- 1223.
[3] Keles GC, Sumer M, Cetinkaya BO, Tutkun F, Simsek SB. Effect of autogenous cortical bone grafting in conjunction with guided tissue regeneration in the treatment of intraosseous periodontal defects. Eur J Dent 2010; 4(4): 403-411.
[4] Dong N, Song JL, Feng G, Liu MY, Deng F. Repair effect preliminary study of low intensity pulsed ultrasound on the Beagle dog periodontitis tissue. J Sichuan Univ (Med Edition) 2012; 43(2): 183-187.
[5] Mostafa NZ, Uludağ H, Dederich DN, Doschak MR, El-Bialy TH. Anabolic effects of low-intensity pulsed ultrasound on human gingival fibroblasts. Arch Oral Biol 2009; 54(8): 743-748
[6] Li N, Lu L,Song JL, Deng F, Zhao CL, Wang ZB. Low intensity pulsed ultrasound combined periodontal tissue flap transplantation on the Beagle dog bone defect type periodontal disease. J Tissue Engin Res China 2012; 16(5): 766-769.
[7] Ikai H, Tamura T, Watanabe T, Itou M, Sugaya A, Iwabuchi S, et al. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J Periodont Res 2008; 43(2): 212-216.
[8] Liu T, Song JL, Liu MY, Wang ZB, Zhao CL, Deng F. Histologic effect of low intensity pulsed ultrasound on the Beagle dog mandible third premolar with chronic periodontitis. Ultrasonic Med Miscellaneous China 2011; 27(11): 970-973.
[9] Yang Z, Ren LX, Song JL, Zhou J, Li FQ, Zhao CL, et al. Stimulation effect of low intensity pulsed ultrasound on the expression of human periodontal ligament cells BMP-2. China Me Cosmetol 2011; 20(5): 767-770.
[10] Zheng H, Lu L, Song JL, Gao X, Deng F, Wang ZB. The animal experiment of low intensity pulsed ultrasound combined periodontal guided tissue regeneration technique promote dental window defect. Chin Oral Med J 2011; 46(7): 431-436.
[11] Kobayashi Y, Sakai D, Iwashina T, Iwabuchi S, Mochida J. Lou- intensity pulsed ultrasound stimulates cell proliferation, proteoglycan synthesis and expression of growth factor- related genes in human nuclesus pulposus cell line. Eur Cell Mater 2009; 17(3): 15-22.
[12] Ma ZG, Pang B, Mao LX, Xia YH. CBCT study of dental density change of patients with periodontal disease before and after orthodontics. Chin Orthodontics Sci Mag 2010; 12(17) :197-200.
[13] Xue H, Peng H, Yang DD, Wang HY, Wu LP. Low intensity ultrasound on BMP-2 expression in rat alveolar bone reconstruction. Influence Heilongjiang Med Sci 2007; 30(6): 9-10.
[14] Liu T, Song JL, Liu MY, Wang ZB, Zhao CL, Deng F, et al. Histological effect of the low intensity pulsed ultrasound on the mandibular third molar teeth of Beagle dogs with chronic periodontitis. China Ultrasonic Med J 2011; 27(11): 970- 973.
[15] Sun SM, Pan YP. Periodontal bone density change after dental periodontal basic treatment with PLANMECA digital layer surface machine. J Oral Med 2012; 32(7): 393-395.
[16] de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG. In vivo effect of photodynamic therapy on periodontal bone loss in dental furcati ons. J Periodontol 2008; 79(6) : 1081-1088
[17] Liu X, Sui XG, Hong W. Comprehensive application of low intensity pulsed ultrasound in healing elderly hip fracture process. China Old Mag 2010; 30(3): 393-394.
[18] Chen G, Chen J. Engineering bone research progress of low intensity pulsed ultrasound to promote the tissue vascularization reconstruction. Chin J Orthopaedic Surg 2010; 18(17): 1450-1452.
[19] Wang C, Zeng RS. Application of low intensity pulsed ultrasound in the jaw distraction osteogenesis. International J Oral Med 2007; 34(5): 374-375.
[20] Li H, Yang YJ, Lv XN. progress in the field of periodontal treatment by low intensity pulsed ultrasound. J Tooth Pulp J Periodontol 2012; 22(12): 733-734.
[21] Yu HS, Chen WZ, Wang Ya, Ding XY, Liu Z, Luo YP. Experimental study of different parameters low intensity pulsed ultrasound to promote stem cell proliferation in bone marrow mesenchymal. China Ultrasonic Medical J 2011; 27(11): 967-970.
[22] Xiao Y, Mareddy S, Crawford R. Clonal characterization of bone mar-row derived stem cells and their application for bone regeneration. Inter J Oral Sci 2010; 2 (3): 127-135.
ment heading
10.1016/S1995-7645(14)60049-3
*Corresponding author: Xiao-Dong Gai, Ph.D., Professor, Deputy Director, Oral Medical School of North China University, Jilin 132011, China.
Tel: 15947964716
E-mail: guxqitg@163.com
Funding project: It is supported by National Science Foundation (Grant no.81170632).
Periodontal bone defect
Repair
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats
- Correlation of expression of STAT3, VEGF and differentiation of Th17 cells in psoriasis vulgaris of guinea pig
- Effect of anesthesia on cognitive status and MMP-2 expression in rats
- Ultrasonic diagnosis and vasoactive substances examination in patients with cirrhosis
- Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
- Expression of PI3-K, PKB and GSK-3β in the skeletal muscle tissue of gestational diabetes mellitus
