Establishment and identification of induced pluripotent stem cells in liver cancer patients
Da-Ming Zhang, Jian-Jun Li, Peng Yan, Jian-Ting Hu
Department of Laparoscopic Surgery, People’s Hospital of Zhengzhou, Zhengzhou 450003, China
Establishment and identification of induced pluripotent stem cells in liver cancer patients
Da-Ming Zhang, Jian-Jun Li, Peng Yan, Jian-Ting Hu*
Department of Laparoscopic Surgery, People’s Hospital of Zhengzhou, Zhengzhou 450003, China
Objective: To induce pluripotent stem (IPS) cells from fibrocytes that are separated from liver cancer patients. Methods: The fibrocytes were reprogrammed to IPS cells by lentiviral vector, stained and identified by immunohistochemistry. Results: The IPS cells were successfully established from fibrocytes after infection, and IPS cell clones formed in round shape under a microscopy. The induction rate was 0.013%±0.007%. No tumor formed at the back of nude mice within 8 weeks after the inoculation of cell clones. However, tetatoma appeared in nude mice within 1 week after IPS inoculation. A few tumors formed in nude mice within 4 weeks after the inoculation of cell clones. However, subcutaneous tumors formed within 1 week after IPS inoculation. The induced IPS cells showed three germ layers in tetatoma. Nanog and OCT4 in the induced IPS cells showed hypomethylation. SSEA-A, TRA-1-6-, TRA-1-81 and Nanog were highly expressed in the induced IPS cells, indicating the IPS cells possessed the similar ability as the stem cells. Conclusion: The IPS cells of liver cancer patients can be established effectively from fibrocytes and can be cultured stably in vitro, which provides an approach for the treatment of intermediate or advanced stage liver cancer.
ARTICLE INFO
Article history:
Received 10 January 2014
Received in revised form 15 February 2014
Accepted 15 March 2014
Available online 20 April 2014
Fibrocyte
1. Introduction
Liver cancer is a malignant cancer in liver, including primary and metastasized cancer. China is a country where hepatitis B outbreaks frequently, and a large number of patients develop liver cancer based on liver cirrhosis. Besides, there is usually no obvious symptom in the early stage. The patients are already in the intermediate or advanced stage when being diagnosed, and liver transplantation is the only treatment[1]. The problem is that there are too limited liver donors to meet the needs of most patients. Since 1990s, researchers have established induced pluripotent stem (IPS) cells from fibrocytes to provide a novel approach for the treatment of liver cancer[2]. We established and identified pluripotent stem cells induced from from fibrocytes in liver cancer patients.
2. Materials and methods
2.1. Materials
Liver tissues were excised from liver cancer patients during April, 2009-April, 2011 in People’s Hospital of Zhengzhou, China. The pMXs-IRES-Sox2, pMXs-IRESKlf4 and pMXs-IRES-Oct4 virus vectors were obtained from Addgene Company. The plasmid extraction kit was purchased from Omega Company. LipofectamineTM2000 was purchased from Invitrogen. Dulbecco’s minimum essential medium (DMEM) with high glucose and DMEM/F12 were purchased from the Hyclone Corporation. LIF factors, SSEA-1, Oct4 and AKP staining reagents were purchased from CHEMICON International, Inc.
2.2. Culture of fibrocytes
The liver tissue was cut into pieces, digested and separated by 2.5 g/L pancreatin. DMEM with high glucose and DMEM/ F12 were used for cell culture and resuspension. The cells were then inoculated into common culture bottles and thermostatically cultured at 37 ℃. After 1 day, the mediumwas changed or subcultured according to the density of cells[3-5]. The fibrocytes of the 1st and 2nd generation were selected and cryopreserved for further use.
2.3. Induction and packaging of vectors
Retroviruses plasmid vectors of Sox2, Klf4, Oct4 and C-MYC were extracted using plasmid DNA extraction kit. The above fibrocytes were virus-packaged by LipofectamineTM2000 and then dyed by green fluorescent protein to observe the efficiency of transfection[6].
2.4. Induction of IPS cells
The fibrocytes of the 1st and 2nd generation were thawed and cultured by DMEM with high glucose and DMEM/F12. The medium was exchanged once every day. The cells were inoculated into six well plates at a density of 3×104cells/well after 3 days. Then, the cells were culture for 6 hours in DMEM containing 10% FBS. The cells were transfected until the confluence area was up to 60%[7]. The transfection continued for 18 hours. The cells were transfected again after 6-hour culture, followed by further culture. Then the cells were passaged and identified after cloning.
2.5. Identification of IPS cells
The IPS cells were fixed by 4% paraformaldehyde and dyed according to the introduction of AKP staining kit after 20 minutes post fixation[8,9]. After 20-minute incubation, the cells were washed by PBS and sealed by glycerin to be observed under a microscope.
2.5.1. RT-PCR
The RT-PCR primers were designed referring to the relevant references. The primer sequences are shown in Table 1.
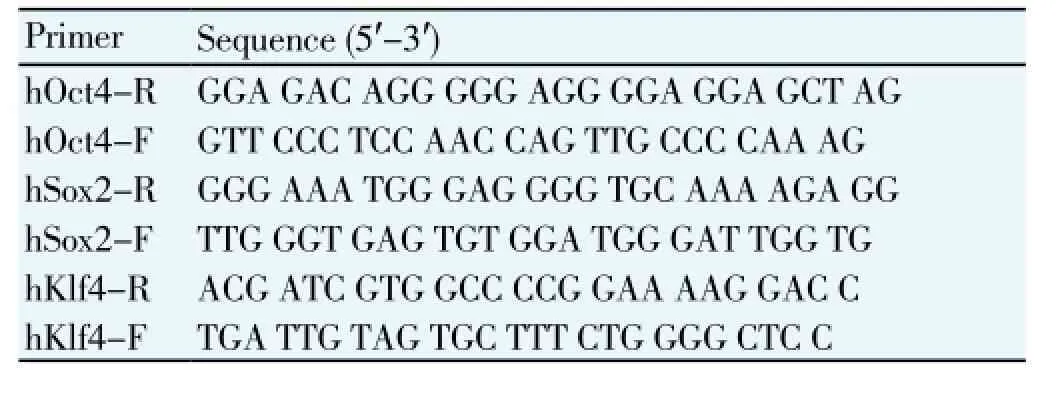
Table 1 Sequences of RT-PCR primers.
2.5.2. Identification of stem cell marker proteins
SSEA-4, TRA-1-60, TRA-1-81, and specific proteins (OCT4 and Nanog) related to stem cell expression were detected by immunohistochemistry. Nanog protein was detected by Western blotting. Methylation level ofNanoggene promoter region was detected by bisulfite-genome sequencing method. All kits were purchased from Shanghai Stan (stem) Biological Technology Co., Ltd.
2.5.3. Teratoma assay
First, 1×106-5×106IPS cells were subcutaneously injected into SCID Beige mouse. The formation of subcutaneous teratoma was observed after 1 month. The tumor tissue was sliced and observed to analyze each germ layer.
2.5.4. Methylation assay
G9a protein methylation transferase inhibitor was used to treat receptor cells to induce reprogramming.
2.5.5. In vivo identification assay
The successfully established IPS cells were inoculated into the back of nude mice to observe the formation of teratoma and subcutaneous tumor[10,11].
3. Results
3.1. Infection of fibrocytes and establishment of IPS cells
The fibrocytes were infected effectively, and the expression of green fluorescence was high, showing high infection ability. The IPS cells were successfully established after fibrocytes were infected, and round IPS cell clones were observed under the microscopy (Figure 1). The induction rate was 0.013%±0.007%.
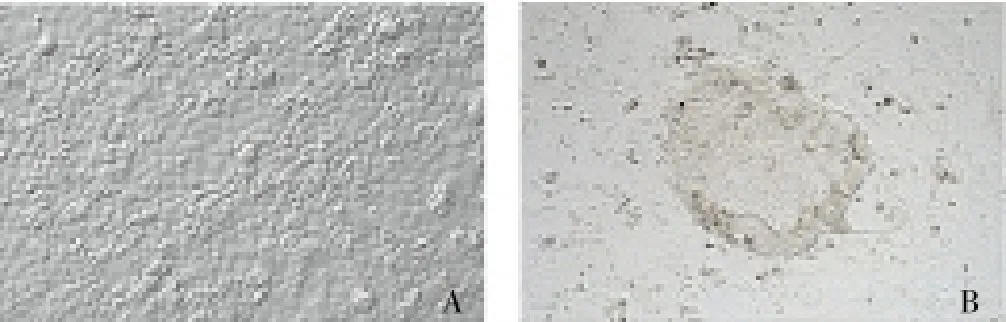
Figure 1. Microscopic observation of normal fibrocytes (A) and pluripotent stem cells induced from fibrocytes (B) (×400).
3.2. Detection of stem cell marker proteins and chromosome in established IPS cells
Obvious expression of SSEA-4, TRA-1-60, TRA-1-81 and Nanog proteins was observed (Figure 2). The IPS cells had normal 46xy chromosome, which was the same as that in the fibrocytes.
3.3. Teratoma formation in nude mice
No tumor formation was found at the back of nude mice within 8 weeks after the inoculation of cell clones. However, teratoma formed in all nude mice within 1 week post the inoculation of IPS cells. The diameter of tumor was around 2 cm after 8 weeks. The nude mouse was killed and the tumorwas obtained for observation. After subcutaneous inoculation of IPS cells into the nude mouse, HE staining was used to observe the teratoma. Three germ layers were found. There were gland cells in endoderm, cartilage tissue in mesoderm, and hair follicle structure in ectoderm (Figure 3).
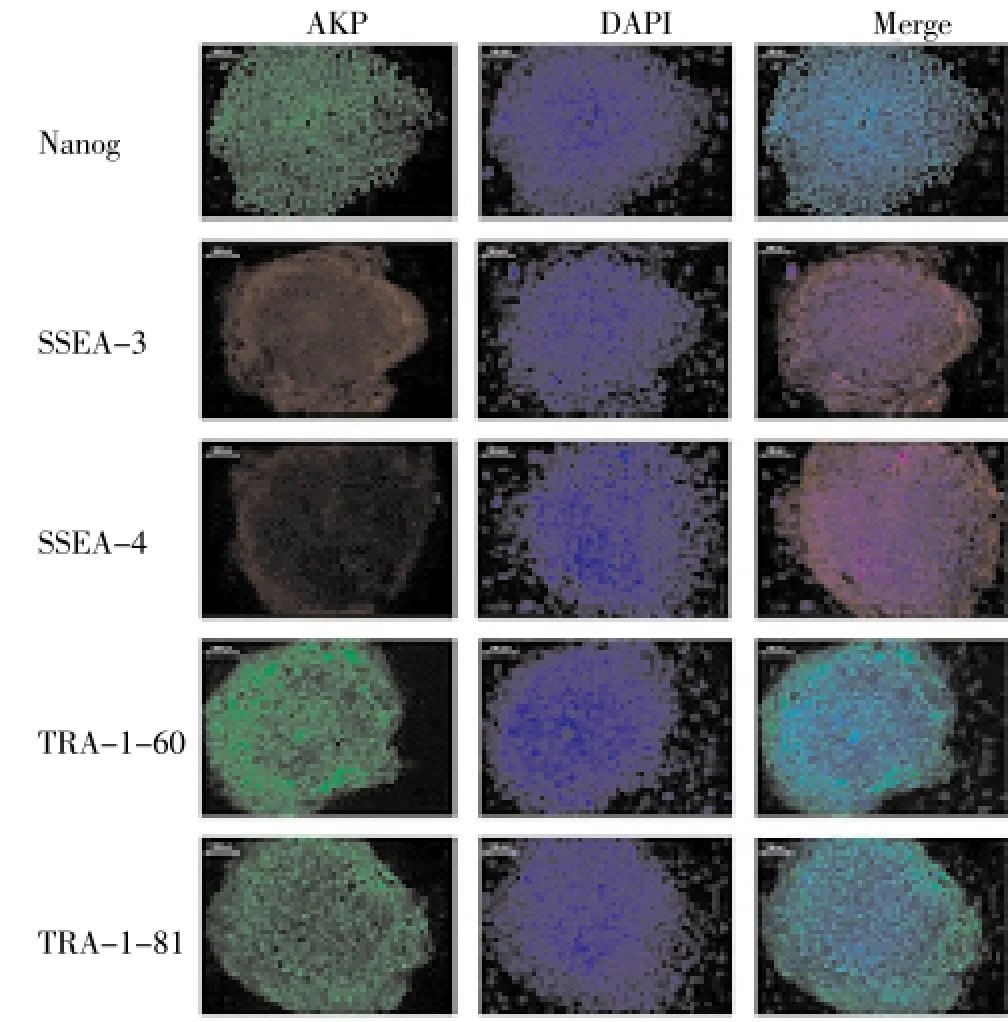
Figure 2. Expression of stem cell marker proteins in the induced pluripotent stem cells.
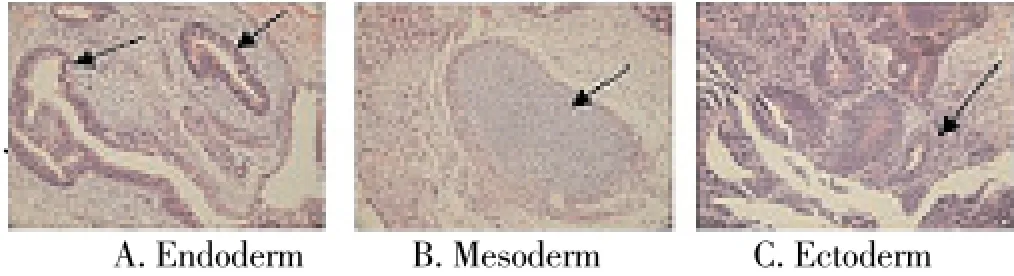
Figure 3. HE staining of teratoma in nude mouse after inoculation of induced pluripotent stem cells.
3.4. Subcutaneous tumor formation in nude mice
No tumor formed subcutaneously in the nude mice within 2 weeks post the inoculation of fibrocyte cell clones, and only one of the five mice had tumors during the last two weeks. In contrast, subcutaneous tumors appeared within 1 week after the inoculation of IPS cells in all nude mice. The diameter of tumor was less than 1 cm, and the tumor was not excised for the observation.
3.5. Methylation of fibrocytes and IPS cells
Figure 4 shows the methylation of fibrocytes and IPS cells. The Nanog and OCT4 showed high methylation status in the fibrocytes but low methylation status in the IPS cells, indicating that the IPS cells had the same ability as stem cells.

Figure 4. Comparison of methylation of Nanog and OCT4 between fibrocytes and induced pluripotent stem (IPS) cells.
4. Discussion
IPS cells is high self-renewal and has high ability of multidirectional differentiationin vitro, which may have wide applicable prospects in the treatment of various diseases, establishment of disease models and research of pathogenic mechanisms[12]. Due to the few obvious symptoms of early stage liver cancer, most of patients have entered intermediate or advance stage when being diagnosed. The liver cancer patients may be too late to receive surgical treatment. The patients can only receive conservative treatment or wait for liver donors. However, compared to the large group of liver cancer patients, the amount of liver donors is comparative less, and thus the life quality of liver cancer patients cannot be guaranteed[13-15]. Subsequently, to establish IPS cells by using fibrocytes has become a focal point to promote the regeneration of liver and has attracted more attention in clinic.
IPS cells have been successfully inducedin vitroin the study of mouse cell sequencing, which made the establishment of individual specific IPS cell possible[16-18]. The methods using transposable genes and lentiviral vector show good effects on the establishment of IPS cells and help the obtainment of therapeutic IPS cells[19,20]. We established the IPS cells of human liver cancer by using this method, and the induction rate was acceptable. The carcinogenicity of IPS cells was also decreased to avoid the risk of regenerative cells induced tumor emergence[21,22]. Nonetheless, the related mechanisms are still uncertain[23]. By observing the formation of teratoma and subcutaneous tumor, we found the IPS cells had high potency of differentiationin vivo. The induced IPS cells had similar pluripotency as embryonic stem cells. (i) The fibrocytes had a similar strong infection capability as pluripotency stem factors. (ii) The stem cell marker proteins including SSEA-4, TRA-1-60, TRA-1-81 and Nanog were highly expressed in both the IPS cells and pluripotency stem cells, as evidenced by RT-PCR. (iii) Teratoma appeared in nude mouse within 1 week after the inoculation of IPS cells and there were three germ layers of tissue cells, showing a strong teratoma formation ability. (iv) According to the methylation results, the methylation level of Nanog and OCT4 was low in the IPS cells, indicating potency of differentiation. Thus, future studies should be focused on controlling the differentiation direction of IPS cells so as to provide cells of artificial liver. The key pointsto establish IPS cells are as follows: control on the induction level of programming factor; selection of transcription factor activity time; selection of the fibrocyte resource; and control on induction level[24-26]. In the establishing process of IPS cells, the selection of small molecular substances should be controlled according to the transcription factor level in fibrocytes, in order to increase the induction rate and decrease the carcinogenicity of cells[27].
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgment
This work was supported by Science and Technology Project of Henan Provincial Department of Health (NO: 2011020038).
[1] Tomokuni A, Eguchi H, Hoshino H, Dewi DL, Nishikawa S, Kano Y, et al. Effect of in vivo administration of reprogramming factors in the mouse liver. Oncol Lett 2013; 6(2): 323-328.
[2] Batista LF, Artandi SE. Understanding telomere diseases through analysis of patient-derived iPS cells. Curr Opin Genet Dev 2013; 23(5): 526-533.
[3] Saito A, Ochiai H, Okada S, Miyata N, Azuma T. Suppression of Lefty expression in induced pluripotent cancer cells. FASEB J 2013; 27(6): 2165-2174.
[4] Comba P, Pirastu R, Conti S, De Santis M, Iavarone I, Marsili G, et al. Environment and health in Taranto, southern Italy: epidemiological studies and public health recommendations. Epidemiol Prev 2012; 36(6): 305-320.
[5] Maclean GA, Menne TF, Guo G, Sanchez DJ, Park IH, Daley GQ, et al. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc Natl Acad Sci U S A 2012; 109(43): 17567-17572.
[6] Ishikawa T, Banas A, Teratani T, Iwaguro H, Ochiya T. Regenerative cells for transplantation in hepatic failure. Cell Transplant 2012; 21(2-3): 387-399.
(1)管理意识淡薄。运输企业正式编排在册员工在减少,所以员工大多是临时雇佣工。这也人员对人事档案没有了解,认为是今天高兴在干活,不高兴就离职。企业也认为企业和驾驶员是雇佣关系,随时可以解雇。所以没必要投入资金对驾驶员的档案进行管理,从而企业中没有形成档案管理。
[7] Russo FP, Parola M. Stem cells in liver failure. Best Pract Res Clin Gastroenterol 2012; 26(1): 35-45.
[8] Ishikawa T, Hagiwara K, Ochiya T. Generation and hepatic differentiation of human iPS cells. Methods Mol Biol 2012; 826: 103-114.
[9] Kato I, Niwa A, Heike T, Fujino H, Saito MK, Umeda K, et al. Identification of hepatic niche harboring human acute lymphoblastic leukemic cells via the SDF-1/CXCR4 axis. PLoS One 2011; 6(11): e27042.
[10] Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, et al. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res 2011; 71(14): 4742-4747.
[11] Lee CH, Brubaker LM, Gerber DA, Ku YM, Kim YH, Shin SS, et al. MRI findings of recurrent hepatocellular carcinoma after liver transplantation: preliminary results. J Magn Reson Imaging 2011; 33(6): 1399-1405.
[12] Eiseman J, Lan J, Guo J, Joseph E, Vucenik I. Pharmacokinetics and tissue distribution of inositol hexaphosphate in C.B17 SCID mice bearing human breast cancer xenografts. Metabolism 2011; 60(10): 1465-1474.
[13] Sun C, Liu YK. Induced pluripotent cancer cells: progress and application. J Cancer Res Clin Oncol 2011; 137(1): 1-8.
[14] Gai H, Nguyen DM, Moon YJ, Aguila JR, Fink LM, Ward DC, et al. Generation of murine hepatic lineage cells from induced pluripotent stem cells. Differentiation 2010; 79(3): 171-181.
[15] Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, et al. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci U S A 2010; 107(1): 40-45.
[16] Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T. Stem cells for hepatic regeneration: the role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther 2010; 5(2): 182-189.
[17] Ochiya T, Yamamoto Y, Banas A. Commitment of stem cells into functional hepatocytes. Differentiation 2010; 79(2): 65-73.
[18] Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 2009; 41(12): 1350-1353.
[19] Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, et al. Phenotypic correction of murine hemophilia A using an iPS cellbased therapy. Proc Natl Acad Sci U S A 2009; 106(3): 808-813.
[20] Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science 2008; 322(5903): 945-949.
[21] Ahlén G, Derk E, Weiland M, Jiao J, Rahbin N, Aleman S, et al. Cleavage of the IPS-1/Cardif/MAVS/VISA does not inhibit T cell-mediated elimination of hepatitis C virus non-structural 3/4A-expressing hepatocytes. Gut 2009; 58(4): 560-569.
[22] Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells 2008; 26(10): 2467-2474.
[23] Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol 2008; 18(12): 890-894.
[24] Ram EV, Meshorer E. Transcriptional competence in pluripotency. Genes Dev 2009; 23(24): 2793-2798.
[25] Caperuto EC, Tomatieli RV, Colquhoun A, Seelaender MC, Costa Rosa LF. Beta-hydoxy-beta-methylbutyrate supplementation affects Walker 256 tumor-bearing rats in a time-dependent manner. Clin Nutr 2007; 26(1): 117-122.
[26] Camnasio S, Delli Carri A, Lombardo A, Grad I, Mariotti C, Castucci A, et al. The first reported generation of several induced pluripotent stem cell lines from homozygous and heterozygous Huntington’s disease patients demonstrates mutation related enhanced lysosomal activity. Neurobiol Dis 2012; 46(1): 41-51.
[27] Chen L, Kasai T, Li Y, Sugii Y, Jin G, Okada M, et al. A model of cancer stem cells derived from mouse induced pluripotent stem cells. PLoS One 2012; 7(4): e33544.
ment heading
10.1016/S1995-7645(14)60032-8
*Corresponding author: Jian-Ting Hu, MD, Department of Laparoscopic Surgery, People’s Hospital of Zhengzhou, No. 33, Yellow River Road, Zhengzhou 450003, China.
E-mail: 876893310@qq.com
Foundation project: This work was supported by Science and Technology Project of Henan Provincial Department of Health (NO: 2011020038).
Liver cancer
Induced pluripotent stem cells
Establishment
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats
- Correlation of expression of STAT3, VEGF and differentiation of Th17 cells in psoriasis vulgaris of guinea pig
- Effect of anesthesia on cognitive status and MMP-2 expression in rats
- Ultrasonic diagnosis and vasoactive substances examination in patients with cirrhosis
- Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines
- Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
