Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
Rui Wang, Chang-zheng Zhu, Ping Qiao, Jian Liu, Qiang Zhao, Kui-jie Wang, Tingbao Zhao
1Department of Spinal Cord Repairing, Jinan Military General Hospital, Jinan 250031, China
2Department of Neurology, People’s Hospital of Zhangqiu, Zhangqiu 250200, China
3Department of Orthopedics, People’s Hospital of Zhangqiu, Zhangqiu 250200, China
Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
Rui Wang1#, Chang-zheng Zhu2#, Ping Qiao3, Jian Liu3, Qiang Zhao3, Kui-jie Wang2, Tingbao Zhao1*
1Department of Spinal Cord Repairing, Jinan Military General Hospital, Jinan 250031, China
2Department of Neurology, People’s Hospital of Zhangqiu, Zhangqiu 250200, China
3Department of Orthopedics, People’s Hospital of Zhangqiu, Zhangqiu 250200, China
Objective: To evaluate of the curative effect of human umbilical cord mesenchymal stem cells (hUC-MSCs) on rat acute radiation pneumonitis. Methods: Fourty rats were randomly divided into control group, radiation group, stem cell prevention group, stem cell treatment group and prednisone treatment group. All rats except those in the control group were radiated with X ray to establish the acute radiation pneumonitis damage model. The hUC-MSCs cultured in vitro was administrated to the rats of the prevention group via tail vein (1×106cells/kg BW) 24 h before the radiation, while the same administration was performed in the rats of the treatment group 24 h after the radiation. After 24 h post the radiation, the rats in the radiation group were given 0.4 mL physiological saline, and those in the prednisone group were given 1 mg/kg prednisone. All rats were observed and executed 72 h after the radiation to detect lung histological changes. Results: After the administration of hUC-MSCs, the survival status of the rats in the prevention group and treatment group was obviously better than that in the control group. As shown by the histological staining, the morphology, proliferation activity and bronchial state of lung tissues were better in the prevention group and treatment group than in the control group. Conclusion: The hUC-MSCs have definite therapeutic effects on acute radiation pneumonitis in rats.
ARTICLE INFO
Article history:
Received 10 January 2014
Received in revised form 15 February 2014
Accepted 15 March 2014
Available online 20 April 2014
Human umbilical cord mesenchymal stem cell
1. Introduction
Radiation pneumonitis refers to the inflammatory reaction caused by injuries of the normal lung tissue within the radiation field after radiotherapy of lung cancer, esophageal cancer, breast cancer, malignant lymphoma or other chest and neck tumors. Usually, irritating dry cough appears as the main symptom of acute patients[1], while for chronic patients, lung infection can be easily stimulated due to their weak immunity, further generating extensive lung fibrosis[2] and causing damage of the respiratory function and even lethal respiratory failure. Clinically, adrenocortical hormone is more often used for the treatment of radiation pneumonitis[3] with good curative effects but many side effects. Currently, there is still no effective means to prevent and treat acute and chronic radiation lung injury.
Human umbilical cord mesenchymal stem cells (hUCMSCs) are a kind of stem cell characterized by selfrenewal, proliferation and multi-directional differentiation potency[4]. Presently, it is widely used for the treatment of multiple organ injury[5] clinically with the advantages of convenience, extensive sources, sufficient quantity, low immunogenicity, no ethical controversy, fast proliferation, strong differentiation ability and extensive application prospect. In this experiment, the curative effect of hUCMSCs on rat acute radiation pneumonitis was observed, providing a clinical basis for the prevention and treatment of radiation-induced diseases.
2. Materials and methods
2.1. Materials
Mouse anti-human CD34-PE, CD45-PE, CD29-PE, CD44-PE, CD73-PE, CD90-PE, and CD105-PE antibodies were purchased from American Cell Signaling Technology Co., Ltd. The Swedish Elekta Precise linear accelerator and FACSCanto II flow cytometer were purchased from Becton Dickinson Medical Instrument Co., Ltd. TCS-SPZ inverted fluorescence confocal microscopy was purchased from German Leica Company.
2.2. Animal grouping and radiation
Fourty healthy male Wistar rats at the age of 2 months and with weight of (200±20) g were provided by the Laboratory Animal Research Center of Shandong University and randomly divided into five groups, namely, the control group, radiation group, stem cell prevention group, stem cell treatment group and prednisone treatment group, eight rats in each group.
The rats except those in the control group were fixed on the plastic foam board by rubber bands, with chest exposed. They were given chloral hydrate (4-5 mL/kg BW) for peritoneal injection anesthesia. The Swedish Elekta Precise linear accelerator (X-ray) was used for irradiation performed under the following conditions: source skin distance, 120 cm; absorbed dose rate, 300 mu/min; radiation field, 10 cm × 20 cm; X-ray energy, 6 mv; and total effective biological dose,8 GY[6].
2.3. In vitro culture of hUC-MSCs
The hUC-MSCs were culturedin vitro[7]. In this aseptic operation, 30-40 cm long healthy neonatal umbilical cord was put into aseptic saline solution containing 0.1% penicillin and streptomycin. Before the experiment, the umbilical cord was rinsed with 75% (v/v) ethanol and cut into 1.5-2.0 cm pieces. After peeling of umbilical cord skin and artery blood vessel, these pieces were washed 2-3 times in the saline solution, cut into pieces, and then put into a centrifuge tube. Then, 2 times volume of 0.2% Type I collagen enzyme and 100 μL of 0.1% penicillin and streptomycin were added, followed by overnight digestion at 37 ℃. In the next morning, 2.5 g/L trypsin with 0.5 g/L EDTA was added, followed by further digestion for 30 min with 37℃. Subsequently, the digestion products were fully diluted with 8 times volume of saline solution, mixed well and put into centrifuge tubes separately, followed by centrifugation at 3 000 r/min for 20 min. They were washed again with normal saline and centrifuged at 2 000 r/min for 10 min. The supernatants were discarded and the cell pellet was resuspended in a certain amount of culture medium.
The cells were inoculated at a density of 1×106cells /cm2and incubated in 5% (v/v) CO2at 37 ℃. Digestion and passage were performed when the convergence area reached 90%. The mixture containing PBS and 2.5 g/L trypsin with 0.5 g/L EDTA at a volume ratio of 1:4 was used for digestion. When most of the cells became round, culture medium was added to stop digestion. The cell suspension was transferred into centrifuge tubes and centrifuged at 1 500 r/min for 10 min. Then, the cells were collected and diluted with culture medium for cell count. The cells were inoculated at a density of 3 000-6 000 cells/cm2in an ncubator with 5% (v/v) CO2at 37 ℃. Generally, the cells of the first five generations were used for the experiment.
2.4. Flow cytometry
After digestion, the hUC-MSCs suspension was centrifuged at 1 300 r/min for 8 min. Then the cells were resuspended in PBS and made into single cell suspension by gently blowing. Later, 15 μL of mouse anti-human CD34-PE, CD45-PE, CD29-PE, CD44-PE, CD73-PE, CD90-PE and CD105-PE antibody were added into each tube containing 50 μL single cell suspension respectively and incubated at room temperature in darkness for 30 min. Then, 1 mL PBS was added into each tube and centrifugated at 1 500 r/min for 6 min. After removal of the supernatants, 2 mL PBS was added into each tube and centrifugated again at 1 500 r/min for 6 min. After the supernatants were discarded, the cell pellets were finally resupended in 400 μL PBS and detected by flow cytometry.
2.5. Administration and observation
The rats in the stem cell prevention group were injected with 0.4 mL of hUC-MSCs suspension (1×106cells/kg BW) via tail vein 24 h before the radiation, while the same administration was done in the rats of the stem cell treatment group 24 h after the radiation. The rats in the radiation group and prednisone treatment group were given normal saline (0.4 mL per rat) and prednisone (1 mg/kg.d) via tail vein 24 h after the radiation, respectively.
The rats were fed in separate cages under the same conditions, and their mental states and manure were observated. They were weighted and killed 72 h after radiation. Under aseptic conditions, the right lung tissue was selected to make slices for HE staining and immunohistochemical staining, and the results were observed under an inverted microscope.
3. Results
3.1. Morphological observation of hUC-MSCs
As observed under the inverted microscope, the hUCMSCs became adherent after 1 d post the inoculation, and more adherent cells appeared on the 2nd day. After removal of suspension cells, the adherent cells began to proliferate on the 3rd day and spread into elliptic, short shuttle-like, polygonal and irregular type (Figure 1). On the 14th day, the cells concentrated in the colony center and overspread the bottle bottom. The 3rd-5th generations of cells showed a uniform long shuttle-like type and arranged in a spiral or radical pattern (Figure 2), which were the typical morphological features of mesenchymal stem cells.

Figure 1. Human umbilical cord mesenchymal stem cells cultured in vitro (×100).(A) Day 3 of the first generation. (B) The 3rd generation.
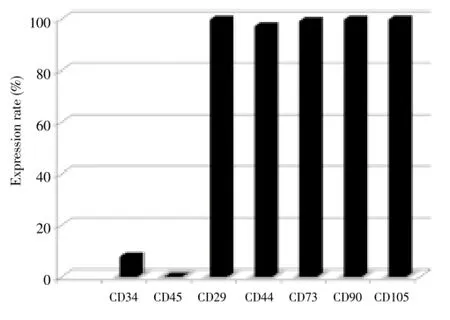
Figure 2. Positive expression rate of CD molecules of mesnchymal stem cells.
3.2. Identification of mesnchymal stem cells
The low expression of CD34, negative expression of CD45, and positive expression of CD29, CD44, CD73, CD90 and CD105 were observed in the isolated hUC-MSCs, as detected by flow cytometry (Figure 3).
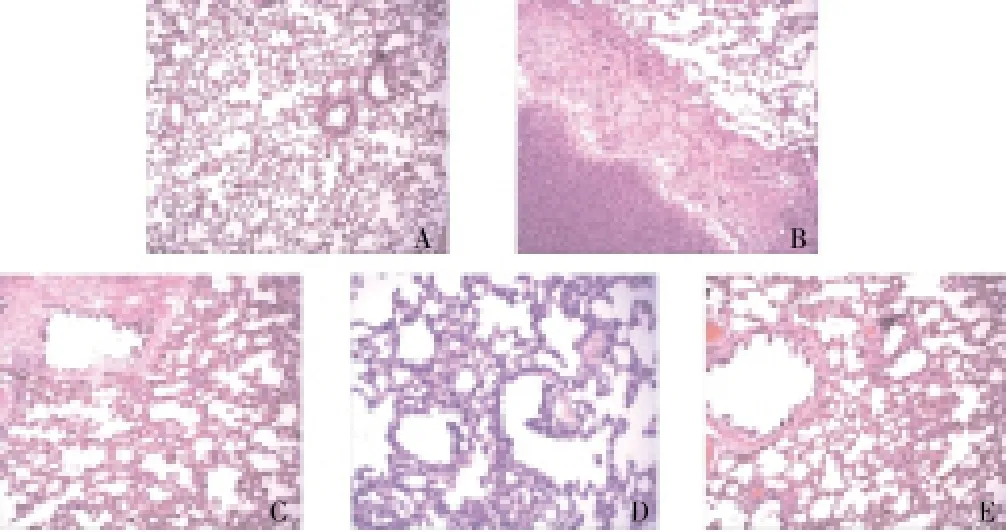
Figure 3. Lung tissue of rats (HE staining, ×100).(A) Control group; (B) Radiation group; (C) Stem cell prevention group; (D) Stem cell treatment group; (E) Prednisone treatment group.
3.3. Macroscopic observations of experimental animals
After the radiation, one rat suffered from tachypnea and died after 4 h. On the 1st day after radiation, the rats in the stem cell prevention group and treatment group respired normally and had the best mental status, with increased diet and drink, active action and fighting phenomenon. These rats bit the glove when being taken from the cages and they had bright eyes and swift response, while the rats in other groups had sleepiness and discharged thin stool. On the 2nd day after radiation, the rats in the stem cell prevention group and treatment group showed good mental status. No obvious changes except thin stool were found in the prednisone treatment group, while the rats in other groups still had sleepiness. On the 3rd day after radiation, the rats in each group had thin stool, which was more serious in the radiation group. All rats had varying yellow hairs on their backs and tail skin shedding with irregular white spots, but the rats in the treatment group showed the best mental status. Totally, 72 h after the radiation, the number of dead rats in the control group, radiation group, stem cell prevention group, stem cell treatment group and prednisone treatment group was 1, 2, 0, 1, and 2, repectively. Remarkable weight loss was observed in the radiation group but not in other groups.
3.4. Efficacy of hUC-MSCs against radiation pneumonitis
Compared with the normal lungs, the rat which died on the day of radiation had an obviously larger volume, more surface exudates after longitudinal cut, scattered hemorrhagic spots and pulmonary edema. After simultaneous perfusion, all rats except those in the control group had a small amount of pleural effusion. As shown by HE staining, the rats in the control group had normal alveolar size and shape, no congestive edema and inflammatory infiltration in bronchial mucus membrane, and no secretion in the tube (Figure 4A). The rats in the radiation group had abnormal alveolar size and shape,massive local inflammatory infiltration, small local abscess and peripheral hyperplasia of fibrous tissue (Figure 4B). The rats in the prevention group had mostly normal alveolar size and shape, local alveolar ectasia, dilation and congestion of bronchial wall and lung interstitial vessels, and no secretion in the bronchial lumen (Figure 4C). The rats in the treatment group had relatively normal alveolar size and shape, mild congestion and edema of bronchial wall and lung interstitial, and small inflammatory infiltration around the bronchial (Figure 4D). The rats in the prednisone treatment group had mostly normal alveolar size and shape, local alveolar ectasia, mild dilation and congestion of bronchial wall and lung interstitial, and no obvious secretion in the bronchial lumen (Figure 4E).
4. Discussion
In recent years, the incidence of lung cancer and other thoracic and neck tumors have been remaining at a high level, leading to an increasing number of patients with radiotherapy induced acute or chronic radiation pneumonitis[8]. Presently, adrenocortical hormone is mostly used for the prevention and treatment of acute or chronic radiation pneumonitis clinically, and it is very effective in the early stage of disease to reduce damages of alveolar epithelial cells and microvessel as well as lung tissue exudation and edema[9]. Our research indicated that the rats in the prednisone group presented better mental status and had mostly normal alveoli, local alveolar ectasia and no obvious secretion in the bronchial lumen as shown by lung histological staining. Although the treatment showed good curative effects, hormone therapy often causes osteoporosis and other side effects[10,11]. Therefore, it is unfavorable for prophylaxis or long-term use. At present, atomized inhalation is more often adopted for hormone therapy and can remarkably alleviate symptoms and reduce side effects. Cyclophosphamide[12] and methotrexate[13] are widely used. Surgical treatment is also considered but with less clinical use for its poor curative effect[14]. With the development of the modernization of traditional Chinese medicines, researches about the preventive effects of herbal medicines on radiation damage have been progressing around the world.Radix isatidis[15],Cordyceps sinensis[16] and Chinese herb extracts like quercetin[17], lycopene[18] and curcumin[19] show good radioprotection effects.
Currently, researches on stem cell and regenerative medicine have become the most eye-catching area in natural science[20]. Adoption of stem cell therapy has many advantages like low toxicity or nontoxicity. Preferable therapeutic effects can also be achieved without completely knowing the exact pathogenesis of the disease. Autologous stem cell transplantation can avoid the immunological rejection. A lot of studies confirm that hUC-MSCs can be induced to differentiate into various adult cells[21].In vivo, stem cells can be differentiated into endothelial cells, dopaminergic neurons, skeletal muscle cells, islet cells,etc[22-26].In vitro, they can be differentiated into osteocytes, chondrocytes, hepatocytes and myocardial cells,etc[27-29], and they have amazing effect on the diseases that cannot be well treated with traditional therapy.
In our study, the injection of a certain amount of mesenchymal stem cells before the radiation could alleviate the radiative damages to the alveolar endothelial cell and small vessels, thus reducing the acute complications after radiation. In this experiment, no obvious difference was found in curative effects between the treatment group and hormonal treatment group, and the effect in the prevention group was not as good as that in the stem cell treatment group and prednisone treatment group but was better than that in the radiation group, indicating an important role of stem cells in preventative treatment. The analysis of the pathological sections showed that the stem cells could repair and replace the injured alveolar epithelial cells to alleviate the pulmonary edema and pulmonary arterial hypertension, and it is effective in the treatment of radiation pneumonitis with no poisonous side effect, compared with hormone therapy. Stem cells bring a new hope to the prevention therapy of radiation pneumonitis, but the clinical time and dose of administration still need studies because of the inactivation effect of radials on stem cells.
In conclusion, the hUC-MSCs was effective in preventing and treating radioactive pneumonia in Wistar rats. The administration of stem cells after radiation was more effective on the treatment of radiation pulmonary injury and on the alleviation of acute pulmonary edema, even curing radiation pneumonitis to some extent. Stem cells could secrete various repairing factors with lasting effect during the culture and survival postin vivoimplantation, but the specific factors and their roles needs further studies.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by Wu Zuze Science and Technology Development Foundation of Beijing.
[1] Nakajima N, Sugawara Y, Kataoka M, Hamamoto Y, Ochi T, Sakai S, et al. Differentiation of tumor recurrence from radiationinduced pulmonary fibrosis after stereotactic ablative radiotherapy for lung cancer: characterization of 18F-FDG PET/CT findings. Ann Nucl Med 2013; 27: 261-270.
[2] Liu L, Zhang MN. Radioactive pneumonia and pulmonary fibrosis. J Clin Int Med 2011; 28: 227-229.
[3] Yan RM, Jing HM, Li EZ, Shen WX. The combined therapy of radiation induced lung injury. Chin J Radiol Med Protect 2007; 27: 563-565.
[4] Liu L, Chai J, Han Y, Sun T, Li D, Zhao J. Research progress of biological characteristics and advantages of Wharton’s jellymesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2011; 25: 745-749.
[5] Tao R, Han MF, Chai JK. Research progress and prospect of application of human umbilical cord mesenchymal stem cells. Bull Acad Military Med Sci 2010; 34: 293-296.
[6] Chen BY, Li SG, Xiao MB, Jiang F, Ni WK, Ni RZ, et al. The mechanisms of APRD in treating radiation-induced lung fibrosis in rats. Chin J Radiol Med Protect 2012; 32: 475-480.
[7] Fan XB, Liu TQ, Hao YJ, Liu Y, Ma XH, Cui ZF. Optimization for dissociation and culture of mesenchymal stem cells derived from umbilical cord blood. Prog Biochem Biophys 2008; 35: 905-913.
[8] Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol 2012; 51: 975-983.
[9] Chaaban S, Salloum V. Chronic eosinophilic pneumonia in a breast cancer patient post radiation therapy: A case report. Respir Care 2013. [Epub ahead of print].
[10] Sarli MA, Zanchetta MB, Rey PG, Spivacow FR. Severe osteoporosis treatment with teriparatide. Medicina (B Aires) 2013; 73: 428-432.
[11] Ponnapakkam T, Katikaneni R, Sakon J, Stratford R, Gensure RC. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov Today 2013. pii: S1359-6446(13)00253-5. doi: 10.1016/ j.drudis.
[12] Ochoa R, Bejarano PA, Glück S, Montero AJ. Pneumonitis and pulmonary fibrosis in a patient receiving adjuvant docetaxel and cyclophosphamide for stage 3 breast cancer: A case report and literature review. J Med Case Rep 2012; 6: 413.
[13] Martos VP, Vonlanthen B. Fatal pneumonitis during low-dose methotrexate treatment. Z Rheumatol 2012; 71: 75-77.
[14] Kunwar A, Jain VK, Priyadarsini KI, Haston CK. A selenocysteine derivative therapy affects radiation-induced pneumonitis in the mouse. Am J Respir Cell Mol Biol 2013; 49: 654-661.
[15] You WC, Lin WC, Huang JT, Hsieh CC. Indigowood root extract protects hematopoietic cells reduces tissue damage and modulates inflammatory cytokines after total-body irradiation: does indirubin play a role in radioprotection. Phytomedicine 2009; 16: 1105-1111.
[16] Liu WC, Wang SC, Tsai ML, Chen MC, Wang YC, Hong JH, et al. Protection against radiationinduced bone mar-row and intestinal injuries by Cordyceps sinensis, a Chinese herbal medicine. Radiat Res 2006; 166(6): 900-907.
[17] Benković V, Knezević AH, Dikić D, Lisicić D, Orsolić N, Basić I, et al. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh Hig Rada Toksikol 2009; 60: 129-138.
[18] Srinivasan M, Devipriya N, Kalpana KB, Menon VP. Lycopene: An antioxidant and radioprotector against gamma-radiation-induced cellular damages in cultured human lymphocytes. Toxicology 2009; 262: 43-49.
[19] Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 2010; 62: 919-930.
[20] Sykova E, Forostyak S. Stem cells in regenerative medicine. Laser Ther 2013; 22: 87-92.
[21] Liu LY, Hou YS, Chai JK, Hu Q, Duan HJ, Yu YH, et al. Basic fibroblast growth factor/vascular endothelial growth factor in the serum from severe burn patients stimulates the proliferation of cultured human umbilical cord mesenchymal stem cells via activation of Notch signaling pathways. J Trauma Acute Care Surg 2013; 75: 789-797.
[22] Can A, Karahuseyinoglu S. Concise review: human umbilical cordstroma with regard to the source of fetus-derived stem cells . Stem Cells 2007; 25: 2886-2895.
[23] Orlova VV, Drabsch Y, Freund C, Petrus-Reurer S, van den Hil FE, Muenthaisong S, et al. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler Thromb Vasc Biol 2013. [Epub ahead of print]
[24] Kim HS, Kim J, Jo Y, Jeon D, Cho YS. Direct lineage reprogramming of mouse fibroblasts to functional midbrain dopaminergic neuronal progenitors. Stem Cell Res 2013; 12: 60-68.
[25] Han ZX, Shi Q, Wang DK, Li D, Lyu M. Basic biological characteristics of mesenchymal stem cells derived from bone marrow and human umbilical card. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013; 21: 1248-1255.
[26] Li G, Zhang XA, Wang H, Wang X, Meng CL, Chan CY, et al. Comparative proteomic analysis of mesenchymal stem cells derived from human bone marrow, umnilical cord, and placenta: implication in the migration. Proteomics 2009; 9: 20-30.
[27] Sonowal H, Kumar A, Bhattacharyya J, Gogoi PK, Jaganathan BG. Inhibition of actin polymerization decreases osteogeneic diffrentiation of mesenchymal stem cells through p38 MAPK pathway. J Biomed Sci 2013; 20: 71.
[28] Krueger WH, Tanasijevic B, Barber V, Flamier A, Gu X, Manautou J, et al. Cholesterol-secreting and statin-responsive hepatocytes from human ES and iPS cells to model hepatic involvement in cardiovascular health. PloS One 2013; 8: e67296.
[29] Burt RK, Chen YH, Verda L, Lucena C, Navale S, Johnson J, et al. Mitotically inactivated embryonic stem cells can be used as an in vivo feeder layer to nurse damaged myocardium after acute myocardial infarction: A preclinical study. Circ Res 2012; 111: 1286-1296.
ment heading
10.1016/S1995-7645(14)60034-1
*Corresponding author: Ting-Bao Zhao, PhD, Professor, Department Director of Spinal Cord Repairing, Jinan Military General Hospital, Jinan 250031, China.
Tel: +86-531-51665443
E-mail: woaifei371328@163.com
#These authors contributed equally to this work.
Foundation project: This paper is supported by Wu Zuze Science and Technology Development Foundation of Beijing.
Radiation pneumonitis
Rat
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
- Effect and mechanism of salvianolic acid B on the myocardial ischemiareperfusion injury in rats
