Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients
Jun-Jie Chen, Jie-Feng Huang, Wen-Xi Du, Pei-Jian Tong
Department of Orthopedics, First Hospital Affiliated to Zhejiang University of Traditional Chinese Medicine, Hangzhou 310006, Zhejiang, China
Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients
Jun-Jie Chen△, Jie-Feng Huang△, Wen-Xi Du, Pei-Jian Tong*
Department of Orthopedics, First Hospital Affiliated to Zhejiang University of Traditional Chinese Medicine, Hangzhou 310006, Zhejiang, China
Objective: To investigate the expression and significance of MMP-3 in synovium of knee joint at different stage in osteoarthritis (OA) patients. Methods: Knee synovial tissue were collected in 90 OA patients (the OA group). Patients in the OA group was divided into 3 subgroups: gradeⅠsubgroup (n=30), grade Ⅱsubgroup (n=30), grade Ⅲ subgroup (n=30). Thirty patients served as control group. Immunohistochemical assay was used to detect the expression of MMP-3 protein in the knee synovial tissue. Results: MMP-3 protein was detected in all knee synovial tissue. The expression of MMP-3 protein in the OA group was significantly higher that in the normal synovium (P<0.05), and the MMP-3 protein was mainly located in the cytoplasm. There was significant difference in the expression of MMP-3 protein between the grade Ⅲ subgroup and the gradeⅠ, grade Ⅱsubgroups (all P<0.05). The expression of MMP-3 protein was positively related to the severity of OA (r=0.912, P<0.05). Conclusions: The expression of MMP-3 protein are closely related to pathogenic mechanism of OA. It may be an important indicator of early diagnosis and the activity of the disease of osteoarthritis.
ARTICLE INFO
Article history:
Received 10 December 2013
Received in revised form 15 January 2014
Accepted 15 February 2014
Available online 20 April 2014
Osteoarthritis
1. Introduction
Osteoarthritis (OA) is a kind of degenerative joint diseases involved in all articular tissues, showing the chief manifestations such as articular cartilage disorders, conduction stress load abnormalities, and progressive cartilage degradation, eventually triggering cartilage thinning, fibrosis, erosions, fissures, ulcers and articular surface disappearance[1-4]. OA occurs predominantly in the middle-aged and the elderly, with high pain prevalence and high morbidity, which seriously affects their quality of life and brings about heavy burden to the society and family[5,6]. As the pathogenesis of OA remains unclear, the patients can not be radically cured. In addition, evidence suggests that metabolic imbalance between articular cartilage degradation and synthesis is the leading cause of OA cartilage degeneration. Matrix metalloproteinase family has attracted increasing attention as the promoter of cartilage extracellular matrix and basement membrane components degradation. Matrix metalloproteinase 3 (MMP3) is secreted from chondrocytes and synovial cells, it can not only degrade a variety of extracellular matrix, but also activate other protease serine[7-9]. It is known that MMP3 is expressed in OA synovium, however, there is little evidence available on the difference of MMP3 expression in synovial membrane at different stages of OA[10]. This study aims to verify the correlation between MMP3 and human OA and to explore the potential mechanism. We detected MMP3 expression in the synovium of knee joint of OA patients at varying degrees with immunohistochemical method[11-13], in an effort to investigate the expression and significance of MMP3 in the pathogenesis of OA.
2. Materials and methods
2.1. Clinical data
According to the diagnostic criteria formulated byAmerican Rheumatism Association[14], 90 OA patients were selected as the OA group. Then the involved patients were divided into three subgroups based on their lesions, which was assessed using the criteria of Kellgren and Lawrence: mild lesion group (n=30), moderate lesion group (n=30), and severe lesion group (n=30). There were 36 males and 54 females, aged from 18 years to 81 years, with a mean of 58.3 years. There were 30 non-OA patients in control group, including 14 cases with simple knee joint meniscus injury, 9 cases with syndromatic synovial plica, and 7 amputated cases, aged 15-45 years, with a mean of 34.1 years.
The synovial specimens were harvested following knee arthroscope, knee joint replacement or amputation between March 2012 and April 2013. The patients were informed of the sample harvesting and gave informed consent. The experimental proposal was approved by the Hospital Ethics Committee in China.
2.2. Main reagents
The antibodies used for immunohistochemistry were rabbit anti-human polyclonal antibody (1:50; Abcam Company, Cambridge, USA) and horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Envision kit was provided by Dako Company (Denmark).
2.3. Immunohistochemical staining
All specimens were fixed in 10% formalin, embedded in paraffin, and sliced into serial sections in 4 μm thickness. Immunohistochemical staining was performed according to the instructions of Envision kit, followed by antigen retrieval. TBS, instead of the first antibody, was used as blank controls.
2.4. Assessment criteria
Immunohistochemical staining results were assessed with semi-quantitative integration method, namely the scores are graded according to the number of positive cells and the staining intensity of positive cells per slice, and then MMP3 expression intensity was determined based on the sum of the above two scores. The staining intensity score was recorded as 0 point (no stains), 1 point (light yellow), 2 points (brown yellow), and 3 points (brown stains). The percentage of positive cells was the ratio of the number of positive cells to the total cell number. Each slice was observed in ten vision fields at 40 × 10 magnification and was classified according to the proportion of stained cells as follows: 0 points, no staining or only < 10% cells stained; 1 point, 10%-25% positive cells; 2 points, 25%-50% positive cells; 3 points, 50%-70% positive cells; 4 point, 70%-100% positive cells. All slices were examined by two independent pathologists using a double-blind review method.
2.5. Statistical analysis
Data were statistically analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA) and are expressed as mean±SD. Difference between groups was compared using analysis of variance and intragroup data were compared usingt-test. The correlation analysis was performed using Pearson correlation analysis. A P < 0.05 was considered statistically significant difference.
3. Results
3.1. MMP3 expression in synovium
A small amount of light yellow-stained MMP3 were found in the cytoplasm of synovial tissue in the control group, and brown particles distributed in extracellular matrix (Figure 1A). MMP3 was mainly expressed in the cytoplasm of synovial tissue in the OA patient group, with a large number of brown particles; brown-yellow or brown particles were also visible in the cytoplasm, endothelial cells and inflammatory cells were apparently stained, synovial cells, vascular endothelial cells, monocytes and macrophages were strongly expressed in synovial lining layer and synovial sublining layer (Figures 1B-D). MMP-3 expression in OA groups (OA group: 2.09±0.16) were significantly higher than that in the control group (0.62±0.13), and it also showed significant differences in varying degree of OA: severe lesion group (3.02±0.19)> moderate lesion group (2.13±0.22)> mild lesion group (1.54±0.17).Correlation analysis showed that OA severity was positively correlated with MMP3 expression (r= 0.912, P < 0.05).
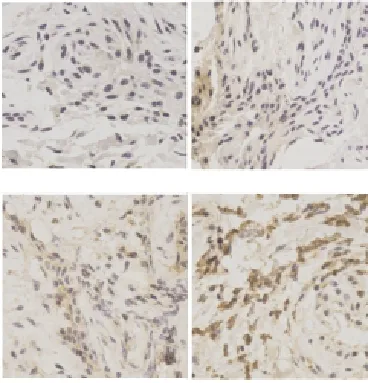
Figure 1. MMP-3 expression.A: control group (× 400); B: mild lesion group (× 400); C moderate lesion group (× 400); D severe lesion group (× 400).
4. Discussion
During the OA articular cartilage degeneration process, metabolic imbalance between degradation and synthesis of articular cartilage is the main reason for the degeneration. Among them, MMP functions to promote extracellular matrix and basement membrane components degradation[15,16]. MMP family are a class of zinc ion-dependent endopeptidases that are localized in a wide variety of connective tissue, it can degrade various extracellular matrix components such as collagen, proteoglycans, fibronectin and laminin[17-19].
MMP3 is also known as stromelysin-1, MMP3 encoding gene is located on chromosome 11, MMP is secreted from chondrocytes and synovial cells as a 59/57 kDa zymogen that is proteolytically processed to the 45 and 28 kDa active forms. MMP can degrade the majority of collagens and matrix proteins such as proteoglycan and laminin, which contribute to damage articular cartilage. Importantly, it also activates other plasminogens and accordingly activates MMP1, MMP9, MMP13, thus producing “waterfall-like”amplification effect and facilitating the destruction of cartilage[20-24]. A previous study[25] showed that, MMP3 levels in OA patient’s blood and synovial fluid were higher than that in healthy people, and the increment was consistent with the extent of cartilage damage; MMP3 was highly expressed in OA synovium, suggesting that MMP3 expression in synovium is closely linked with the degradation of joint tissue in OA patients. The present study found that MMP-3 protein expression in synovial tissue of OA patient was significantly increased compared with control group, and the expression level was positively correlated with the severity of OA. This evidence indicated that MMP3 is progressively increasing as OA course and highlighted the contribution of MMP3 in OA pathogenesis. It is previously reported that, when MMP-3 is involved in OA cartilage damage process, synovial macrophages play a crucial role in the early MMP activity and induce MMP production in synovium, rather than in cartilage[26,27]. The aforementioned studies revealed that synovial changes in OA are not just the result of MMP3-mediated cartilage degradation; MMP-3 may play an independent important role in OA synovial tissue. Some scholars confirmed that miR-155 overexpression in RA synovial tissue could inhibit MMP3 expression[28]. Papet al[29] found a positive correlation between MMP3 and uPA expression in cartilage tissue at different degrees of OA and they played a synergic role on promoting cartilage degradation, as detected by immunohistochemical staining. The above findings show that the abnormal intrinsic regulatory mechanisms in synovial tissue may stimulate MMP-3 secretion to various extents, activate other MMPs and produce amplification effect, thus generating many inflammatory cytokines such as interleukin-1. These inflammatory cytokines are secreted into the cartilage and can significantly increase MMP activity and content. When the increment exceeds the degree of increasing TIMPs, MMPs/TIMPs will loss the balance and degrade cartilage matrix type Ⅰ, Ⅱ, gelatin and fibronectin[13], thus promoting the degradation of articular cartilage matrix and inducing OA. Through a predetermined way, interleukin-1 and other inflammatory cytokines inhibit the expression of transcription factor, which is essential for cartilage regeneration, ultimately inhibit cartilage synthesis and restoration, and further deteriorate the destruction of articular cartilage[30,31]. MMP3’s function in synovium and cartilage allows to accelerate OA.
The present study showed that MMP3 expression in OA patients had significant differences compared with control group, and MMP-3 levels was positively correlated with OA severity. This evidence suggests that MMP-3 play a potential crucial role in OA pathogenesis and development, and MMP-3 inhibitors can delay OA or remove synovial lesions by arthroscopic surgery. This can not only relieve pain and improve function, but also reduce inflammation factors production and slow down OA, so that patients will avoid premature artificial joint replacement and the resulting high cost. In addition, MMP-3 is considered an indicator of early diagnosis and disease activity of OA patients.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Felson DT, Neogi T. Osteoarthrit is: is it a disease of cartilage or of bone? Arthritis Rheum 2009; 50(2): 341-344.
[2] Lohmander LS. What can we do about osteoarthritis? Arthritis Res 2010; 2(2): 95-100
[3] Mandell BF, Lipani J. Refractory osteoarthritis. Differential diagnoses and therapy. Rheum Dis Clin North Am 2008; 21(1): 163-178.
[4] Martel-Pelletier J, lsch DJ, lletier JP. talloproteinases and inhibitors in arthritic diseases. Best Pract Res Clin Rheuma-tol 2012; 15: 805-829.
[5] Alfano D, Franco P, Vocca I, Gambi N, Pisa V, Mancini A, et al.The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb Haemost 2005; 93(2): 205-211.
[6] Tchetverikov I, Ronday HK, Van El B, Kiers GH, Verzijl N, TeKoppele JM, et al. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis 2009; 3(7): 881-883.
[7] Yoshihara Y, Nakamura H, Obata K. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 2010; 59(6): 455-461.
[8] hrens D, Koch AE, Pope RM. xpression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum 2011; 39(9): 1576-1587.
[9] liuSun H, Wang X. Association of increased expression of macrophage elastase (matrix metalloproteinase 12) with rheumatoid arthritis. Arthritis Rheum 2008; 50(10): 3112-3117.
[10] Hitchon CA, Danning CL, Illei GG. Gelatinase expression and activity in the synovium and skin of patients with erosive psoriatic arthritis. J Rheumatol 2012; 29(1): 107-117.
[11] Wang WS, Dong JB. OA patients blood and joint fluid of MMP -3, uPA level detection and clinical significance. J Shandong Med 2011; (3): 271-274.
[12] Biota AB, van Lent PL, Libregts S, Holthuysen AE, van der Kraan PM, van Rooen N, et al. Crucial role ofmac-rophages in matrix metalloproteinase mediated cartilage destine tion during experimental osteoarthritis: involvement of matrix metal loproteinase. Arthritis Rheum (1): 147-157.
[13] Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 2008; 58(4): 1001-1009.
[14] Pap G, Eberhardt R, Rocken C, Nebelung W, Neumann HW,Roessner A, et al. xpression of stromelysin and urokinase type plasminogen activator protein in resection speciments and biopsies at different stages of osteoarthritis of the knee. Pathol Res Pntct 2000; 196(4): 219-226.
[15] Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 2011; 44(3): 585-594.
[16] Okada Y, Shinmei M, Tanaka O. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest 2012; 66(6): 680-690.
[17] Freemont AJ, Hampson V, Tilman R. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis 2010; 56(9): 542-549.
[18] Serni U, Fibbi G, Anichini E, Zamperini A. Plasminogen activator and receptor in osteoarthritis. J Rheumatol Suppl 2009; 43: 120-122.
[19] Ronday HK, Smits HH, Van Muijen GN. Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Br J Rheumatol 2009; 35(5): 416-423.
[20] Sun J, Hakobyan N, Valentino LA. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood 2008; 112(12): 4532-4541.
[21] Wehling N, Palmer GD, Pilapil C. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum 2009; 60(3): 801-812.
[22] Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a highdensity co-culture microenvironment. Arthritis Res Ther 2010; 12(4): 101-104.
[23] Shakibaei M, John T, Schulze-Tanzil G. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol 2007; 73(9): 1434-1445.
[24] Henrotin Y, Clutterbuck AL, Allaway D. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage 2010; 18(2): 141-149.
[25] Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2012; 39(1-2): 237-246.
[26] Malemud CJ. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging 2010; 27(2): 95-115.
[27] Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2011; 39(1-2): 237-246.
[28] Malemud CJ, Goldberg VM. Future directions for research and treatment of osteoarthritis. Front Biosci 2009; 4: 110-112
[29] Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, et al.Interleukin-1 beta and tumor necrosis factor-alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappa B dependent pathways. Arthritis Rheum 2009; 60(3): 801-812.
[30] Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a highdensity co-culture microenvironment. Arthritis Res Ther 2010; 12(4): 127-129.
[31] Shakibaei M, John T, Schulze-Tanzil G. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol 2009; 73(9): 1434-1445.
ment heading
10.1016/S1995-7645(14)60042-0
*Corresponding author: Pei-Jian Tong, Ph.D., Professor, Doctoral Supervisor, Department of Orthopedics, First Hospital Affiliated to Zhejiang University of Traditional Chinese Medicine, Hangzhou 310006, Zhejiang, China.
Email: Tongpeijian@163.com△: Both are first authors.
Foundation project: It is supported by Zhejiang Provincial Administration of Traditional Chinese Medicine (20132 a039).
MMP-3
Immunohistochemical
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Establishment and identification of induced pluripotent stem cells in liver cancer patients
- Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer
- Experimental treatment of radiation pneumonitis with human umbilical cord mesenchymal stem cells
- Protection effect of Xuanfudaizhetang on reflux esophagitis in rats
- Effect of peroxisome proliferator-activated receptor gamma agonist on heart of rabbits with acute myocardial ischemia/reperfusion injury
- Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats
