有机聚合物负载的手性催化剂研究与进展
徐圆圆, 程探宇, 魏 星, 李晓敏, 陈倩芸, 刘国华
(上海师范大学 生命与环境科学学院,上海 200234)
0 引 言
半个世纪之前Merrifield第一次报道了通过用聚苯乙烯为载体固相合成多肽链[1],自此之后以聚合物为载体被广泛的应用于简易合成、产物提纯等领域.类似于Merrifield树脂这种非均相的聚苯乙烯仍然应用于各种固相合成,还应用于各种反应试剂或催化剂的固载方面,而且越来越多的大分子材料应用于这些领域.Hudson将在1999年之前使用聚合物固载的主要研究者的工作做了非常全面的综述[2,3],另外包括聚乙二醇、非交联聚苯乙烯、聚乙烯、聚丙酸等一些可溶性的聚合物的应用也有较多的综述文献[4-7].近年来,随着医药合成领域的快速发展,手性催化剂也随之发展迅速,而聚合物应用与手性催化剂的固载也越来越多.本文作者主要综述了有机聚合物固载型手性催化剂的研究进展.
1 聚合物负载的有机小分子手性催化剂
早在20世纪化学家就发现了生物碱[8]和L-脯氨酸[9]的不对称催化作用.随后的几十年间手性小分子催化剂引起了广大的关注,并且发展迅速.近年来有机小分子手性催化剂越来越多的被负载在聚合物上,使之能够降低催化剂成本、更加利于回收.以近年来应用较为广泛的金鸡纳碱、咪唑啉酮和脯氨酸为例,介绍聚合物负载的有机小分子手性催化剂.
1.1 金鸡纳碱
2008年Itsuno等报道了一种新颖的固载季铵盐手性催化剂的方法,他们将聚合物链上引入磺酸根负离子,然后与季铵盐催化剂以离子键相结合形成聚合物负载的金鸡纳碱类似物催化剂1[10](图1).该催化剂可以催化N-(二苯基亚甲基)氨基乙酸叔丁酯和苄溴(或烯丙基溴)反应,收率高达95%(91% 对映体过量),但是该催化剂对碘甲烷反应活性不高,收率45%(70% 对映体过量ee).作者推测催化剂在催化过程中,离子蔟起了较为重要的作用.它促使了底物的烯醇化,并且活化了苄溴,反应后释放出催化剂并得到手性产物.

图1 负载型金鸡纳碱催化剂
随后Itsuno等又报道了金鸡纳碱季铵盐与二磺酸根负离子形成的高聚物催化剂2[11-12](图2),在室温条件下、碱性环境中,加入催化剂2(10 mol%)可以使N-(二苯基亚甲基)氨基乙酸叔丁酯不对称烷基化,而且收率和ee值都比较理想.作者认为该催化剂可以拓展为一种平台,适用于各种以离子键相结合的催化剂.
Haraguchi在2012年报道了与Itsuno相似的高聚物催化剂3[13](图2),作者以金鸡纳碱季铵盐为催化单体,用二卤素有机物通过共价键将金鸡纳碱相连形成高聚物催化剂.金鸡纳碱的二聚体催化N-(二苯基亚甲基)氨基乙酸叔丁酯不对称烷基化时可以得到较好的收率(>90%)和ee值(>80%).但是当二聚体再次以二卤素化合物相连形成高聚物催化剂后,其催化效率有所下降,其中收率和ee值都下降了约10%(图2).

图2 自聚型金鸡纳碱催化剂
1.2 咪唑啉酮
早在2002年,Cozzi[8]等就将咪唑啉酮的酪氨酸衍生物固载在可溶性的聚乙二醇上,用于催化Diels-Alder反应.该催化剂对映选择性较好(92% ee),另外它还可以重复使用4次,但是催化效率和对映选择性都有所降低.在2004年Benaglia和Cozzi同样将咪唑啉酮的衍生物固载在聚乙二醇上得到催化剂4[14](图3),它可用于催化N-苄基苯硝酮和丙烯醛在酸性条件下的Diels-Alder反应.负载催化剂4与负载前催化剂对映选择性没有区别,但是催化效率有所降低,另外该催化剂可以方便的回收利用3次.
Haraguchi等将咪唑啉酮衍生得到其季铵盐,然后通过离子键负载在有磺酸根负离子功能化的交联聚苯乙烯上得到催化剂5[9](图3).该催化剂可以催化环戊二烯和肉桂醛的环加成反应,得到较好的对映选择性(91% ee),而且它的重复使用无需经过重生步骤.Pericàs等通过Click反应将咪唑啉酮的衍生物固载与聚苯乙烯上得到催化剂6[15](图3),其可在0.5 mol/L的三氟乙酸的THF体系中催化氮取代的吡咯进行烷基化.该催化剂重复使用6次而其对映选择性基本没有变化,但是催化效率有所降低(图3).
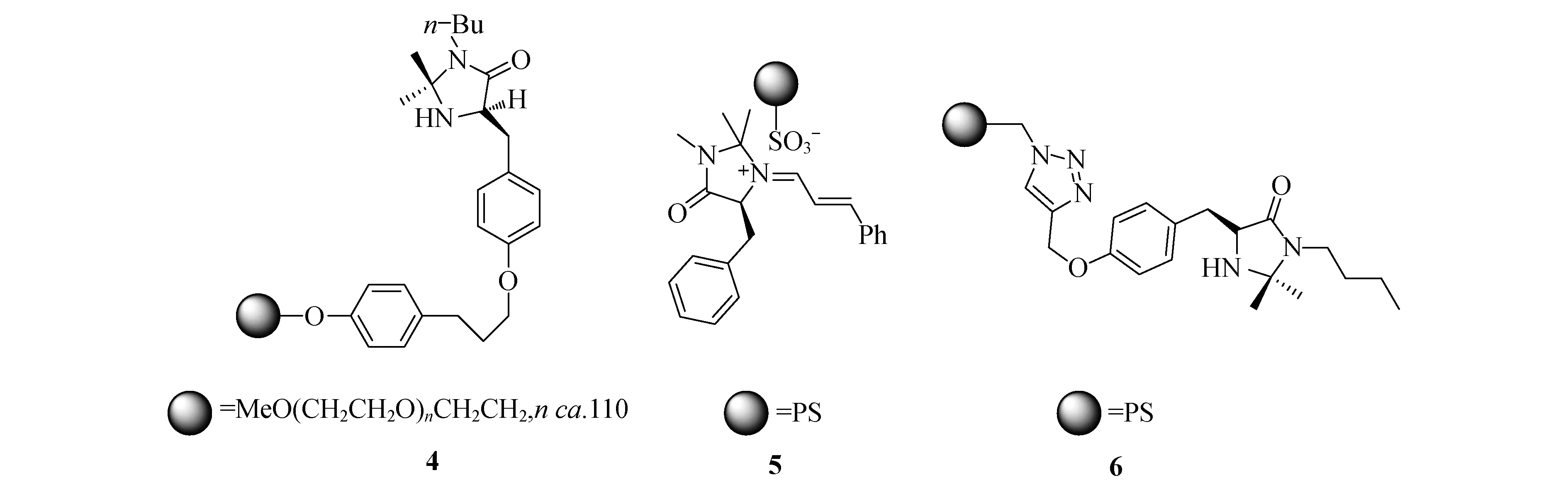
图3 负载型咪唑啉酮催化剂
1.3 脯氨酸
L-脯氨酸及其衍生物是一类最简单有效的有机小分子催化剂,它可以催化aldol反应[16]、Robinson缩环反应[17]、Mannich反应[18]、Michael反应[19]、Diels-Alder反应[20]和一些其他反应[21-26].(2S,4R)-4-羟基脯氨酸被固载在PEG500上形成可溶性的催化剂7(图4),它可以用于催化aldol缩合和Mannich反应[27,28],而且脯氨酸固载前后的催化效率和对映选择性没有明显变化.用于硝基苯甲醛和丙酮的缩合反应,催化剂7重复使用3次而ee值没有变化,但是收率有比较明显的降低(68%~51%).另外该催化剂还可以用于催化2-硝基丙烷和环己烯酮的加成反应[29],而用于β-硝基苯乙烯与酮的加成时对映选择性(40% ee)比均相脯氨酸(60% ee)低.
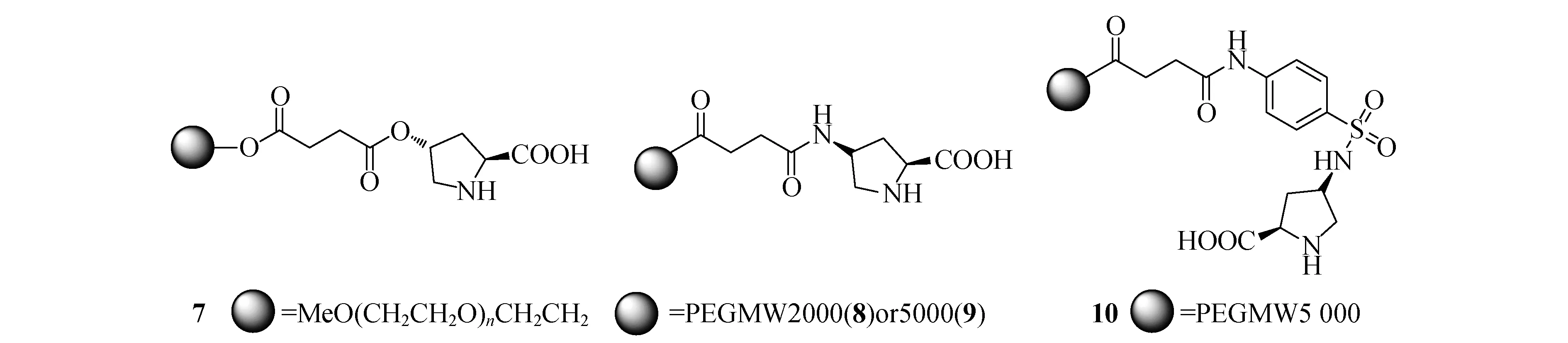
图4 负载型脯氨酸催化剂
Zhao等报道了与7相似的催化剂8~10[29],研究发现对于环己酮与β-硝基苯乙烯的反应,催化剂10无论是效率还是对映选择性都比8和9要好.催化剂可以通过加入非极性溶剂析出,过滤回收后可再次使用四次,但是收率(92-24%)和对映选择性(60-<10%)都有非常明显的降低.
除了将脯氨酸固载在可溶性的聚乙二醇上,不溶性的聚苯乙烯类树脂也作为一类载体被广泛的使用.各种脯氨酸的衍生物通过Click反应固载在树脂上,用于aldol反应[30-31]、α-胺氧化反应[32]、Michael加成反应[33]、α-硒化反应[34]等.
2 聚合物负载的手性有机金属催化剂
手性金属络合物催化剂在不对称催化中始终扮演着及其重要的角色,2001年度的诺贝尔化学奖就授予了从事手性催化的Knowles、Noyori和Sharpless 3位化学家.但是随着工业的发展,由于手性金属络合物催化剂尤其是重金属催化剂容易造成的环境污染,而且他们的成本一般相对较高,这些原因大大的限制了其发展.而近年来手性催化剂的固载技术缓解了这些问题,而且越来越多的手性催化剂被固载与有机聚合物和介孔硅胶材料上.本文作者以固载与有机聚合物上的钴、钌和铑等重金属催化剂为例介绍近年来有机聚合物负载金属手性催化剂的发展情况.
2.1 钴
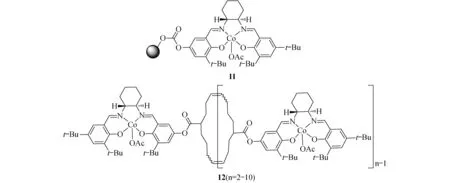
图5 负载型手性钴催化剂
Jacobsen等在1999年将手性的Co(Salen)固载与聚苯乙烯上得到催化剂11[35](图5),该催化剂可以在二氯甲烷中水解端基的环氧乙烷得到手性(>92% ee)的二醇化合物,另外它可以重复使用5次.几年后Jones和Weck以大环相连的低聚物12[36](图5),催化单元同样为手性钴催化剂,该催化剂是通过烯烃的扩环得到.在有氧的酸性条件下,12可以水解单一构型的端基环氧乙烷,得到手性的环氧乙烷和手性的二醇化合物,而且该催化剂的催化活性(收率>42%)和对应选择性(>98% 对映体过量值)都非常好.值得一提的是这种以大环相连的低聚物比以烯烃链直接聚合得到的催化剂催化活性高很多.
2.2 钌
金属钌是一种非常重要的过渡金属元素,它可以催化较多种类的反应.而很多该类催化剂都被负载在无机介孔硅胶材料[37-41]和聚合物材料[42-48]上.在此简单介绍几个比较有代表性的手性钌催化剂.催化剂13[45](图6)是以聚苯乙烯树脂为载体、吡啶双噁唑啉为手性配体与金属钌配位形成的手性负载型催化剂.该催化剂可以有效的催化苯乙烯和重氮乙酸乙酯的环丙化,得到主要为反式的环丙烷产物,ee值高达85%,但是13的催化剂效率较低(收率<40%).Haraguchi课题组在2009年报道了以TsDPEN为手性配体的催化剂14[43](图6),他们选择两类聚苯乙烯载体,分别为疏水性的交联聚苯乙烯和两亲性的交联聚苯乙烯.疏水性的催化剂在有机溶剂(如二氯甲烷、乙腈)可以有效的催化亚胺的氢转移得到手性的仲胺,在水环境中催化效率较差.而两亲性的催化剂在水环境中的催化效率较高,但是在有机环境中基本没有催化活性.催化剂15[42](图6)同样为聚苯乙烯为载体的手性催化剂,它和13类似催化苯乙烯和重氮乙酸乙酯的环丙化反应,而且该催化剂反应活性和对映选择性都非常好(收率>90%,>90% ee).
2.3 铑
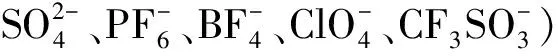
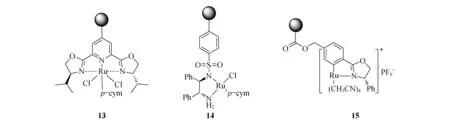
图6 负载型金属钌催化剂
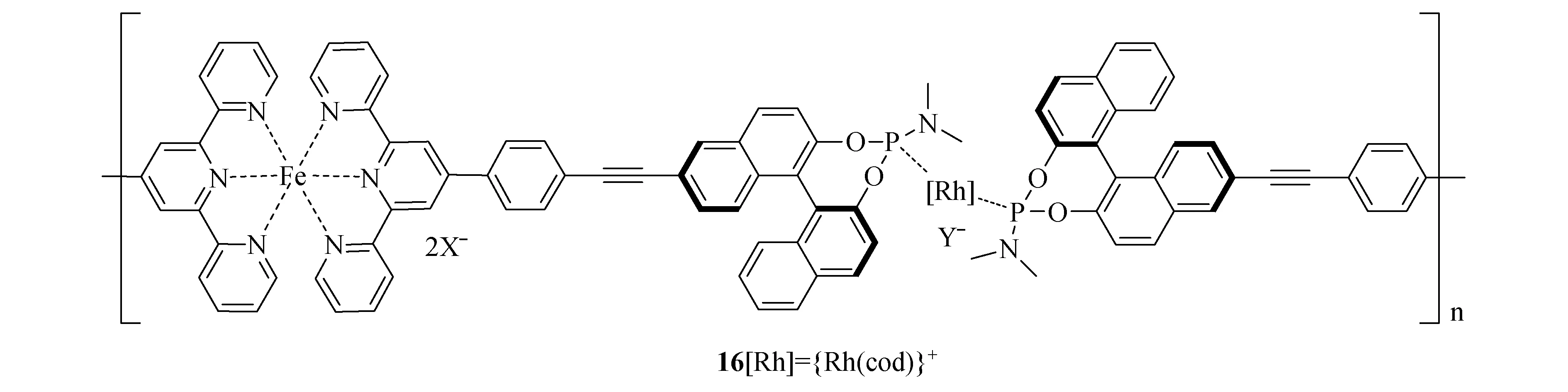
图7 自组装型金属铑催化剂
2.4 其他金属
除了以上介绍的常被用于有机聚合物金属非均相催化剂外,还要一些金属也被化学家们用于有机聚合物的非均相催化.例如铜离子,手性的噁唑啉配体可以聚合成高聚物形成非均相催化剂[55],或者通过多个噁唑啉配体与铜离子配位相连形成类似于MOF的材料[56].这两种催化剂都可以催化苯乙烯和重氮乙酸乙酯的环丙化,但是回收再利用比较差.还有Pd[57-58]和Mo[59-61]都曾被用于有机聚合物非均相催化剂,但是结果比均相的催化剂活性或对映选择性差.
3 展 望
手性催化剂目前正在高速发展的阶段,但是由于成本、环境等问题大大的限制了其实际应用.而非均相催化剂在一定程度上可以克制以上缺点,使手性催化剂慢慢走向工业化.而有机聚合物制备简单、廉价易得,另外有机聚合物种类繁多,具有各种各样的形状,所以有机聚合物非均相手性催化剂具有较好的前景.但是目前其还在起步阶段,存在一些缺点,例如回收再利用次数不如无机介孔负载的手性催化剂多,还有就是聚合物使用单一(多为聚苯乙烯).相信在化学家们的不懈努力下,其一定会走向实际应用、走向工业化.
参考文献:
[1] MERRIFIELD R B.Solid phase peptide synthesis I.The synthesis of a tetrapeptide[J].J Am Chem Soc,1963,85(14):2149-2154.
[2] HUDSON D.Matrix assisted synthetic transformations:a mosaic of diverse contributions II the pattern is completed[J].J Comb Chem,1999,1(6):403-457.
[3] HUDSON D.Matrix assisted synthetic transformations:a mosaic of diverse contributions.I.the pattern emerges[J].J Comb Chem,1999,1(5):333-360.
[4] TOY P H,JANDA K D.Soluble polymer-supported organic synthesis[J].Acc Chem Res,2000,33(8):546-554.
[5] BERGBREITER D E,SUNG S D.Liquid/Liquid Biphasic recovery/reuse of soluble polymer-supported catalysts[J].Adv Synth Catal,2006,348(12-13):1352-1366.
[6] DICKERSON T J,REED N N,JANDA K.D.Soluble polymers as scaffolds for recoverable catalysts and reagents[J].Chem Rev,2002,102(10):3325-3344.
[7] BERGBREITER D E.Using soluble polymers to recover catalysts and ligands[J].Chem Rev,2002,102(10):3345-3384.
[8] PRACEJUS H.Organische Katalysatoren,LXI.Asymmetrische synthesen mit ketenen,I alkaloid-katalysierte asymmetrische synthesen von α-Phenyl-propionsäureestern[J].Justus Liebigs Ann Chem,1960,634(1):9-22.
[9] EDER U,SAUER G,WIECHERT R.New type of asymmetric cyclization to optically active steroid CD partial structures[J].Angew Chem Int Ed,1971,10(7):496-497.
[10] ARAKAWA Y,HARAGUCHI N,ITSUNO S.An immobilization method of chiral quaternary ammonium salts onto polymer supports[J].Angew Chem Int Ed,2008,47(43):8232-8235.
[11] ITSUNO S,PAUL D K,SALAM M A,et al.Main-chain ionic chiral polymers:synthesis of optically active quaternary ammonium sulfonate polymers and their application in asymmetric catalysis[J].J Am Chem Soc,2010,132(9):2864-2865.
[12] PARVEZ M M,HARAGUCHI N,ITSUNO S.Molecular design of chiral quaternary ammonium polymers for asymmetric catalysis applications[J].Org Biomol Chem,2012,10(14):2870-2877.
[13] HARAGUCHI N,AHAMED P,PARVEZ M M,et al.Synthesis of main-chain chiral quaternary ammonium polymers for asymmetric catalysis using quaternization polymerization[J].Molecules,2012,17(6):7569-7583.
[14] PUGLISI A,BENAGLIA M,CINQUINI M,et al.Enantioselective 1,3-dipolar cycloadditions of unsaturated aldehydes promoted by a poly(ethylene glycol)-supported organic catalyst[J].Eur J Org Chem,2004(3):567-573.
[15] RIENTE P,YADAV J,PERICAS M A.A click strategy for the immobilization of macmillan organocatalysts onto polymers and magnetic nanoparticles[J].Org Lett,2012,14(14):3668-3671.
[16] ALLEMANN C,GORDILLO R,CLEMENTE F R,et al.Theory of asymmetric organocatalysis of aldol and related reactions:rationalizations and predictions[J].Acc Chem Res,2004,37(8):558-569.
[17] BUI T,BARBAS C F.A proline-catalyzed asymmetric Robinson annulation reaction[J].Tetrahedron Lett,2000,41(36):6951-6954.
[18] HAYASHI Y,TSUBOI W,ASHIMINE I,et al.The direct and enantioselective,one-pot,three-component,cross-mannich reaction of aldehydes[J].Angew Chem Int Ed,2003,42(31):3677-3680.
[19] LIST B,POJARLIEV P,MARTIN H J.Efficient proline-catalyzed michael additions of unmodified ketones to nitro olefins[J].Org Lett,2001,3(16):2423-2425.
[20] RAMACHARY D B,CHOWDARI N S,BARBAS C F.Amine-catalyzed direct self Diels-Alder reactions of α,β-unsaturated ketones in water:synthesis of pro-chiral cyclohexanones[J].Tetrahedron Lett,2002,43(38):6743-6746.
[21] LIST B.Direct catalytic asymmetric α-amination of aldehydes[J].J Am Chem Soc,2002,124(20):5656-5657.
[22] IMBRIGLIO J E,VASBINDER M M,MILLER S J.Dual catalyst control in the amino acid-peptide-catalyzed enantioselective baylis-hillman reaction[J].Org Lett,2003,5(20):3741-3743.
[23] UTSUMI N,ZHANG H,TANAKA F,et al.A way to highly enantiomerically enriched aza-morita-baylis-hillman-type products[J].Angew Chem Int Ed,2007,46(11):1878-1880.
[24] WANG J,LI H,MEI Y,LOU B,et al.Direct,facile aldehyde and ketone α-selenenylation reactions promoted by l-prolinamide and pyrrolidine sulfonamide organocatalysts[J].J Org Chem,2005,70(14):5678-5687.
[25] BØGEVIG A,SUNDÉN H,CRDOVA A.Direct catalytic enantioselective α-aminoxylation of ketones:a stereoselective synthesis of α-hydroxy and α,α′-dihydroxy ketones[J].Angew Chem Int Ed,2004,43(9):1109-1112.
[26] BROCHU M P,BROWN S P,MACMILLAN D W C.Direct and enantioselective organocatalytic α-chlorination of aldehydes[J].J Am Chem Soc,2004,126(13):4108-4109.
[27] BENAGLIA M,CINQUINI M,COZZI F,et al.Poly(ethylene glycol)-supported proline:a versatile catalyst for the enantioselective aldol and iminoaldol reactions[J].Adv Synth Catal,2002,344(5):533-542.
[28] BENAGLIA M,CELENTANO G,COZZI F.Enantioselective aldol condensations catalyzed by poly(ethylene glycol)-supported proline[J].Adv Synth Catal,2001,343(2):171-173.
[29] BENAGLIA M,CINQUINI M,COZZI F,et al.Poly(ethylene-glycol)-supported proline:a recyclable aminocatalyst for the enantioselective synthesis of γ-nitroketones by conjugate addition[J].J Mol Catal A:Chem,2003,204-205,157-163.
[30] FONT D,JIMENO C,PERICS M A.Polystyrene-supported hydroxyproline:an insoluble,recyclable organocatalyst for the asymmetric aldol reaction in water[J].Org Lett,2006,8(20):4653-4655.
[31] FONT D,SAYALERO S,BASTERO A,et al.Toward an artificial aldolase[J].Org Lett,2007,10(2):337-340.
[32] FONT D,BASTERO A,SAYALERO S,et al.Highly enantioselective α-aminoxylation of aldehydes and ketones with a polymer-supported organocatalyst[J].Org Lett,2007,9(10):1943-1946.
[33] ALZA E,CAMBEIRO X C,JIMENO C,et al.Highly enantioselective michael additions in water catalyzed by a PS-saupported pyrrolidine[J].Org Lett,2007,9(19):3717-3720.
[34] GIACALONE F,GRUTTADAURIA M,MARCULESCU A M,et al.Polystyrene-supported proline and prolinamide.Versatile heterogeneous organocatalysts both for asymmetric aldol reaction in water and α-selenenylation of aldehydes[J].Tetrahedron Lett,2007,48(2):255-259.
[35] ANNIS D A,JACOBSEN E N.Polymer-supported chiral co(salen)complexes:synthetic applications and mechanistic investigations in the hydrolytic kinetic resolution of terminal epoxides[J].J Am Chem Soc,1999,121(17):4147-4154.
[36] ZHENG X,JONES C W,WECK M.Ring-expanding olefin metathesis:a route to highly active unsymmetrical macrocyclic oligomeric co-salen catalysts for the hydrolytic kinetic resolution of epoxides[J].J Am Chem Soc,2007,129(5):1105-1112.
[37] LIU G,SUN Y,WANG J,et al.Microwave-assisted tandem allylation-isomerization reaction catalyzed by a mesostructured bifunctional catalyst in aqueous media[J].Green Chem,2009,11(9):1477-1481.
[38] LIU G,WANG J,HUANG T,et al.Mesoporous silica-supported iridium catalysts for asymmetric hydrogenation reactions[J].J Mater Chem,2010,20(10):1970-1975.
[39] LIU G,GU H,SUN Y,et al.Magnetically recoverable nanoparticles:highly efficient catalysts for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium[J].Adv Synth Catal,2011,353(8):1317-1324.
[40] SUN Y,LIU G,GU H,et al.Magnetically recoverable SiO2-coated Fe3O4nanoparticles:a new platform for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium[J].Chem Commun,2011,47(9):2583-2585.
[41] ZHANG H,JIN R,YAO H,et al.Core-shell structured mesoporous silica:a new immobilized strategy for rhodium catalyzed asymmetric transfer hydrogenation[J].Chem Commun,2012,48(63):7874-7876.
[42] ABU-ELFOTOH A-M,PHOMKEONA K,SHIBATOMI K,et al.Asymmetric inter- and intramolecular cyclopropanation reactions catalyzed by a reusable macroporous-polymer-supported chiral ruthenium(II)/phenyloxazoline complex[J].Angew Chem Int Ed,2010,49(45):8439-8443.
[43] HARAGUCHI N,TSURU K,ARAKAWA Y,et al.Asymmetric transfer hydrogenation of imines catalyzed by a polymer-immobilized chiral catalyst[J].Org Biomol Chem,2009,7(1):69-75.
[44] FERRAND Y,LE MAUX P,SIMONNEAUX G.Macroporous chiral ruthenium porphyrin polymers:a new solid-phase material used as a device for catalytic asymmetric carbene transfer[J].Tetrahedron-Asymmetry,2005,16(23):3829-3836.
[45] CORNEJO A,FRAILE J M,GARCA J I,et al.The first immobilization of pyridine-bis(oxazoline)chiral ligands[J].Org Lett,2002,4(22):3927-3930.
[46] LEE S-H,KIM H-J,CHOI D,et al.Highly purified cyclic olefin polymer by ROMP;Insituhydrogenation with ruthenium supported catalyst[J].Macromol Res,2012,20(8):777-779.
[47] DENG J,LU C,YANG G,et al.Preparation of microgel-supported chiral catalysts and their application in the asymmetric hydrogenation of aromatic ketones[J].React Funct Polym,2012,72(6):378-382.
[48] TERASHIMA T,OUCHI M,ANDO T,et al.Transfer hydrogenation of ketones catalyzed by PEG-armed ruthenium-microgel star polymers:microgel-core reaction space for active,versatile and recyclable catalysis[J].Polym J,2011,43(9):770-777.
[49] OOHARA T,NAMBU H,ANADA M,et al.A polymer-supported chiral fluorinated dirhodium(II)complex for asymmetric amination of silyl enol ethers[J].Adv Synth Catal,2012,354(11-12):2331-2338.
[50] TAKEDA K,OOHARA T,ANADA M,et al.A Polymer-supported chiral dirhodium(II)complex:highly durable and recyclable catalyst for asymmetric intramolecular C-H insertion reactions[J].Angew Chem Int Ed,2010,49(39):6979-6983.
[51] CANDEIAS N R,AFONSO C A M,GOIS P M P.Making expensive dirhodium(ii)catalysts cheaper:Rh(ii)recycling methods[J].Org Biomol Chem,2012,10(17):3357-3378.
[52] YU L,WANG Z,WU J,et al.Directed orthogonal self-assembly of homochiral coordination polymers for heterogeneous enantioselective hydrogenation[J].Angew Chem Int Ed,2010,49(21):3627-3630.
[53] CORKUM E G,HASS M J,SULLIVAN A D,et al.A highly reusable rhodium catalyst-organic framework for the intramolecular cycloisomerization of 1,6-enynes[J].Org Lett,2011,13(13):3522-3525.
[54] ARAKAWA Y,CHIBA A,HARAGUCHI N,et al.Asymmetric transfer hydrogenation of aromatic ketones in water using a polymer-supported chiral catalyst containing a hydrophilic pendant group[J].Adv Synth Catal,2008,350(14-15):2295-2304.
[55] BURGUETE M I,FRAILE J M,GARCA-VERDUGO E,et al.Polymer-supported bis(oxazolines)and related systems:toward new heterogeneous enantioselective catalysts[J].Ind Eng Chem Res,2005,44(23):8580-8587.
[56] GARCIA J I,LOPEZ-SANCHEZ B,MAYORAL J A.Linking homogeneous and heterogeneous enantioselective catalysis through a self-assembled coordination polymer[J].Org Lett,2008,10(21):4995-4998.
[57] SONG C E,YANG J W,ROH E J,et al.Heterogeneous pd-catalyzed asymmetric allylic substitution using resin-supported trost-type bisphosphane ligands[J].Angew Chem Int Ed,2002,41(20):3852-3854.
[58] NAKANO H,TAKAHASHI K,FUJITA R.Polymer-supported chiral phosphinooxazolidine ligands for palladium-catalyzed asymmetric allylic alkylations and Diels-Alder reactions[J].Tetrahedron-Asymmetry,2005,16(12):2133-2140.
[59] KROLL R M,SCHULER N,LUBBAD S,et al.A ROMP-derived,polymer-supported chiral Schrock catalyst for enantioselective ring-closing olefin metathesis[J].Chem Commun,2003,12(1):2742-2743.
[60] WANG D,KROELL R,MAYR M,et al.Polymer-supported chiral Schrock catalysts immobilized via the arylimido ligand[J].Adv Synth Catal,2006,348(12-13):1567-1579.
[61] HULTZSCH K C,JERNELIUS J A,HOVEYDA A H,et al.The first polymer-supported and recyclable chiral catalyst for enantioselective olefin metathesis[J].Angew Chem Int Ed,2002,41(4):589-590.

