Synthesis of shape-controlled ZnSn(OH)6 and gas sensing properties
HAN Lixian,DU Mengjuan,LI Yusheng,YE Bin,YU Xibin*
(1.College of life and Environment Sciences,Shanghai Normal University,Shanghai 200234,China;2.Department of Applied Mathematics,Shanghai University of Finance and Economics,Shanghai 200433,China)
1 Introduction
The fabrication of nano-or micro-size particles with controlling morphologies,orientations,and dimensionalities has attracted much attention,because the physical and chemical properties of materials can be strongly influenced by their sizes and shapes[1-11].The study of composite metal oxides(CMOs)is always interesting to researchers because their performances are superior over single metal oxides in many cases[12-13],such as gas-sensor materials.For instance,polyhedral zinc hydroxystannate(ZHS),CdIn2O4[14]and EuFeO3nanoparticles have been used as gas-sensor materials[15].
Zinc hydroxystannate(ZHS),an important member of CMOs,is a kind of perovskite structure tending to form face-centered-cubic(FCC)crystal structure.Up to now,zinc hydroxystannate has been widely used as fire retardant and smoke inhibitor,photocatalyst,inorganic filler,and flame-retardant[15-18].Furthermore,ZHS thermal decomposition products including ZnSnO3[19-20],crystalline SnO2and Zn2SnO4can be used in lithium ion battery anodes,gas sensors and photocatalysts[19-20].
Nano-microparticles of ZHSwith different shapes such as nanocage,14-faceted polyhedra,and wire-like have been synthesized by various synthesis routes,including thermal decomposition[21]hydrothermal synthesis[22-23]low temperature ion exchange[24]and different surfactants have assisted wet-chemical methods at low temperature[25].However,the synthesis strategies mentioned above usually need complex operating procedures,expensive raw materials and further heat treatment.Considering their excellent features,such as high sensitivity,short recovery time and good reproducibility,a novel route is highly required for the fabrication of the hierarchical architectures assembled with nanostructured building blocks of ZHS.So far,the preparations of ZHS through low-cost,convenient routes are still a challenge.In addition,to our knowledge,the shapecontrolled synthesis and the gas sensing investigation of ZHS MCs with different shapes and a facile method have not been reported,especially ZHSMCs self-assembled by nanoparticles.
Herein,we demonstrate that different shapes of ZHS(microcube,microsphere)with uniform size could be on a large scale through precipitation process.It is worthy to note that neither complicated steps nor advanced experimental conditions or equipments were used,making this process easy.Then,the formation mechanisms of the ZHScrystallites were studied via a series of time-dependent experiments.In the following section,UV-vis absorption spectra have been used to discuss the influences of gas sensor based on ZHSMCs including oxygen vacancy defects,the gas adsorption,band gap energy,and active surface area.However,the band gap energy of spherical ZHScrystallites was less than that of nanocubic crystallites.It is clearly that the sensors based on spherical ZHSMCs exhibited faster response,higher sensitivity,and shorter recovery times toward HCHO gas than those based on ZHS nancubic ZHS because of more oxygen vacancy defects,less band gap energy,and larger active surface area.The as-prepared MCs should be significant for exploiting new gas-sensing materials in the future.
2 Experimental Section
2.1 Materials
All reagents were analytic grade from Aladdin reagent(China)Co.,LtdS.and used as received without further purification.Deionized Water(PURELAB Plus,PALL)with a resistivity of 18 M?cm was used throughout.
2.2 Preparation of ZHS Cubic Crystallites
In a typical experiment,2.8754 g(10 mmol)of zinc sulfate heptahydrate(ZnSO4.7H2O)was added into 100 mL of deionized water(DW),and the solution was stirred at room temperature until ZnSO4.7H2O was dissolved completely.Then appropriate sodium stannate trihydrate(Na2SnO3.3H2O)solution was dropped into ZnSO4.7H2O solution,making the molar ration of[Zn]/[Sn]=1∶1.After the completion of the above steps,the mixed solution was stirred for 5 h.After the reaction,the precipitates were collected by centrifugation,washed with DWfor several times to remove residual ions in the products.The final products were then dried in air at 100℃ for 8 h before characterization.
2.3 Preparation of ZHS Spherical Crystallites
Before adding Na2SnO3.3H2O solution,the adequate ammonia was dropped into the ZnSO4solution.The other synthesizing process was the same as the preparation of ZHSnanocubic crystallites.
2.4 General Characterization
X-ray powder diffraction(XRD)pattern was recorded using a Japan Regaku D/max cA X-ray diffractometer equipped with graphite monochromatized Cu Kα radiation(λ =1.5418Å)irradiated with a scanning rate of 4 deg/min.The Field-emission scanning electron microscopic(FESEM)images were obtained using a JEOL JSM-7500F microscope operated at an acceleration voltage of 15 kV.A JEOL JEM-200CX microscope operating at 160 kV in the bright-field mode was used for Transmission Electron Microscopy(TEM).Selected area electron diffraction(SAED)pattern was performed on a JEOL JEM-2010 electron microscope operating at 200 kV.
2.5 Measurements of gas-sensing
The gas-sensing properties were measured using a static test system of WS-60A made by Hanwei Electronics Co.Ltd.,Henan Province,China.The sensors were fabricated by a modifying method described in the reference[20].Before the sensitivity measurement,the samples were connected to the 5 V dc source and the heat voltage was maintained at 5 V till the stabilization of the base line voltage.
In our gas-sensing measurements,a given amount of test gas was injected into a closed chamber,and the sensor was put into the chamber for the measurement of the sensitive performance.After each measurement,the sensor was exposed to the atmospheric air by opening the chamber.Sensitivity was defined as S=Ra/Rg,where Rawas the average resistance in air and Rgwas the average resistance in the test gas.The response and recovery time were defined as the time taken by the sensor to achieve 90%of the total resistance change in the case of adsorption and desorption,respectively.
3 Results and Discussion
3.1 Structural Characterization
The composition and phase purity of the as-obtained products are first examined by X-ray powder diffraction(XRD)patterns.Fig.1 shows the XRD patterns of typical ZHSwith microcube(a)and microsphere(b),respectively.The XRD patterns of the ZHSpowders are quite similar.All of the diffraction peaks can be indexed to the standard ZHS with the perovskite structure(JCPDS No.20-1455),confirming that the assynthesized samples have a typical FCC crystal structure.According to data of XRD,the lattice parameters calculated via the XRD data for ZHSmicrocube and microsphere are corresponding to 7.7656 Å and 7.7456 Å,respectively.Peaks at around 22.8,40.2,and 52.6 correspond to the(200),(220),and(420)planes of the as-obtained products.By comparison,the diffraction peak intensity of sphereical product is stronger than that of microcube.No impurity phases are detected from the XRD pattern,indicating that two types ZHS crystallites with high purity could be obtained under current synthetic conditions.
3.2 Morphology Characterization
Many researchers have reported ZHS structures with different morphologies could be obtained hydrothermally under appropriate reaction conditions[22-23].In this work,the microcube ZHScrystallites were obtained simply by the reaction between ZnSO4.7H2O and Na2SnO3·3H2O,while the spherical ZHS MCs were obtained by introducing NH4OH into the reaction system.
The morphology and structure details of the as-obtained ZHSproducts are investigated by SEM,FESEM,and TEM techniques.It can be clearly seen from Fig.2A that the ZHSproducts are entirely composed of a large-scale of uniform and monodisperse MCs.High-magnification FESEM image(Fig.2B)shows that the products consist of homogeneous nanocubes with side lengths of about 500-600 nm.A representative TEM micrograph of cubic ZHScrystallites is shown in Fig.2C,clearly showing that the single nanoparticle has perfect cubic profile with very clear edges and corners.The corresponding selected area electron diffraction(SAED)pattern of cubic crystallites(as shown in Fig.2D)confirms that the nanocubes have good crystallinity and there is no secondary phase.The corresponding selected area electron diffraction(SAED)pattern of cubic crystallites(as shown in Fig.2D)confirms that the nanocubes have well-crystallized structure.The diffraction spots of the typical FCC crystal structure could be indexed to{020},{220}and{200}panels of the ZnSn(OH)6(ZHS)microcrystallites.
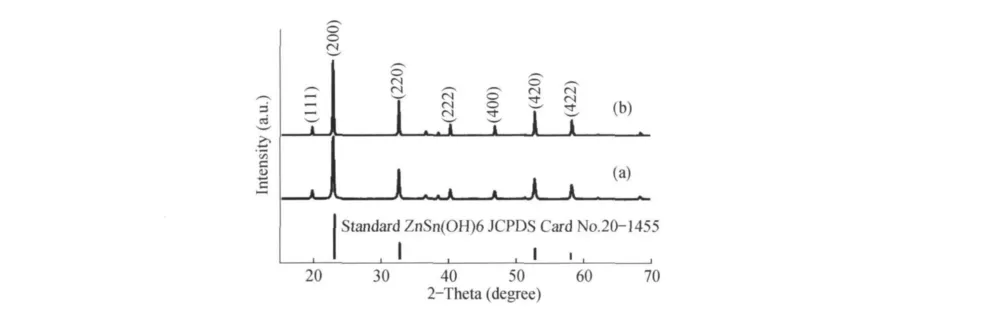
Figure 1 XRD patterns of the as-prepared ZHSproducts:nanocube(a),nanosphere(b)and Standard XRD pattern of ZHS(JCPDSNo.20-1455)
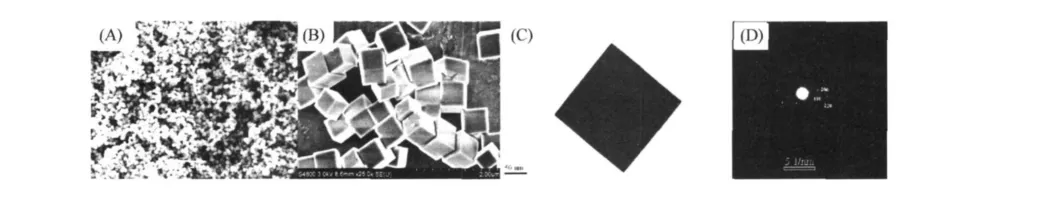
Figure 2 SEM,TEM and SAED images of the ZHSnanocubic crystallites:(A)low magnification SEM image;(B)high magnification FESEM image;(C)high magnification TEM image;(D)SAED pattern
In the further process,spherical ZHSMCs were obtained with adding appropriate amount of ammonia in the reaction solution.Fig.3A shows the panoramic morphologies of the typical sample.The results indicate that the product consists of monodisperse spherical crystallites with glossy surface in the size range of 500~600 nm.HoweverF,high magnification FESEM images(Fig.3B and 3C)display that the as-obtained spherical ZHS crystallite is not as smooth as the former shown(Fig.3A).A clear grain boundary can be observed on the surface of ZHSmicrospheres(Fig.3B and C),indicating that the as-obtained spherical ZHS crystallites are constituted by the oriented aggregation of small ZHSnanoparticles.More structure information of spherical ZHScrystallites is researched by TEM.As shown in high magnification TEM images(Fig.3C),the surface of the as-obtained ZHS spherical crystallites is rough.Many nanoparticles attach on the surface of ZHSmicrospheres.It also demonstrates the as-obtained spherical ZHScrystallites are composed of small ZHSnanoparticles with diameter of 5~10 nm diameter,which validates the observation results of FESEM tests(Fig.3B).The selected area electron diffraction(SAED)pattern(Inset Fig.3C)taken from the edge of the sphere marked by a circle exhibits the clear diffraction lattices,revealing the single-crystalline nature of the sample with a preferential grown direction.The diffraction spots could also be indexed to{020},{220},and{200}panels of the ZnSn(OH)6(ZHS)microcrystallites.
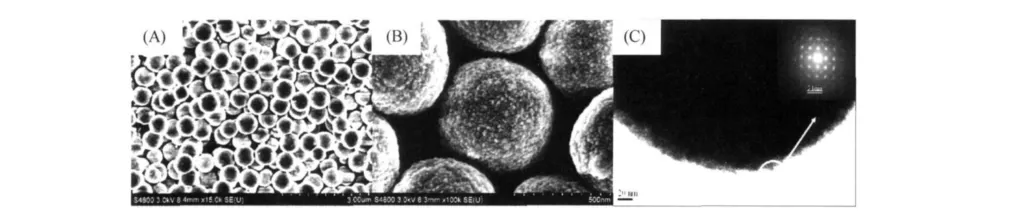
Figure 3 SEM and TEM images of the spherical ZHSMCs:(A)low magnification FESEM image;(B)high magnification FESEM image;(C)high magnification TEM image,inset in(C)SAED pattern
3.3 Optical Absorption and BET
The optical absorption properties of ZHS MCs semiconductor were measured by The UV-vis absorption spectra.As shown in Fig.4,the Egof two samples are 5.02eV(microcube)and 4.90eV(microsphere)which are calculated on the basis of the corresponding absorption edges.This indicates that the spherical ZHS MCs have less band gap energy,which may help the O2adsorption on the ZHS surface to trap electrons from the conduction band of ZHS and enhance the sensing performance.The surface area of these morphologies are shown in the Table 1.The surface area of spherical ZHS MCs is larger than that of the microcubic shape(as shown in Table 1).It is reported that″surface accessibility″becomes one of crucial factors to maintain the high sensitivity of the gas sensor[26]. Hence,the sensor based on spherical ZHS MCs should exhibit higher sensitivity.

Figure 4 The UV-visible absorption spectrum of(a)nanocube and(b)nanosphere samples
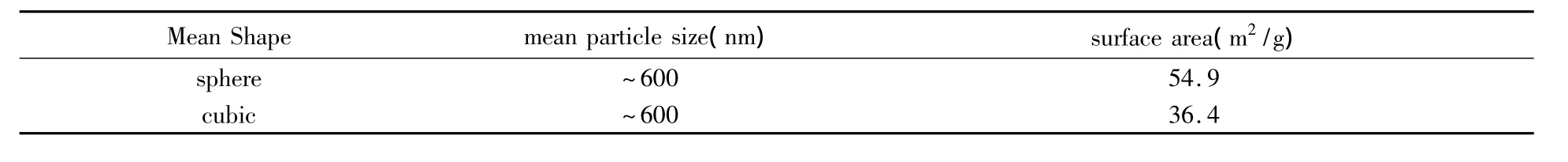
Table 1 Active surface srea of ZHSMCs
3.4 Gas sensor performance
According to the sensing mechanism of Wolkenstein’s model for semiconductors[27],when the sensors are exposed to air,the surface of ZHSsensors could adsorb oxygen species to ionize into O-(ads)or O2-(ads).This is because oxygen atom owns the strong electronegativity from the conduction band of ZHS.Hence,the concentration of electrons in the conduction band would decrease and the resistance of the material would increase.And then a chemical reaction would take place between HCHO and O2-(ads),which leads to a relatively strong activation on the surface of the ZHS:
HCHO(gas)+O2-(ads)→H2O(g)+CO2(g)+2e-.
As to say,the oxygen vacancy,the band gap energy,and the active surface area may have affects on the gas sensor.spherical ZHS MCs may present better gas sensor performance compared with cubic ZHS MCs,because spherical ZHSMCs have more oxygen vacancy,less band gap energy,and larger BET surface area.
Gas sensor performance based on ZHS MCs to HCHO with the similar size of microcube(line a)and microsphere(line b)are Shown in the Fig.5.The typical response curves of ZHS-based gas sensors with different shapes to increasing concentration of HCHOare shown in the Fig.5A.It is obvious that the sensitivity of gas sensors increases rapidly with the increase of HCHO concentrations,revealing that the sensitivities of the ZHS-based gas sensors are excellent to HCHO.But the sensitivity of the spherical ZHS MCs increases faster than that of the cubic MCs with the same response time and the same HCHO concentration.
As shown in Fig.5B,it is clear that ZHSbased sensors have a wide detection range for HCHO(from10 to 100 ppm).The sensitivity of the spherical ZHSMCs especially increases faster with the same concentration of HCHO.At the same time,the recovery time of sensor based on spherical ZHS sphere MCs is shorter.Hence,the sensors based on spherical ZHS MCs are much more sensitive than those based on ZHS cubic MCs.The detection limit of the as-prepared ZHS sensors can reach as low as several parts per million for HCHO.Meanwhile,the recovery time of sensor based on spherical ZHSsphere MCs is the shortest.
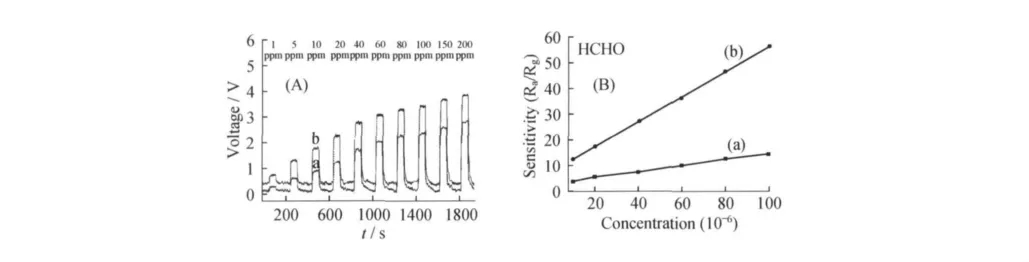
Figure 5 (A)Sensitivities of the sensors based on ZHSMCs with different shapes to increased concentrations of HCHO.(B)Typical response curves of ZHSNCs sensors of different shapes to HCHO with increasing concentrations.In parts A and B,(a)and(b)correspond to the ZHSNCs of nanocube and nanosphere,respectively
4 Conclusion
In conclusion,the successful synthesis of uniform ZHSMCs with different shapes via a facile process was proposed.It was found that the micro-cube morphology evolved to microsphere with adding different concentrations of NH4OH.The gas sensors based on both of the two morphologies exhibited good sensor performance toward HCHO gas.The sensor based on spherical ZHS MCs demonstrated faster response,higher sensitivity and shorter recovery time owing to more oxygen vacancy defects,less band gap energy,and larger active surface area.The as-synthesized ZHSMCs make them ideal candidates for HCHO gas-sensing devices.
[1]PENG X G,MANNA L,YANG W D,et al.Shape control of CdSe nanocrystals[J].Nature,2000,404:59-61.
[2]BUDAI J D,WHITE C W,WITHROW S P,et al.Controlling the size,structure and orientation of semiconductor nanocrystals using metastable phase recrystallization [J].Nature,1997,390:384-386.
[3]COZZOLI P D,MANNA L,CURRI M L,et al.Shape and phase control of colloidal ZnSe nanocrystals[J].Chemistry of Materials,2005,17(6):1296-1306.
[4]GONG Q,QIAN X,CAO H,et al.Novel shape evolution of BaMoO4microcrystals[J].The Journal of Physical Chemistry B,2006,110(39):19295-19299.
[5]TIAN Y,LIU H,ZHAO G,et al.Shape-controlled electrodeposition of gold nanostructures[J].The Journal of Physical Chemistry B,2006,110(46):23478-23481.
[6]FANG X,BANDO Y,YE C,et al.Shape-and size-controlled growth of ZnS nanostructures[J].The Journal of Physical Chemistry C,2007,111(24):8469-8474.
[7]NANDWANA V,ELKINSK E,POUDYAL N,et al.Size and shape control of monodisperse FePt nanoparticles[J].The Journal of Physical Chemistry C,2007,111(11):4185-4189.
[8]TAN T T,SELVAN ST,ZHAO L,et al.Size control,shape evolution,and silica coating of near-infrared-emitting PbSe quantum dots[J].Chemistry of Materials,2007,19(13):3112-3117.
[9]WANG F,TANGR,YUH,et al.Size-and shape-controlled synthesis of bismuth nanoparticles[J].Chemistry of Materials,2008,20(11):3656-3662.
[10]ZHANG H,XU JJ,CHEN H Y.Shape-controlled gold nanoarchitectures:synthesis,superhydrophobicity,and electrocatalytic properties[J].The Journal of Physical Chemistry C,2008,112(36):13886-13892.
[11]BAO N,SHEN L,AN W,et al.Formation mechanism and shape control of monodisperse magnetic CoFe2O4nanocrystals[J].Chemistry of Materials,2009,21(14):3458-3468.
[12]WHITBY R L D,BRIGATTI K S,KINLOCH I A,et al.Novel Mg2SiO4structures[J].Chemical Communications,2004(21):2396-2397.
[13]ZHANG T,JIN C G,QIAN T,et al.Hydrothermal synthesis of single-crystalline La0.5Ca0.5MnO3nanowires at low temperature[J].Journal of Materials Chemistry,2004,14:2787-2789.
[14]MAHANUBHAV M D,PATIL L A.Studies on gas sensing performance of CuO-modified CdIn2O4thick film resistor[J].Sensors and Actuators B:Chemical,2007,128(1):186-192.
[15]SIEMONSM,SIMON U.High throughput screening of the propylene and ethanol sensing properties of rare-earth orthoferrites and orthochromites[J].Sensors and Actuators B:Chemical,2007,126(1):181-186.
[16]YANG L,HU Y,YOU F,et al.A novel method to prepare zinc hydroxystannate-coated inorganic fillers and its effect on the fire properties of PVC cable materials[J].Polymer Engineering & Science,2007,47(7):1163-1169.
[17]ZHANG B,JIAO Y,XU J Z.A study on the flame-retardance of poly(vinyl chloride)incorporated with metal hydroxystannates[J].Journal of Applied Polymer Science,2009,112(1):82-88.
[18]FU X,WANG X,DING Z,et al.Hydroxide ZnSn(OH)6:A promising new photocatalyst for benzene degradation[J].Applied Catalysis B:Environmental,2009,91(1-2):67-72.
[19]RONG A,GAO X P,LI G R,et al.Hydrothermal synthesis of Zn2SnO4as anode materials for Li-ion battery[J].The Journal of Physical Chemistry B,2006,110(30):14754-14760.
[20]ZHANG W H,ZHANG W D.Fabrication of SnO2– ZnO nanocomposite sensor for selective sensing of trimethylamine and the freshness of fishes[J].Sensors and Actuators B:Chemical,2008,13(2):403-408.
[21]WROBEL G,PIECH M,DARDONA S,et al.Seedless synthesis and thermal decomposition of single crystalline zinc hydroxystannate cubes[J].Crystal Growth & Design,2009,9(10):4456-4460.
[22]ZHANG Y,GUO M,ZHANG M,et al.Hydrothermal synthesis and characterization of single-crystalline zinc hydroxystannate microcubes[J].Journal of Crystal Growth,2007,308(1):99-104.
[23]FAN H,AI S,JU P.Room temperature synthesis of zinc hydroxystannate hollow core-shell microspheres and their hydrothermal growth of hollow core-shell polyhedral microcrystals[J].Cryst Eng Comm,2011,13:113-117.
[24]KOVACHEVA D,PETROV K.Preparation of crystalline ZnSnO3from Li2SnO3by low-temperature ion exchange[J].Solid State Ionics,1998,109(3-4):327-332.
[25]WANG L,TANG K,LIU Z,et al.Single-crystalline ZnSn(OH)6hollow cubes via self-templated synthesis at room temperature and their photocatalytic properties[J].Journal of Materials Chemistry,2011,21:4352-4357.
[26]GENG B,FANG C,ZHAN F,et al.Synthesis of Polyhedral ZnSnO3Microcrystals with Controlled Exposed Facets and Their Selective Gas-Sensing Properties[J].Small,2008,4(9):1337-1343.
[27]HAICK H,AMBRICO M,LIGONZO T,et al.Controlling semiconductor/metal junction barriers by incomplete,nonideal molecular monolayers[J].Journal of the American Chemical Society,2006,128(21):6854-6869.

