4-HNE通过抑制TNF-α介导的NF-κB活化诱导酒精性肝损伤*
于晨辉, 杜仲燕, 高 佳, 王伟茜, 窦晓兵
(浙江中医药大学生命科学学院,浙江 杭州 310053)
4-HNE通过抑制TNF-α介导的NF-κB活化诱导酒精性肝损伤*
于晨辉, 杜仲燕, 高 佳, 王伟茜, 窦晓兵△
(浙江中医药大学生命科学学院,浙江 杭州 310053)
目的通过体外细胞及体内动物实验,研究肿瘤坏死因子α(TNF-α)诱导的4-羟基壬烯酸(4-HNE)致敏肝细胞发生死亡的作用及机制。方法以人肝细胞株HepG2及小鼠原代肝细胞为细胞模型,通过乳酸脱氢酶(LDH)释放及MTT比色法检测4-HNE对TNF-α诱导的肝细胞死亡的作用,利用Western blotting技术检测细胞内4-HNE与蛋白质形成的加合物水平,通过Western blotting和ELISA技术检测细胞核内NF-κB(p65)的表达及其与DNA结合活性。以C57BL/6小鼠为动物模型,利用HE染色、ELISA、Western blotting及TUNEL等技术,检测长期摄入酒精前后动物肝组织形态、甘油三酯(TG)水平、4-HNE水平、TNF-α水平及血浆丙氨酸氨基转移酶(ALT)活性的变化。结果(1) 4-HNE可以显著增加HepG2细胞及小鼠原代肝细胞对TNF-α杀伤作用的敏感性,从而使TNF-α诱导4-HNE致敏的肝细胞死亡。(2) 4-HNE可显著提高HepG2细胞内4-HNE-蛋白质加合物的水平。(3) 4-HNE抑制HepG2细胞内TNF-α介导的NF-κB活化。(4) 长期摄入酒精导致小鼠肝细胞内4-HNE和TNF-α水平升高,引起肝细胞内TG水平升高,血浆ALT活性升高,肝细胞死亡增多。结论长期摄入酒精使肝细胞发生氧化应激,其产物4-HNE可作为一种肝细胞致敏因子,通过抑制肝细胞内TNF-α介导的NF-κB抗细胞凋亡信号通路,诱导酒精性肝损伤。这可能是一种新的酒精性肝病发病机制。
酒精性肝病; 4-羟基壬烯酸; 肿瘤坏死因子α; 核因子κB; 肝细胞
酒精性肝病(alcoholic liver disease, ALD)是由于长期过度饮酒导致的肝脏疾病[1]。近年来我国ALD的发病率及死亡率也呈逐年增长趋势,已成为仅次于病毒性肝炎的第二大肝病。有研究显示,酒精性肝硬化占肝硬化发病总数的比例已从1999年的10.8%上升为2003年的24.0%[2-3]。虽然许多研究已证实,氧化应激在ALD的发生发展过程中起着十分关键的作用,抗氧化剂对ALD具有一定的预防作用[4],但是ALD的发病机制复杂,氧化应激可以影响细胞内许多信号调控通路[5],其具体机制尚未完全明确,很大程度上限制了抗氧化剂在ALD临床治疗上的应用。目前为止,没有任何能够安全有效治疗ALD的临床用药。长期摄入酒精导致肝细胞发生氧化应激和脂质过氧化反应,4-羟基壬烯酸(4-hydroxynonenal, 4-HNE)是氧化应激和脂质过氧化反应的主要产物之一,在ALD的发生发展过程中起着至关重要的作用[6]。许多临床研究、流行病学调查及动物实验研究均表明,ALD患者血浆中4-HNE水平显著增加。此外,1989年,McClain等最初发现酒精性肝炎患者体内的肿瘤坏死因子α(tumor necrosis factor α, TNF-α)明显高于正常人[7]。TNF-α具有多种生物学功能,在生理状态下,TNF-α介导的抗凋亡通路的活化使肝细胞对TNF-α处于耐受状态。然而在酒精等某些病理因素作用下,肝细胞可以变得对TNF-α敏感,导致细胞的死亡[8]。以上研究提示,长期摄入酒精可产生氧化应激,而氧化应激是诱发肝细胞死亡的重要触发机制,4-HNE作为其主要产物之一,是否也可以通过增加肝细胞对TNF-α介导的细胞毒作用的敏感性,诱导肝细胞死亡呢?在本研究中我们采用人肝细胞株HepG2和小鼠原代肝细胞作为细胞模型、长期摄入乙醇的C57BL/6小鼠作为动物模型,检测4-HNE对TNF-α诱导的肝细胞死亡的作用,从细胞信号调控及蛋白质翻译后修饰水平进一步揭示ALD的发病机制,为ALD的临床治疗提供新的策略,也为以4-HNE为靶点的新型药物研发提供重要的理论指导和依据。
材 料 和 方 法
1材料
1.1动物 8周龄雄性C57BL/6小鼠,体重(25.0±0.5)g,由Jackson Laboratory提供。
1.2细胞 人肝细胞株HepG2购自中科院上海细胞库;小鼠原代肝细胞购自Celsis IVT。
1.3试剂 4-HNE购自Cayman Chemical。TNF-α购自Perro Tech。LDH测试盒购自Key Gen。β-actin 抗体购自Santa Cruz。4-HNE抗体购自R&D。NF-κB(p65)抗体购自CST。ELISA试剂盒购自Pierce。其它实验试剂均购自Sigma Aldrich。
2方法
2.1LDH测定 按2×108/L细胞密度制备单细胞悬液,每孔1 mL接种于24孔培养板中,每组重复3孔进行细胞培养及药物孵育。根据LDH检测试剂盒说明测定细胞培养液中LDH的活性,分析各组间的细胞毒性作用。
2.2MTT比色法 制备单细胞悬液,细胞按2×108/L密度接种于96孔培养板内,每孔100 μL,每组重复5孔。置37 ℃、5%CO2培养箱中培养24 h,待细胞贴壁后,弃掉原培养基,向每孔加入含4-HNE的培养基100 μL,预处理2 h, 再加入TNF-α孵育16 h。加入20 μL MTT在培养箱内培养4 h后,弃去孔中所有液体,各孔加入150 μL DMSO,振荡10 min后在酶标仪570 nm波长处测定吸光度。
2.3Western blotting 细胞培养及药物孵育后,PBS洗涤细胞2次,加入蛋白裂解液,置冰浴裂解30 min。以12 000 ×g、4 ℃离心10 min。取上清,用BCA试剂盒测定提取液中的蛋白质浓度。将各组调整至相同蛋白浓度后,取20 μg样本经SDS-PAGE电泳、转移电泳、抗体免疫、显影、曝光等步骤检测蛋白表达情况(β-actin作为内参照)。
2.4ELISA检测 细胞培养药物孵育后按试剂盒(碧云天)说明书操作抽提核蛋白,根据ELISA试剂盒(Pierce)说明书操作,经包被,样本孵育,封闭,Ⅰ抗、Ⅱ抗孵育,显色,终止等步骤检测细胞核内NF-κB(p65)与96孔板中特异性DNA序列的结合活性。
2.5动物模型制备 采用含乙醇的液体饲料(alcohol fed,AF)及不含乙醇但含有与AF组相同热卡的对照组饲料(pair fed,PF)喂饲小鼠5周(试喂1周后,从第2周开始乙醇液体饲料中乙醇含量从占总热卡的30%递增到36%,每周增加2%),以建立正常对照组(PF)和ALD模型组(AF)。小鼠的进食量和体重每天、每周分别记录,5周后处死小鼠,提取并保存肝组织和血浆。
3统计学处理
利用SPSS 10.0统计软件,数据以均数±标准差(mean±SD)表示,组间均数比较采用单因素方差分析。以P<0.05为差异有统计学意义。
结 果
14-HNE显著增加肝细胞对TNF-α杀伤作用的敏感性
用4-HNE(0~60 μmol/L)预处理肝细胞2 h,再加入40 μg/L TNF-α孵育16 h,LDH检测结果显示,40 μg/L TNF-α或20 μmol/L 4-HNE单独作用HepG2肝细胞,细胞活性与对照组相比无明显差异(P>0.05),若先用20 μmol/L 4-HNE预处理HepG2肝细胞2 h,再加入40 μg/L TNF-α孵育16 h,可检测到细胞活性显著降低(P<0.05),且呈现明显的剂量效应,见图1A。MTT比色法检测4-HNE对TNF-α介导的肝细胞增殖的作用,得到了与LDH检测相一致的结果,见图1B。先用20 μmol/L 4-HNE预处理HepG2肝细胞2 h,再加入40 μg/L TNF-α孵育16 h,荧光显微镜下被Hoechst 33342染色的细胞数量明显减少,见图1C。MTT检测4-HNE对TNF-α介导的小鼠原代肝细胞的增殖作用,得到了与HepG2细胞相一致的结果,见图1D。

Figure 1. 4-HNE significantly increased the sensitivity of hepatocytes to the killing effect of TNF-α. A: HepG2 cells were pretreated with 4-HNE (20, 40 or 60 μmol/L) for 2 h, followed by 40 μg/L TNF-α incubation for 16 h, and then the activity of LDH in cell medium was determined (n=3); B: HepG2 cells were pretreated with 20 μmol/L 4-HNE for 2 h, followed by TNF-α (10 or 40 μg/L) incubation for 16 h, and then the proliferation of the cells was tested by MTT assay(n=6); C: HepG2 cells were pretreated with 20 μmol/L 4-HNE for 2 h, followed by 40 μg/L TNF-α incubation for 16 h, and then Hoechst 33342 staining was performed (×40); D: primary mouse hepatocytes were pretreated with 20 μmol/L 4-HNE for 2 h, followed by 40 μg/L TNF-α incubation for 24 h, and then MTT assay was conducted(n=6). Mean±SD.*P<0.05,**P<0.05vscontrol (0 μmol/L 4-HNE + 0 μg/L TNF-α);△P<0.05vs20 μmol/L 4-HNE + 40 μg/L TNF-α or 40 μmol/L 4-HNE alone;#P<0.05vs40 μmol/L 4-HNE + 40 μg/L TNF-α or 60 μmol/L 4-HNE alone.
图14-HNE显著增加肝细胞对TNF-α杀伤作用的敏感性
24-HNE对HepG2细胞内4-HNE-蛋白质加合物形成的影响
不同浓度的4-HNE作用于HepG2细胞,随着4-HNE浓度的增高,细胞内4-HNE-蛋白质加合物明显增多,当4-HNE为80 mol/L时,4-HNE与蛋白质形成加合物的量是对照组的4.83倍(P<0.01),见图2A。用20 μmol/L 4-HNE作用HepG2细胞2 h,细胞内4-HNE-蛋白质加合物明显增多(P<0.05),作用4 h表达量进一步增加,约为对照组的10倍(P<0.01),作用8 h与4 h比无明显变化,见图2B。
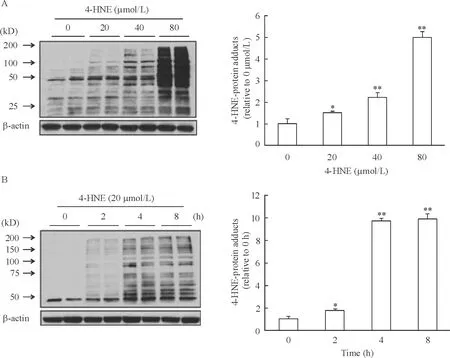
Figure 2. Effect of 4-HNE on 4-HNE-protein adduct formation in HepG2 cells detected by Western blotting. A: HepG2 cells were incubated with exogenous 4-HNE at the indicated concentrations for 4 h; B: HepG2 cells were incubated with exogenous 4-HNE (20 μmol/L) for the indicated time periods. Mean±SD.n=3.*P<0.05,**P<0.01vscontrol (0 μmol/L or 0 h).
图24-HNE对HepG2细胞4-HNE-蛋白质加合物形成的影响
34-HNE抑制HepG2细胞内TNF-α介导的NF-κB活化
用20 μmol/L 4-HNE预处理细胞2 h,再用100 μg/L TNF-α孵育4 h,细胞核内NF-κB(p65)与DNA的结合活性比单独用100 μg/L TNF-α孵育4 h明显降低(P<0.05),见图3A。随着4-HNE浓度的增高,NF-κB(p65)的活性进一步降低,对照组NF-κB(p65)的活性是4-HNE 40 μmol/L组的2.02倍(P<0.05),见图3B。
Western blotting结果显示,随着4-HNE浓度的增高,HepG2细胞核内NF-κB(p65)蛋白的表达明显降低,见图3C。
4乙醇对小鼠肝组织内4-HNE、TNF-α、甘油三酯(triglyceride,TG)和血浆丙氨酸氨基转移酶(alanineaminotransferase,ALT)的影响
与正常喂食组小鼠相比,实验组小鼠肝脏内TG的含量显著升高(图4A),并且肝脏出现脂肪水解现象(图4B)。同时发现,摄入乙醇提高了实验组小鼠血浆内ALT的活性(图4C)。TUNEL检测显示,实验组小鼠比正常组小鼠表现出明显的肝细胞凋亡现象(图4D)。肝脏内TNF-α水平也明显升高(图4E)。此外,用含乙醇的食物喂养5周后小鼠肝细胞内4-HNE-蛋白质加合物水平明显提高(图4F)。

Figure 3. 4-HNE inhibitied TNF-α-mediated NF-κB activation in HepG2 cells. A: HepG2 cells were pretreated with 20 μmol/L 4-HNE for 2 h, followed by 100 μg/L TNF-α incubation for 4 h, and then intranuclear NF-κB (p65) DNA binding activity were measured by ELISA; B: HepG2 cells were pretreated with 4-HNE (20 or 40 μmol/L) for 2 h, followed by 40 μg/L TNF-α incubation for 4 h, and then intranuclear NF-κB (p65) DNA binding activity were measured by ELISA; C: HepG2 cells were pretreated with 4-HNE (20 or 40 μmol/L) for 2 h, followed by 100 μg/L TNF-α incubation for 4 h, and then NF-κB (p65) protein expression was detected by Western blotting. Mean±SD.n=3.*P<0.05,**P<0.01vscontrol (0 μmol/L 4-HNE+0 μg/L TNF-α);△P<0.05vs40 μg/L TNF-α alone;#P<0.05vs20 or 40 μmol/L 4-HNE alone;▲P<0.05vs20 μmol/L 4-HNE + 40 μg/L TNF-α.
图34-HNE抑制HepG2肝细胞内TNF-α介导的NF-κB活化
讨 论
肝脏是酒精代谢的主要部位,长期摄入酒精会导致肝细胞发生氧化应激和脂质过氧化。血浆中4-HNE的生理浓度是0.3~0.7 μmol/L[9-10],ALD患者的血浆中4-HNE水平显著增加,肝炎早期4-HNE水平是生理浓度的2.8倍,肝硬化时期的4-HNE水平是生理浓度的8.4倍[11],肝脏的局部细胞为了应对氧化应激,浓度甚至可高达100 μmol/L[12-13]。本研究中我们用20~60 μmol/L 4-HNE来模拟早期ALD的体内4-HNE水平。虽然4-HNE对许多类型细胞的毒性作用已被广泛证实,但在病理状态下,肝细胞对4-HNE的耐受力将增强,这是因为肝细胞对4-HNE具有较强的降解代谢功能[14]。本研究体外实验数据表明,20~40 μmol/L 4-HNE单独作用于人肝细胞HepG2和小鼠原代肝细胞并没有引起细胞死亡或仅引起很小程度死亡,但TNF-α可以诱导4-HNE致敏的肝细胞显著杀伤;体内实验也证实,长期摄入酒精可以引起肝组织内TNF-α和4-HNE水平显著升高,从而诱导ALD。上述结果均表明TNF-α诱导4-HNE致敏的肝细胞产生细胞毒作用可能是导致ALD患者肝损伤的一个新机制。
TNF-α水平过高是ALD的另一个病理因素。乙醇会增加肠的通透性,引起巨噬细胞吞噬脂多糖增加,导致TNF-α水平升高。NF-κB是用以应对TNF-α刺激的细胞生死存亡之间一个重要的转换枢纽。在TNF-α的诱导下,缺乏NF-κB p65亚基或IKK复合物重要组成部分的小鼠在妊娠中期就会因肝细胞凋亡而死亡[15]。此外,IκBα突变的肝细胞对TNF-α诱导的杀伤作用更敏感[16]。本研究结果表明,4-HNE可以抑制TNF-α诱导的肝细胞内NF-κB的激活,从而增加肝细胞对TNF-α的敏感性,诱导肝细胞死亡,这个结论被以下2个实验结果所支持:(1)4-HNE降低了p65与DNA的结合活性; (2)4-HNE减少了细胞核内p65的含量。虽然前期研究有报道4-HNE对NF-κB活化的影响,但本研究结果与以前的结果存在不一致之处。4-HNE具有明显的细胞类型和刺激因素依赖性,4-HNE可以激活血管平滑肌细胞NF-κB的活性[17],而在THP-1单核细胞中,4-HNE通过抑制IκBα的磷酸化来抑制NF-κB的活性,这种抑制作用受到脂多糖、白细胞介素1β和佛波酯的刺激,而不是TNF-α的刺激[18]。目前为止,本文首次报道4-HNE可以抑制TNF-α诱导的肝细胞内NF-κB活性。

Figure 4. Effects of long-term (5 weeks) alcohol intake on the morphology and functions of mouse hepatic tissues, and the hepatic levels TNF-α and 4-HNE-protein adducts. A: hepatic triglyceride (TG) content; B: histological changes of hepatic tissues (HE staining, ×100); C: plasma alanine aminotransferase (ALT) activity; D: hepatocyte apoptosis in hepatic tissues (TUNEL, ×100); E: hepatic TNF-α level; F: Western blotting analysis of hepatic 4-HNE-protein adduct formation. PF: pair fed; AF: alcohol fed. Mean±SD.n=3.*P<0.05vsPF.
图4长期摄入酒精对小鼠肝组织形态、功能、TNF-α水平和4-HNE-蛋白质加合物形成的影响
4-HNE影响蛋白质功能的主要机制之一是通过与蛋白质形成加合物,这一过程称为蛋白质的羰基化。本研究体内和体外实验结果均表明,外源4-HNE的加入及慢性酒精摄入均可以引起肝细胞内4-HNE与蛋白质形成加合物水平的升高,这与前期报道相一致[19-21]。这些均表明4-HNE在ALD的发生发展过程中起着至关重要的作用。
本研究提示,长期摄入乙醇使肝细胞发生氧化应激,其产物4-HNE可能作为肝细胞致敏因子,通过抑制肝细胞内TNF--α介导的NF-κB抗细胞凋亡信号通路而诱导ALD。本文为脂质过氧化作用和TNF-α诱导的肝细胞损伤作用之间潜在的相互联系及ALD的发病机制提供了新的思路,为ALD的临床治疗提供新的策略,也为以4-HNE为靶点的新型药物研发提供了重要的理论指导和依据,但4-HNE抑制NF-κB活性的确切机制和作用靶点仍需进一步研究。
[1] 贾 青, 张维东,王朝霞,等. 不同剂量乙醇对小鼠早期肝纤维化的影响及机制研究[J]. 中国病理生理杂志, 2010, 26(9):1801-1806.
[2] 刘 阳, 迟宝荣. 酒精性肝硬化237例临床分析[J]. 吉林医学, 2004, 25(4):40-42.
[3] 中华医学会肝病学分会脂肪肝和酒精性肝病学组.酒精性肝病诊疗指南[J].胃肠病学, 2010, 15(10):617-621.
[4] Wheeler MD, Kono H, Yin M, et al. Delivery of the Cu/Zn-superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats [J]. Gastroenterology, 2001, 120(5):1241-1250.
[5] Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms [J]. World J Hepatol, 2009, 1(1):72-78.
[6] Smathers RL, Galligan JJ, Stewart BJ, et al. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease [J]. Chem Biol Interact, 2011, 192(1-2):107-112.
[7] Goldblum SE, Hennig B, Jay M, et al. Tumor necrosis factor α-induced pulmonary vascular endothelial injury [J]. Infect Immun, 1989, 57(4):1218-1226.
[8] McClain CJ, Hill DB, Song Z, et al.S-Adenosylmethionine, cytokines, and alcoholic liver disease [J]. Alcohol, 2002, 27(3):185-192.
[9] Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes [J]. Free Radic Biol Med, 1991, 11(1):81-128.
[10] Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma [J]. J Lipid Mediat Cell Signal, 1995, 11(1):51- 61.
[11] Aleynik SI, Leo MA, Aleynik MK, et al. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease [J]. Alcohol Clin Exp Res, 1998, 22(1):192-196.
[12] Tsukamoto H, Horne W, Kamimura S, et al. Experimental liver cirrhosis induced by alcohol and iron [J]. J Clin Invest, 1995, 96(1):620-630.
[13] Benedetti A, Comporti M, Fulceri R, et al. Cytotoxic aldehydes originating from the peroxidation of liver microsomal lipids: identification of 4,5-dihydroxydecenal [J]. Biochim Biophys Acta, 1984, 792(2):172-181.
[14] Sakuma S, Negoro M, Kifamura T, et al. Xanthine oxidase-derived reactive oxygen species mediate 4-oxo-2-nonenal-induced hepatocyte cell death [J]. Toxicol Appl Pharmacol, 2010, 249(2):127-131.
[15] Tanaka M, Fuentes ME, Yamaguchi K, et al. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β-deficient mice [J]. Immunity, 1999, 10(4):421-429.
[16] Chaisson ML, Brooling JT, Ladiges W, et al. Hepatocyte-specific inhibition of NF-κB leads to apoptosis after TNF treatment,but not after partial hepatectomy [J]. J Clin Invest, 2002, 110(2):193-202.
[17] Lee JY, Je JH, Jung KJ,et al. Induction of endothelialiNOS by 4-hydroxyhexenal through NF-κB activation [J]. Free Radic Biol Med, 2004, 37(4):539-548.
[18] Page S, Fischer C, Baumgartner B, et al. 4-Hydroxynonenal prevents NF-κB activation and tumor necrosis factor expression by inhibiting IκB phosphorylation and subsequent proteolysis [J]. J Biol Chem, 1999, 274(17):11611-11618.
[19] Koteish A, Yang S, Lin H, et al. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation [J]. J Biol Chem, 2002, 277(15):13037-13044.
[20] Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration [J]. Clin Biochem, 1999, 32(7):571-578.
[21] Mandrekar P, Catalano D, White B, et al. Moderate alcohol intake in humans attenuates monocyte inflammatory responses:inhibition of nuclear regulatory factor kappa B and induction of interleukin 10[J]. Alcohol Clin Exp Res, 2006, 30(1):135-139.
InhibitionofTNF-α-mediatedNF-κBactivationby4-hydroxynonenalcontributestoliverinjuryinalcoholicliverdisease
YU Chen-hui, DU Zhong-yan, GAO Jia, WANG Wei-xi, DOU Xiao-bing
(LifeSciencesCollegeofZhejiangChineseMedicalUniversity,Hangzhou310053,China.E-mail:xbdou77@163.com)
AIM: To study the role of 4-hydroxynonenal (4-HNE) in hepatocyte death induced by tumor necrosis factor α (TNF-α).METHODSHuman liver cell line HepG2 and primary mouse hepatocytes were used to establish the cell model. The effect of 4-HNE on TNF-α-induced cell death was determined by lactate dehydrogenase (LDH) release and MTT assays. The intracellular levels of 4-HNE-protein adducts were determined by Western blotting. The intranuclear NF-κB (p65) and its DNA binding activity were detected by Western blotting and ELISA, respectively. Long-term intake of alcohol in C57BL/6 mice was performed to establish the animal model. The histological changes of mouse hepatic tissues and the apoptosis of hepatocytes were observed by HE staining and TUNEL assay, respectively. The hepatic levels of triglyceride (TG), TNF-α and 4-HNE-protein adducts, and the plasma activity of alanine aminotransferase (ALT) were also detected.RESULTS(1) 4-HNE significantly increased the sensitivity of HepG2 cells and primary mouse hepatocytes to the killing effect of TNF-α. (2) 4-HNE significantly increased the intracellular levels of 4-HNE-protein adducts. (3) 4-HNE inhibited TNF-α-mediated NF-κB (p65) activation in HepG2 cells. (4) Long-term intake of alcohol in mice resulted in high hepatic levels of 4-HNE and TNF-α, accompanied with the increases in hepatic TG content, plasma ALT activity and hepatocyte death.CONCLUSIONLong-term intake of alcohol induces oxidative stress and produces 4-HNE as a hepatocyte-sensitizing factor, which inhibits TNF-α-mediated NF-κB anti-apoptotic signaling pathway in hepatocytes, thus inducing alcoholic liver damage.
Alcoholic liver disease; 4-Hydroxynonenal; Tumor necrosis factor alpha; Nuclear factor kappa B; Hepatocytes
R363
A
10.3969/j.issn.1000- 4718.2013.06.016
1000- 4718(2013)06- 1046- 07
2012- 12- 08
2013- 04- 17
国家自然科学基金资助项目(No.81241145);浙江省自然科学基金资助项目(No.LY12C07002);浙江省卫生厅中医药科技计划(No.2013KYA138)
△通讯作者Tel: 0571-86613724; E-mail: xbdou77@163.com

