不同部位绝对不应期电刺激对慢性心力衰竭兔心功能的影响*
张飞飞, 齐晓勇, 李英肖, 刘惠良, 党 懿, 宁 彬, 秦 晨
(1河北医科大学研究生学院, 2河北省人民医院心脏中心,河北 石家庄 050051)
不同部位绝对不应期电刺激对慢性心力衰竭兔心功能的影响*
张飞飞1, 齐晓勇2△, 李英肖2, 刘惠良2, 党 懿2, 宁 彬1, 秦 晨1
(1河北医科大学研究生学院,2河北省人民医院心脏中心,河北 石家庄 050051)
目的观察心脏不同部位绝对不应期电刺激对慢性心力衰竭兔心脏功能及心脏收缩同步性的影响,探讨绝对不应期电刺激治疗慢性心力衰竭的最佳模式。方法40只新西兰大白兔通过升主动脉根部套扎法建立兔慢性心力衰竭模型,将其分为心衰组、左室前壁(LVAW)刺激组、左室后侧壁(LVPLW)刺激组和右室心尖(RVA)刺激组。刺激组发放心脏收缩力调节信号(CCM),每天刺激6 h,连续刺激7 d。观察心脏功能及心脏收缩同步性的变化。结果与心衰组相比,LVAW刺激组、LVPLW刺激组及RVA刺激组的左室收缩末内径、左室舒张末内径和血浆脑钠肽(BNP)水平下降(P<0.05),左室射血分数和左室短轴缩短率升高(P<0.05),其中以LVAW刺激组变化最为明显,其次为LVPLW刺激组与RVA刺激组。室间隔厚度和左室后壁厚度各组之间刺激前后未见明显变化(P>0.05)。采用脉冲多普勒频谱测量主、肺动脉射血前间期之差评价心脏收缩同步性,各组之间刺激前后未见明显变化。结论心脏不同部位绝对不应期电刺激改善心功能的程度不同,左室前壁刺激组最为明显,其次为左室后侧壁与右室心尖刺激组。绝对不应期电刺激并不影响左右心室之间的收缩同步性。
心力衰竭; 绝对不应期; 电刺激; 心功能
慢性心力衰竭(chronic heart failure,CHF)是各种心脏疾病的终末阶段,其发病率高,致死致残率高。CHF的治疗包括药物治疗和非药物治疗,虽然心力衰竭的药物治疗迅速发展,但仍有一部分患者对药物治疗反应欠佳,且其长期疗效有限并存在较高的死亡率。心脏移植、人工心脏虽可以延长患者生命,但其费用昂贵、供体不足难以满足临床的需要。心脏再同步化治疗虽可改善心衰但其适应症有限,且部分患者对其无应答[1]。
心脏收缩力调节(cardiac contractility modulation,CCM)作为一种新的机械装置治疗方法进入人们视线,它是在绝对不应期内给予心脏非兴奋性电刺激(non-excitatory currents,NEC),它不会诱发心肌细胞产生动作电位,但这个刺激可以使心肌细胞的这一次收缩能力得到加强[2],国内外的基础及临床研究均显示,CCM主要通过增强心肌局部收缩力改善心功能,且不同部位刺激对心功能的影响是不同的,但在哪些部位进行刺激具有最好的效果尚不清楚,需进一步研究。本研究通过对心力衰竭兔在体心脏的不同部位给予绝对不应期电刺激,观察其对心脏功能及心脏收缩同步性的影响。
材 料 和 方 法
1动物和材料
选用6月龄新西兰大白兔40只作为实验对象,体重2.5~3.5 kg,雌雄不拘,由河北医科大学动物实验中心提供。采用日本阿洛卡公司Prosound a10超声诊断仪测定各项心脏超声指标;美国巴德公司的MICROPACE EPS320心脏电生理刺激仪发放CCM信号;用上海西塘公司的兔脑钠肽(brain natriuretic peptide,BNP) ELISA试剂盒检测CCM治疗之后各组血浆BNP水平。
2兔心衰模型的建立
3%戊巴比妥钠溶液1 mL/kg麻醉后,于胸骨左缘2、3、4肋间区,打开胸腔,暴露心脏,于升主动脉根部远端1.0 cm处游离升主动脉根部约4~5 mm,测量主动脉周长,环扎缩窄后周长为原周长的60%;术后12周筛选成功的心力衰竭动物模型。心力衰竭模型建立成功的标准:心输出量下降30%或(和)左室舒张末压上升至2.4 kPa(18 mmHg)以上[3]。
40只兔子术前随机分为4组:心衰(heart failure,HF)组、左室前壁刺激(left ventricular anterior wall CCM,LVAW-CCM)组、左室后侧壁刺激(left ventricular posterior lateral wall CCM,LVPLW-CCM)组和右室心尖刺激(right ventricular apex CCM,RVA-CCM)组,每组10只,各组间兔子体重无明显区别(P>0.05)。对照组仅给予主动脉环扎缩窄,左室前壁刺激组预先将小儿用临时起搏电极弯针端缝合于心外膜左室前壁,左室后侧壁刺激组、右室心尖刺激分别预先将小儿用临时起搏电极弯针端缝合于心外膜相应部位,保持裸露导线与心肌组织接触,且与胸壁组织绝缘,直针端通过皮下穿刺固定于颈部,留待以后CCM电刺激。
3CCM刺激
造模成功的动物模型,暴露其颈部电极直针端,于窦性心律下,通过体表心电图的R波触发刺激仪发放CCM刺激(2 ms,7 V,R波感知后延迟30 ms发放),每天刺激6 h,连续刺激7 d。
4观测指标
CCM刺激前后采用无创心脏超声检查,在二维超声引导下取左室长轴切面,在 M-型图像上,采用Teichholz法测定左室收缩末内径(left ventricular end-systolic dimension,LVESD)、左室舒张末内径(left ventricular end-diastolic dimension,LVEDD)、左室短轴缩短率(left ventricular fractional shortening,LVFS)、室间隔厚度(interventricular septal thickness,IVS)、左室后壁厚度(left ventricular posterior wall thickness,LVPW)和左室射血分数(left ventricular ejection fraction,LVEF)。心室间机械延迟 (interventricular mechanical delay,IVMD)的测定:先测定主动脉射血前间期(aortic pre-ejection interval,APEI),应用脉冲频谱多普勒在心尖五腔心切面主动脉瓣口采集脉冲多普勒频谱,测量QRS波群起始点距脉冲多普勒频谱起始的时间, 然后在大血管短轴切面肺动脉瓣口采集脉冲多普勒频谱,测量肺动脉射血前间期(pulmonary wave pre-ejection internal,PPEI),两者之差即IVMD。以上各测量值均取连续3次测量的平均值。采用双抗体夹心ABC-ELISA法进行血液学检查,测定CCM刺激前后血浆BNP水平,观察CCM治疗对心脏功能及血浆BNP浓度的影响。
5统计学处理
运用SAS 8.0统计软件分析。数据用均数±标准差(mean±SD)表示,组间均数比较采用方差分析及SNK-q检验。以P<0.05为差异有统计学意义。
结 果
1动物存活情况
各组术中均死亡1只,心衰组、左室前壁刺激组和右室心尖刺激组各有1只因心衰程度未满足入选标准而剔除;左室后侧壁刺激组2只和右室心尖刺激组1只因严重心衰死亡。术后12周各组存活并满足心衰条件的兔子:心衰组8只,左室前壁刺激组8只,左室后侧壁刺激组7只,右室心尖刺激组7只。
2CCM刺激对心脏功能影响
术后12周CCM刺激之前4组心脏超声各项指标无明显差别(P>0.05),见图1、表1。术后12周再给予CCM持续刺激1周后,与心衰组相比,刺激组LVESD和LVEDD明显下降,以左室前壁刺激组下降最显著(P<0.05);LVEF和LVFS明显升高,以左室前壁刺激组升高最为明显(P<0.05),左室后侧壁刺激组与右室心尖刺激组各指标差异无统计学意义(P>0.05);IVS和LVPW刺激前后与各组之间比较无明显变化(P>0.05),见表1、2。
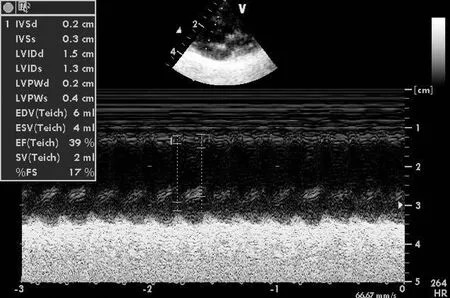
Figure 1. Ultrasonic cardiogram indicators in LVAW-CCM group before CCM.
图1左室前壁刺激组CCM刺激之前心脏超声各指标

表1 各组CCM刺激前心脏超声结果比较

表2 各组CCM刺激后心脏超声结果比较
▲P<0.05vsHF group;●P<0.05vsLVAW-CCM group.
3CCM刺激对血浆BNP的影响
CCM刺激前血浆BNP水平各组相比无明显差别(P>0.05);CCM刺激后血浆BNP水平以左室前壁刺激组降低最明显,其次为左室后侧壁刺激组和右室心尖刺激组(P<0.05),但后2组BNP水平无显著差异(P>0.05)。心衰组BNP水平没有明显变化(P>0.05),见表3。
4CCM刺激对心脏收缩同步性方面的影响
室间收缩同步性评价(IVMD)采用脉冲多普勒频谱,主、肺动脉射血前间期(QRS起始分别至主、肺动脉血流频谱起始的时间)见图2、3,各组之间刺激前后未见明显变化,见表4。
表3各组CCM刺激前后血浆BNP水平比较
Table 3. Comparison of plasma BNP level before and after CCM(μg/L.Mean±SD)

GroupnBeforeCCMAfterCCMHF861.56±6.7061.83±6.44LVAW-CCM863.48±5.6350.47±3.43▲●LVPLW-CCM763.07±5.8656.49±5.19▲●RVA-CCM762.72±5.3856.46±6.25▲●
▲P<0.05vsHF group;●P<0.05vsLVAW-CCM group.

Figure 2. Aortic pre-ejection interval in LVAW-CCM group after CCM.
图2左室前壁刺激组CCM刺激之后主动脉射血前间期

Figure 3. Pulmonary pre-ejection interval in LVAW-CCM group after CCM.
图3左室前壁刺激组CCM刺激之后肺动脉射血前间期
表4各组CCM刺激前后室间收缩同步性比较
Table 4. Comparison of IVMD before and after CCM (ms.Mean±SD)

GroupnBeforeCCMAfterCCMHF811.50±0.3611.48±0.41LVAW-CCM811.38±0.4211.35±0.43LVPLW-CCM711.31±0.4311.33±0.40RVA-CCM711.20±0.4511.21±0.25
讨 论
CCM刺激可在不影响窦性心律的情况下改善心功能,无致恶性心律失常作用。Sabbah等[4]对慢性心衰犬模型给予CCM刺激,结果显示 CCM信号对左室射血分数、左室压力变化峰值和左室面积缩短分数均有明显的改善作用,且无不良影响。进一步的研究评定了CCM治疗慢性心衰的安全性及可行性[5],心衰患者经过3个月CCM治疗后,左室射血分数、6 min步行距离、峰值氧流量、患者明尼苏达生活问卷评估值等都有明显升高[6]。此外,一系列大规模、多中心的临床研究证实,CCM信号可以安全有效地改善心功能,提高患者的运动耐受及生活质量[7-8]。
目前,国内外基于心脏的结构形态、心肌收缩的次序、心肌细胞生理特性以及CCM增强心肌收缩力的局部性对心脏不同部位CCM刺激进行了部分研究[9]。Callans 等[10]将2个 CCM信号电极分别安置在猪心脏左室侧壁和前壁的心外膜,结果显示其对心功能的影响不同,说明CCM 信号刺激对收缩功能的影响与部位相关。另有研究表明对兔室间隔部发放CCM刺激发现可获得与刺激左室前壁类似的增强心肌收缩力的效果,因此CCM电极不一定必须安置在左室游离壁[11]。此外,对正常与心梗兔的左室前壁及后壁给予CCM刺激的研究显示由于心脏几何构型及各部位心肌反应性的差异产生了不同的效果[12]。Pane等[13]将 CCM 信号电极经由心外静脉分别安置在心衰患者心脏的前壁、侧壁和后壁,实验结果显示,CCM 信号可以显著改善左室收缩压、压力变化峰值和主动脉搏出压,其中安置在前壁的 CCM 信号电极使前壁和间隔处心肌收缩期增厚率明显改善。我们研究中也发现, CCM刺激能够改善LVESD、LVEDD、LVEF、LVFS等指标,刺激心脏不同部位改善心功能效果不同,左室前壁CCM刺激对心脏功能的影响优于左室后侧壁与右室心尖CCM刺激。本实验仅给予短时间CCM刺激,CCM刺激之后短期内进行测定,尚未观察到CCM刺激引起血流动力学改变后心脏结构的继发改变。
心脏再同步化治疗(cardiac resynchronization therapy,CRT)为终末期心衰的治疗带来了希望,它通过多部位起搏心脏,改善左右心室收缩的同步性,提升心功能而改善心衰症状[14],提高了心衰患者生活质量、运动耐量,减少了再住院率。然而。最近研究表明心衰患者不伴QRS间期延长,即不存在心脏机械收缩不同步者对CRT表现为无应答。因此临床迫切需要一种新的治疗方法来改善QRS波群正常的心力衰竭患者的生活质量和预后。CCM不同于心脏起搏,它不引起心脏额外收缩,是非兴奋性的。本研究表明,CCM与CRT不同,它对CHF兔左、右心室收缩之间的同步性无影响,而是通过增强心肌收缩力来改善心功能。研究表明,CCM刺激对正常或窄QRS波群心衰的治疗有一定疗效,与QRS波群时间长短无关,是中、重度心衰的最佳选择[15]。
总之,本实验发现,心脏绝对不应期电刺激对心室收缩之间的同步性无影响,它通过提高慢性心力衰竭心肌的收缩力来改善心功能,尤以左室前壁CCM刺激最佳。CCM作为慢性心力衰竭的非药物治疗手段具有良好的应用前景。
[1] Burkhoff D, Parides M, Borggrefe M,et al. “Responded analysis”for assessing effectiveness of heart failure therapies based on measures of exercise tolerance[J]. J Card Fail,2009,15(2):108-115.
[2] Goliasch G, Khorsand A, Schütz M, et al.The effect of device-based cardiac contractility modulation therapy in myocardial efficiency and oxidative metabolism in patients with heart failure[J]. Eur J Nucl Med Mol Imaging,2012,39(3):408-415.
[3] Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy [J]. Cardiovasc Res, 1998, 39(1): 60-76.
[4] Sabbah HN, Haddad W, Mika Y, et al. Cardiac contractility modulation with the impulse dynamic signal:Study in dogs with chronic heart failure [J]. Heart Fail Rev, 2001, 6(1): 45-53.
[5] Abraham WT, Nademanee K, Volosin K,et al. Subgroup analysis of a randomized controlled trail evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure[J]. J Card Fail,2011,17(9):710-717.
[6] Burkhoff D. Does contractility modulation have a role in the treatment of heart failure?[J]. Cur Heart Fail Rep, 2011, 8(4):260-265.
[7] Kadish A,Nademanee K,Volosin K ,et al.Arandomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure[J]. Am Heart J, 2011, 161(2):329-337.
[8] Borgreffe M,Burkhoff D. Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure[J]. Eur J Heart Fail, 2012, 14(7):703-712.
[9] Winter J, Brack KE, Ng GA, et al. The acute inotropic effects of cardiac contractility modulation(CCM) are associated with action potential duration shortening and mediated by β1-adrenoceptor signalling[J]. J Mol Cell Cardiol, 2011,51(2):252-262.
[10] Callans DJ, Fuchs S, Mika Y, et al. Global improvement in left ventricular performances observed with cardiac contractility modulation is the result of changes in regional contractility [J]. Heart Fail Rev, 2001, 6(1): 35-44.
[11] Zhang H,Cui C,Hu D. Effects of electric stimulations applied during absolute refractory period on cardiac function of rabbits with heart failure[J]. J Huazhong Univ Sci Technol Med Sci, 2010, 30(2):155-158.
[12] 张海柱,崔长琮,赵晓静,等. 非兴奋性电刺激对正常及心肌梗死兔心功能的影响及其作用的局部性[J]. 中国病理生理杂志,2004,20(10):1846-1848.
[13] Pane C,Vicedomimi G,Salvati A,et al.Eletical modulation of cardiac contractility: clinical aspects in congestive heart failure[J].Heart Fail Rev,2001,6(1):55-60.
[14] Sweeney MO,van Bommel RJ,Schalij MJ,et al.Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy[J].Circulation,2010,121(5):626-634.
[15] Zhang Q, Chan YS, Liang YJ,et al.Comparison of left ventricular reverse remodeling induced by cardiac contractility and cardiac resynchronization therapy in heart failure patients with different QRS durations[J]. Int J Cardiol,2012,Feb 11.[Epub ahead of print]
Effectsofcardiaccontractilitymodulationappliedtodifferentlocationsofheartoncardiacfunctionsinrabbitswithheartfailure
ZHANG Fei-fei1, QI Xiao-yong2, LI Ying-xiao2,LIU Hui-liang2,DANG Yi2,NING Bin1, QIN Chen1
(1GraduateSchoolofHebeiMedicalUniversity,2DepartmentofCardiology,HebeiGeneralHospital,Shijiazhuang050051,China.E-mail:xiaoyong_q@yahoo.com)
AIM: To investigate the effects of cardiac contractility modulation (CCM) applied to different locations of the heart on cardiac functions and cardiac dys-synchrony in the rabbits with chronic heart failure, and to explore the best pattern of CCM.METHODSForty rabbits were divided into 4 groups according to the location of receiving CCM: heart failure (HF) group, left ventricular anterior wall (LVAW-CCM) group, left ventricular posterior lateral wall (LVPLW-CCM) group and right ventricular apex (RVA-CCM) group. The model of chronic heart failure was made by ligating ascending aortic root of the rabbits. After 12 weeks, the electrical stimulations during the absolute refractory period were delivered in different locations of the heart, lasting 6 h everyday for 7 days. The changes of cardiac functions and cardiac dys-synchrony were observed by cardiac ultrasonic cardiogram before and after CCM stimulation. The plasma level of brain natriuretic peptide (BNP) was detected by ABC-ELISA method. Pulsed-wave Doppler was used to acquire aortic pre-ejection interval (APEI) and pulmonary pre-ejection internal (PPEI), and inter-ventricular mechanical delay (IVMD) was calculated to evaluate the cardiac dys-synchrony.RESULTSCompared with HF group, left ventricular end-systolic dimension (LVESD) and left ventricular end-diastolic dimension (LVEDD) in LVAW-CCM group, LVPLW-CCM group and RVA-CCM group were significantly decreased (P<0.05), while left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were significantly increased (P<0.05), especially in LVAW-CCM group. Interventricular septal thickness (IVS) and left ventricular posterior wall thickness (LVPW) were similar among groups. No significant difference of plasma BNP level before CCM delivery among the 4 groups was observed. However, the plasma BNP level in control group was the highest, followed by LVPLW-CCM group and RVA-CCM group, and LVAW-CCM group was the lowest after CCM delivery. No change of IVMD was observed among groups before and after CCM delivery.CONCLUSIONThe effect of CCM applied to different locations of the heart on cardiac functions is different.The optimal site of CCM delivery is left ventricular anterior wall. No influence of interventricular dys-synchrony was observed during application of CCM.
Heart failure; Absolute refractory period; Electric stimulation; Cardiac functions
R363
A
10.3969/j.issn.1000- 4718.2013.06.007
1000- 4718(2013)06- 0998- 06
2012- 11- 27
2013- 03- 19
河北省自然科学基金资助项目(No.C2010001631)
△通讯作者 Tel: 0311-85988937; E-mail: xiaoyong_q@yahoo.com

