依那西普抑制博来霉素诱导的小鼠肺纤维化*
胡玉洁, 李 理, 李伟峰△, 黄文杰
(1广州军区广州总医院呼吸内科,广东 广州 510010; 2南方医科大学研究生学院,广东 广州 510515)
依那西普抑制博来霉素诱导的小鼠肺纤维化*
胡玉洁1,2, 李 理1, 李伟峰1△, 黄文杰1
(1广州军区广州总医院呼吸内科,广东 广州 510010;2南方医科大学研究生学院,广东 广州 510515)
目的观察肿瘤坏死因子 α(TNF-α)拮抗剂依那西普对博来霉素诱导的肺纤维化小鼠的抑制纤维化作用,并探讨依那西普治疗肺纤维化的可能机制。方法将45只SPF级雌性昆明小鼠随机分为3组:对照组(气管内雾化生理盐水)、纤维化组(气管内博来霉素3 mg/kg溶于100 μL生理盐水内雾化)和依那西普干预组(气管内雾化博来霉素后,4 mg/kg依那西普溶于100 μL生理盐水内腹腔注射,每3 d注射1次)。处理后第28 d收集样本,小鼠左肺置于10%中性甲醛固定,石蜡包埋切片后行HE与Masson染色;右肺碱水解法检测组织羟脯氨酸(HYP)的含量;酶联免疫法检测血清TNF-α和转化生长因子 β(TGF-β)的含量;提取肺组织总蛋白,Western blotting 检测磷酸化ERK1/2、JNK和p38的表达。结果依那西普干预组肺组织病理损伤及气道上皮下胶原沉积较纤维化组减轻,肺叶炎症损伤评分和纤维化评分明显下降(均P<0.01),肺组织HYP含量显著降低(P<0.05),血清TNF-α 和TGF-β的浓度明显减少(均P<0.01),肺组织ERK1/2、JNK和p38蛋白的磷酸化水平也显著下降(P<0.01,P<0.05,P<0.01)。结论依那西普能显著下调TNF-α 和TGF-β的水平,从而抑制ERK1/2、JNK和p38的活化,缓解博来霉素诱导的小鼠肺纤维化病变。
肺纤维化; 肿瘤坏死因子 α; 依那西普; 博来霉素; 丝裂原活化蛋白激酶类
肺间质纤维化发病机制未明,缺乏有效的治疗手段,糖皮质激素和免疫抑制剂/细胞毒性药物疗效仍不明确,且毒副作用大,不利于长期治疗。因此,寻找更加有效、安全的治疗药物成为当前肺纤维化研究的热点。研究发现,无论是在肺间质纤维化患者还是纤维化动物模型,整个病程中均出现了肿瘤坏死因子 α(tumor necrosis factor α,TNF-α)持续性的高表达[1-2]。TNF-α的高表达能促进成纤维细胞的增殖、向肌成纤维细胞的转化以及细胞外基质的沉积[3-4],是重要的促肺纤维化因子之一[5-6]。丝裂原活化蛋白激酶(mitogen-activated protein kinases, MAPKs)是TNF-α下游信号转导的关键环节[7],能参与TNF-α介导的炎症反应和上皮-间质转化过程(epithelial-mesenchymal transition,EMT)[8],但对其在肺纤维化体内实验方面与TNF-α相关性的研究尚不多。本研究建立博来霉素(bleomycin,BLM)诱导的小鼠肺纤维化模型,以代表性的肿瘤坏死因子受体-抗体融合蛋白(TNFR-Fc)依那西普(etanercept,Et)竞争性地阻断TNF-α与受体的结合,探讨依那西普对TNF-α下游MAPKs通路的干预作用及其防治肺纤维化的可行性。
材 料 和 方 法
1材料
10周龄SPF级昆明雌性小鼠45只(购自南方医科大学动物中心),平均体重28 g,饲养于广州军区广州总医院实验动物中心。BLM每支15 mg,日本化药株式会社产品。Et每支25 mg,美国恩利公司产品。动物喉镜、小鼠固定操作台与MicroSprayereTM雾化器(Penn-Century)。Masson三色染色试剂盒 (福州迈新生物技术公司)。BX51光学显微镜(Olympus)。羟脯氨酸(hydroxyproline,HYP)检测试剂盒(南京建成生物工程研究所)。小鼠TNF-α和转化生长因子 β(transforming growth factor -β, TGF-β)酶联免疫试剂盒(武汉博士德生物研究所)。兔抗鼠磷酸化细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)1/2、磷酸化c-Jun N-末端激酶(c-Jun N-terminal kinase, JNK)、磷酸化p38和总JNK、总ERK1/2、总p38 Ⅰ抗(Cell Signaling Technology)。化学发光剂(Millipore)。Multiskan GO全波长酶标仪 (Thermo Scientific)。
2方法
2.1纤维化动物模型的建立、分组和标本处理 实验小鼠随机分为对照组、BLM组和BLM+Et组,每组15只。小鼠腹腔注射水合氯醛麻醉后固定于操作台,以动物喉镜压下小鼠舌根暴露声门,雾化器从声门插入气管并雾化BLM溶液(3 mg/kg,100 μL),对照组雾化等体积生理盐水;BLM+Et组小鼠雾化BLM后给予4 mg/kg Et溶于100 μL生理盐水内腹腔注射,每3 d注射1次,对照组与BLM组予相同体积生理盐水腹腔注射。于处理后第28 d收集标本待测。
2.2肺组织病理学改变 小鼠左肺以10%中性甲醛固定后,石蜡包埋切片,常规行HE染色及Masson胶原染色,胶原染色按照Masson三色改良染色试剂盒产品说明书进行。参照Mikawa等[9]和Ashcroft等[10]法对肺病理损伤和纤维化程度进行评分。
2.3肺组织HYP含量测定 取出右肺,PBS洗去血迹,滤纸吸干后清除周围的结缔组织并充分剪碎,称量100 mg肺组织,加入1 mL裂解液,95 ℃碱水解20 min。后续操作按产品说明书进行。HYP含量以微克/每克肺组织(μg/g)表示。
2.4血清TNF-α和TGF-β含量检测 收集小鼠血液,静置2 h后,4 ℃、5 000 r/min离心10 min,分离上层血清。ELISA检测TNF-α 和TGF-β含量,按照产品说明书进行操作。
2.5肺组织磷酸化ERK1/2、JNK和p38 Western blotting 肺组织匀浆后抽提总蛋白,30 μg/well 蛋白上样进行SDS-PAGE电泳,蛋白转移至PVDF膜上。5%的脱脂奶粉20 mL室温封闭1 h。TBST洗涤液洗膜3次,每次10 min。分别加入抗磷酸化ERK1/2、JNK、p38和总ERK1/2、JNK、p38抗体,4 ℃孵育过夜。TBST洗膜3次,每次10 min。加入HRP标记的II抗37 ℃孵育1 h。TBST洗膜3次,每次10 min。化学发光试剂检测蛋白印迹条带,光密度扫描仪扫描胶片,Gel-Pro Analyzer软件分析结果。
3统计学处理
用SPSS 13.0软件进行统计分析。数据以均数±标准差(mean±SD)表示,数据均进行正态性检验。计量资料符合正态分布者通过单因素方差分析进行总体均数比较,两两比较采用LSD-t检验。显著性水准α=0.05,双侧。
结 果
1HE染色及Masson染色小鼠肺脏病理改变
1.1小鼠肺脏HE染色及炎症损伤评分 处理后第28 d,对照组肺组织结构清晰,肺泡无充血,无炎症细胞浸润,见图1A;BLM组见肺泡内出血,肺泡间隔内成纤维细胞、上皮下肌成纤维细胞增多,炎症细胞浸润,肺泡间隔明显破坏,肺组织大片实变,见图1B;BLM+Et组肺泡结构完整,间隔稍有增厚,见图1C。BLM组炎症评分明显高于对照组(6.80±0.40vs1.43±0.51,P<0.01)和BLM+Et组(6.80±0.40vs3.63±0.42,P<0.01), BLM+Et组与对照组比较差异有统计学意义(3.63±0.42vs1.43±0.51,P<0.01)。
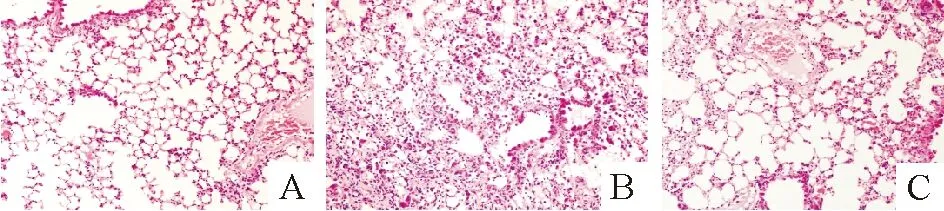
Figure 1. Pathological changes of mouse lung tissues stained with hematoxylin and eosin (×200).A:control group;B:BLM group;C:BLM+Et group.
图1小鼠肺组织HE染色
1.2小鼠肺脏Masson染色及纤维化评分 对照组肺组织无明显炎症细胞浸润和胶原沉积,肺泡结构完整, 见图2A;BLM组小鼠肺组织结构紊乱,间隔增厚,肺泡内炎症细胞浸润,组织内可见大量蓝色胶原沉积,部分纤维化病灶呈片状分布,见图2B;BLM+Et组与BLM组比较肺泡结构基本完整,肺上皮下轻度炎症细胞聚集,肺纤维化程度明显好转,少量蓝色胶原沉积,见图2C。对照组、BLM组和BLM+Et组小鼠纤维化评分分别为1.83±0.76、 7.13±0.32和3.73±0.32,BLM组评分显著高于对照组和BLM+Et组(均P<0.01);BLM+Et组评分较对照组明显增高,差异有统计学意义(P<0.01)。
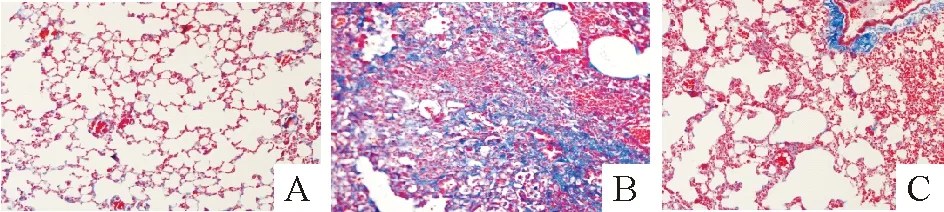
Figure 2. Pathological changes of mouse lung tissues detected by Masson’s trichrome staining (×200).A:control group;B:BLM group;C:BLM+Et group.
图2小鼠肺组织Masson染色
2肺组织HYP含量
BLM组小鼠肺组织HYP含量明显高于对照组[(577.6±98.5) μg/gvs(322.3±30.0) μg/g,P<0.01]; BLM+Et组小鼠肺组织HYP含量较BLM组比较显著下降[(395.6±71.7) μg/gvs(577.6±98.5) μg/g,P<0.05];BLM+Et组与对照组比较差异无统计学意义[(395.6±71.7) μg/gvs(322.3±30.0) μg/g,P>0.05]。
3血清TNF-α含量
BLM处理后,BLM组小鼠血清内TNF-α表达显著高于对照组(P<0.01),Et干预后,小鼠血清内TNF-α含量显著下降(P<0.01),与对照组比较差异无统计学意义(P>0.05),见表1。
4血清TGF-β含量
BLM处理后,BLM组小鼠血清内TGF-β表达显著高于对照组(P<0.01),Et干预后,BLM+Et组小鼠血清内TGF-β的表达较BLM组显著下降(P<0.01),与对照组比较差异无统计学意义(P>0.05),见表1。
表1各组小鼠血清内TNF-α和TGF-β含量的测定
Table 1. The levels of TNF-α and TGF-β in mouse serum (ng/L.Mean±SD.n=15)
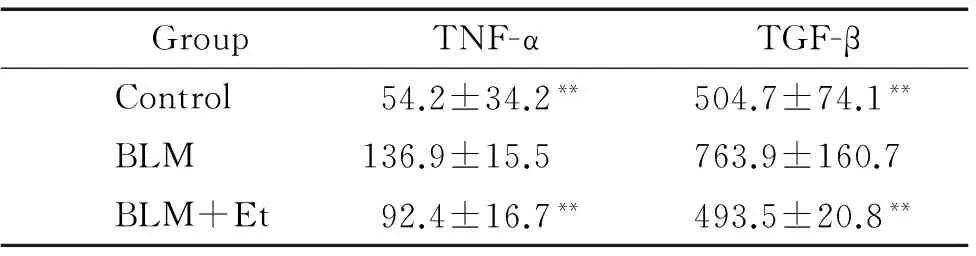
GroupTNF-αTGF-βControl54.2±34.2**504.7±74.1**BLM136.9±15.5763.9±160.7BLM+Et92.4±16.7**493.5±20.8**
**P<0.01vsBLM group.
5Et对磷酸化JNK、ERK1/2和p38蛋白表达的影响
BLM组小鼠肺组织内JNK、ERK1/2和p38的磷酸化水平均显著高于对照组(均P<0.01),给予Et干预后,p-ERK1/2、p-JNK和p-p38的表达较对照组高但比BLM组明显降低(P<0.01,P<0.05,P<0.01),见图3。
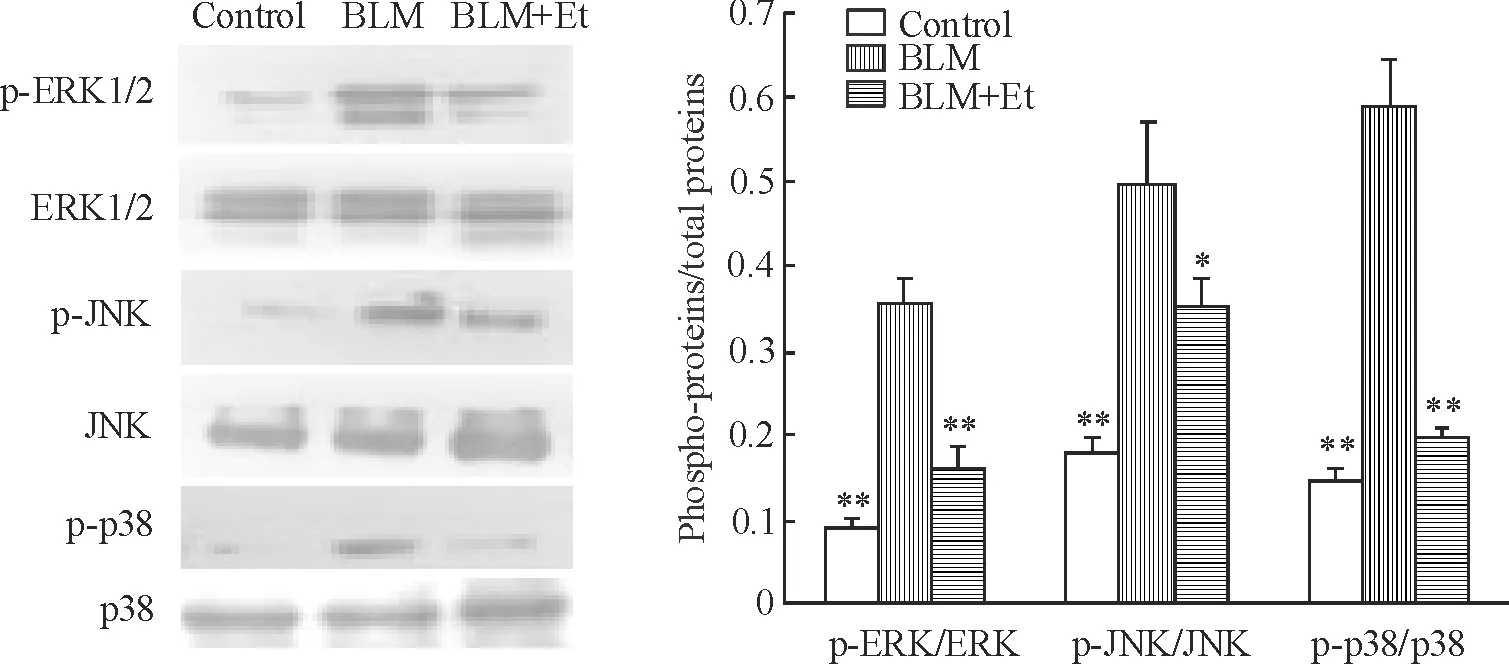
Figure 3. The phosphorylated and total JNK,ERK1/2 and p38 protein expression in mouse lung tissues induced by BLM.Mean±SD.n=6.*P<0.05,**P<0.01vsBLM group.
图3BLM诱导的磷酸化JNK、ERK1/2和p38蛋白及总蛋白的表达
讨 论
TNF-α不仅介导抗肿瘤和免疫调节反应,还能调节成纤维细胞的增殖、分化和细胞外基质的沉积[3-4],参与组织损伤的修复和结缔组织代谢的调节。研究证实,TNF-α转基因小鼠高表达TNF-α,并出现纤维化[3],而转染了psTNFR-I的小鼠肺纤维化的症状较空载体组明显改善[11]。Piguet等[12-13]报道rsTNFR-β能明显改善BLM或二氧化硅诱导的小鼠体内TNF-α mRNA水平的表达,早期抑制TNF-α的过度表达能明显减轻小鼠肺纤维化的病变。以上研究均证实阻断TNF-α信号通路可能是治疗肺纤维化的有效途径。
但是,目前TNF-α拮抗剂与组织纤维化的关系尚存在争议。体外实验中,TNF-α 促进肝脂细胞增殖的同时能抑制胶原蛋白的合成,在一定程度上抑制肝纤维化的进程[14]。单用甲氨蝶呤治疗3年后的风湿性关节炎患者在联用英利昔单抗后出现了严重的间质性肺纤维化,认为英利昔单抗能加重甲氨蝶呤的肺毒性效应[15]。本研究对BLM致纤维化小鼠给予Et腹腔注射,发现小鼠血清TNF-α的表达受到显著抑制,肺部炎症损伤和纤维化病变明显减轻,Masson染色示气道下胶原沉积显著减少,肺组织内代表胶原纤维代谢的产物HYP含量也明显降低,提示Et能有效抑制BLM诱导的小鼠肺纤维化。
MAPKs是细胞内的一类丝氨酸/苏氨酸蛋白激酶,MAPKs信号转导通路将细胞外刺激信号转导至细胞内,引起细胞的增殖、分化、转化和凋亡等生物学效应。研究证实,MAPKs的活化参与了纤维性病变的过程[16-17],ERK1/2、JNK和p38是主要的MAPK通路。TNF-α刺激人皮肤成纤维细胞能诱导MAPKs的活化,ERK1/2的活化又能促进TNF-α的自分泌,上调TNF-α的表达[7],从而介导TNF-α调节的成纤维细胞增殖和EMT过程。EMT是纤维化病灶中成纤维细胞的主要来源之一[18]。p38和JNK的活化也参与了EMT过程,有研究认为单独的p38不能完全诱导EMT,需要与其它MAPKs通路联合作用[7]。本实验中,BLM+Et组明显抑制了BLM诱导的TNF-α高表达和随后ERK1/2、p38和JNK的磷酸化,表明MAPKs通路参与了TNF-α诱导小鼠肺纤维化,并起协同作用。
TGF-β是目前已发现的与肺纤维化发生和形成最为亲密的因子,能通过Smad2、MAPKs和RhoA[19-21]等多种信号通路参与肺纤维化的进程,ERK1/2[22]、JNK[23]和p38[21]的活化是TGF-β促进成纤维细胞的增殖、分化和胶原沉积的重要通路。Sime等[3]认为TGF-β参与TNF-α调节的纤维性病变,而与TNF-α调控的单纯免疫炎症性疾病关系不大。白细胞介素1β、TNF-α等炎症因子能刺激TGF-β I型受体和Smad蛋白的表达[24]。本研究发现,BLM气道雾化后,TGF-β表达明显增高,而Et干预则显著抑制了TGF-β的表达,与之前Sullivan等[25]的研究结果相符。BLM致肺纤维化小鼠体内TNF-α的高表达诱导了TGF-β表达的增高,这可能与TNF-α促进TGF-β受体表达上调[24]和ERK1/2活化有关[25]。本研究中Et可能通过抑制TNF-α的表达及下游ERK1/2的活性,降低了肺纤维的小鼠体内TGF-β的表达。反过来,TGF-β表达上调又能促进MAPKs通路的磷酸化[21-23],形成相互作用的环路。Et通过抑制TGF-β的表达,从而阻断TGF-β下游与纤维化相关的信号级联反应。
本研究证实,Et能明显抑制BLM诱导的小鼠肺纤维化。Et一方面能直接阻断MAPKs信号通路,另一方面通过下调TGF-β的表达,抑制MAPKs通路的活化和纤维化的进程。众所周知,类风湿性关节炎容易累及肺脏,表现为肺间质纤维化,Et在治疗类风湿性关节炎的同时也能控制肺间质纤维化的发生、发展,这为临床治疗肺纤维化提供了新思路。
[1] Ortiz LA, Lasky J, Hamilton RF Jr,et al. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice[J]. Exp Lung Res,1998, 24(6):721-743.
[2] 靳长俊, 辛洪涛, 林殿杰,等.芪丹颗粒剂对大鼠肺纤维化模型的干预作用及对TGF-β1、TNF-α表达的影响[J].中国病理生理杂志,2006,22(4):814-817,820.
[3] Sime PJ, Marr RA, Gauldie D, et al.Transfer of tumor necrosis factor-α to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-β1 and myofibroblasts[J]. Am J Pathol,1998,153(3):825-832.
[4] Chuang MJ, Sun KH,Tang SJ,et al. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells[J]. Cancer Sci, 2008,99(5):905-913.
[5] Oikonomou N, Harokopos V, Zalevski J,et al .Soluble TNF mediates the transition from pulmonary inflammation to fibrosis[J].PLoS One,2006,1(1):e108.
[6] Mukhopadhyay S, Hoidal JR, Mukherjee TK.Role of TNFα in pulmonary pathophysiology[J]. Respir Res, 2006, 7:125.
[7] Vietor I, Schwenger P, Li W,et al. Tumor necrosis factor-induced activation and increased tyrosine phosphorylation of mitogen-activated protein (MAP) kinase in human fibroblasts[J]. J Biol Chem, 1993,268(25): 18994-18999.
[8] Grund EM, Kagan D, Tran CA, et al.Tumor necrosis factor-α regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor κB in fuman endometriotic epithelial cells[J].Mol Pharmacol ,2008,73(5):1394-1404.
[9] Mikawa K, Nishina K, Takao Y, et al. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits[J]. Anesth Analg, 2003, 97(6):1751-1755.
[10] Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale[J]. J Clin Pathol, 1988, 41(4):467-470.
[11] Przybyszewska M, Miloszewska J, Rzońca S,et al.Soluble TNF-α receptor I encoded on plasmid vector and its application in experimental gene therapy of radiation-induced lung fibrosis[J].Arch Immunol Ther Exp,2011, 59(4):315-326.
[12] Piguet PF,Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice[J]. Eur Respir J, 1994, 7(3): 515-518.
[13] Piguet PF, Collart MA, Grau GF, et al. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis[J]. J Exp Med,1989, 170(3):655-63.
[14] Armendariz-Borunda J, Katayama K, Seyer JM. Transcriptional mechanisms of type I collagen gene expression are differentially regulated by interleukin-1 beta, tumor necrosis factor alpha, and transforming growth factor beta in Ito cells[J]. J Biol Chem,1992,267(20):14316-14321.
[15] Villeneuve E, St-Pierre A, Haraoui B.Interstitial pneumonitis associated with infliximab therapy[J]. J Rheumatol, 2006, 33(6):1189-1193.
[16] Tsukagoshi H, Kawata T, Shimizu Y,et al. 4-Hydroxy-2-nonenal enhances fibronectin production by IMR-90 human lung fibroblasts partly via activation of epidermal growth factor receptor-linked extracellular signal-regulated kinase p44/42 pathway[J]. Toxicol Appl Pharmacol,2002,184(3): 127-135.
[17] Chen J, Chen JK, Nagai K,et al. EGFR signaling promotes TGFβ-dependent renal fibrosis[J]. J Am Soc Nephrol, 2012,23(2):215-224.
[18] Willis BC,duBois RM,Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung[ J ]. Proc Am Thorac Soc, 2006, 3(4): 377-382.
[19] Bhowmick NA, Ghiassi M, Aakre M, et al.Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism[J]. Mol Biol Cell,2001,12(1):27-36.
[20] Li JH, Zhu HJ, Huang XR.Smad7 inhibits fibrotic effect of TGF-β on renal tubular epithelial cells by blocking Smad2 activation[J]. J Am Soc Nephrol,2002, 13(6): 1464-1472.
[21] Bhowmick NA, Zent R, Ghiassi M, et al. Integrin β1signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity[J]. J Biol Chem,2001, 276(50): 46707-46713.
[22] Ellenrieder V, Hendler SF, Boeck W, et al.Transforming growth factor β1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation[J].Cancer Res,2001, 61(10): 4222-4228.
[23] 林云灵,李维维,陈良龙.TGF-β1作用后老龄大鼠心肌成纤维细胞p38 MAPK 通路和 JNK 通路的变化[J].中国病理生理杂志,2012,28(8):1420-1423.
[24] Liu X.Inflammatory cytokines augments TGF-β1-induced epithelial-mesenchymal transition in A549 cells by up-regulating TβR-I[J]. Cell Motill Cytoskeleton,2008,65(12):935-944.
[25] Sullivan DE, Ferris M, Pociask D, et al.Tumor necrosis factor-α induces transforming growth factor-β1expression in lung fibroblasts through the extracellular signal-regulated kinase pathway[J]. Am J Respir Cell Mol Biol,2005,32(4):342-349.
Etanerceptattenuatesbleomycin-inducedlungfibrosisinmice
HU Yu-jie1,2, LI Li1, LI Wei-feng1, HUANG Wen-jie1
(1DepartmentofRespiratoryMedicine,GuangzhouGeneralHospitalofGuangzhouMilitaryCommand,Guangzhou510010,China;2GraduateSchoolofSouthernMedicalUniversity,Guangzhou510515,China.E-mail:lwf980622@126.com)
AIM: To evaluate the effect of tumor necrosis factor α (TNF-α) antagonist etanercept on bleomycin-induced lung fibrosis in mice.METHODSForty-five Kunming female mice were randomly divided into 3 groups: the mice in control group were intraperitoneally injected with vehicle and intratracheally administered with saline aerosol, the mice in bleomycin group were intraperitoneally injected with vehicle and intratracheally administered with bleomycin (3 mg/kg) aerosol, and the mice in bleomycin+etanercept group were intraperitoneally injected with etanercept (4 mg/kg) every 3 d and intratracheally administered with bleomycin aerosol. All animals were sacrificed 28 d after treatments. The left lung was fixed in 10% neutral formalin and then stained with hematoxylin-eosin or Masson’s trichrome for the pathological examination. The tissues of right lung were sampled for measuring the content of hydroxyproline (HYP) by the method of alkaline hydrolysis. The serum concentrations of TNF-α and TGF-β were detected by ELISA. Total tissue protein was extracted for examination of ERK1/2, JNK and p38 by Western blotting.RESULTSEtanercept prevented the collagen accumulation under the airway epithelium and decreased the scores of lung inflammation and fibrosis induced by bleomycin with significantly reduced the levels of tissue HYP, serum TNF-α and serum TGF-β. The protein phosphorylations of ERK/JNK/p38 in the lung tissues were remarkably decreased compared with BLM group.CONCLUSIONEtanercept decreases the phosphorylations of ERK1/2/JNK/p38 via inhibiting the expression of TNF-α and TGF-β. Etanercept might be useful in the treatment of pulmonary fibrosis.
Pulmonary fibrosis; Tumor necrosis factor α; Etanercept; Bleomycin; Mitogen-activated protein kinases
R363
A
10.3969/j.issn.1000- 4718.2013.06.014
1000- 4718(2013)06- 1034- 05
2012- 11- 30
2013- 04- 09
广东省自然科学基金资助项目(No.S2011010000511);广东省科技计划项目(No.2010B031600122)
△通讯作者Tel: 020-86653555; E-mail: lwf980622@126.com

