Crystal Structure of PAI-1 in Complex with Gallate①
HONG Ze-Bin LIN Zhong-Hui GONG Li-Hu HUANG Ming-Dong②
a (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China)b (Graduate School of Chinese Academy of Sciences 100039, China)
1 INTRODUCTION
Plasminogen activator inhibitor-1 (PAI-1) is the primary physiological inhibitor of urokinase-type plasminogen activator (uPA)[1]. PAI-1 is well-known for its negative regulator role in the fibrinolytic system (for review see reference[2]). Transgenic mice over-expressing high levels of PAI-1 cause spontaneous thrombosis[3-4], whereas inhibition of PAI-1 activity by gene-deletion[5]or antibodies prevents thrombus formation[6-7]. In addition, extensive studies have shown that PAI-1 plays a key role in cell migration and adhesion, probably by competing with integrin and the uPA receptor for vitronectin binding[8-9], or by serving a role in angiogenesis[10]and stimulating cell proliferation[11]. In PAI-1 genedeficient mice, local invasion and tumor vascularization of transplanted malignant keratinocytes was blocked[10]. In human malignant tumors, the levels of uPA and PAI-1 are significantly higher than those in the corresponding normal tissues[12]. Moreover, uPA and PAI-1 are currently recognized as the most extensively validated biological prognostic factors in breast cancer[13]. PAI-1 is therefore a potential target for treatments of thrombosis and cancer. During the past decades, many efforts have been made to the development of PAI-1 inactivators, and several small molecules (Table 1)[14-20,36-37], monoclonal antibodies[21], peptide[22]and RNA aptamers[23]have been reported.
PAI-1 belongs to the serine protease inhibitor(serpin) family, which shares a unique structural feature including three β-strands, nine α-helixes and a flexible reactive center loop (RCL). PAI-1 inhibits its target proteases following the classical serpin mechanism of protease inhibition[24]. In its active conformation, RCL is flexible and will dock into the active site of the target protease, forming a reversi-ble Michaelis complex. RCL loop is then cleaved by the protease, but remains attached to the protease,forming a metastable acyl enzyme intermediate. The cleaved PAI-1 undergoes a rapid stressed-to-relaxed transition that causes the protease to move from the top to the bottom of PAI-1. At the same time, the active site of protease is distorted to prevent acyl enzyme intermediate from hydrolysis, leading to the loss of catalytic activity of the target protease. In the case of slow stressed-to-relaxed transition, the acyl enzyme intermediate could be hydrolyzed, which results in a RCL-cleaved and inactive PAI-1. Active PAI-1 can undergo spontaneous stressed-to-relaxed conversion by burying its RCL into one of its beta sheets (βA), resulting in inactive PAI-1 at its latent conformation.
In recent years, many small molecular PAI-1 inhibitors have been reported (summarized in Table 1).A novel class of PAI-1 inhibitors has been discovered by Lawrence group recently[17]. This class of PAI-1 inhibitors is polyphenolic compounds, and has IC50 ranging from 7 nM (tannic acid) to 51 µM(Sennoside A). One of the best inhibitors is CDE-066 with an IC50 of 10 nM (Fig. 1). All these compounds contain gallic acid moieties which are a major active component of herbs (Chinese gall).Gallic acid inhibits PAI-1 with an IC50 of 6.6 μM(Fig. 1[17]). Many studies have shown that gallic acid plays a crucial role in the suppression of malignant transformation, cancer development, and inflammation[25-26].
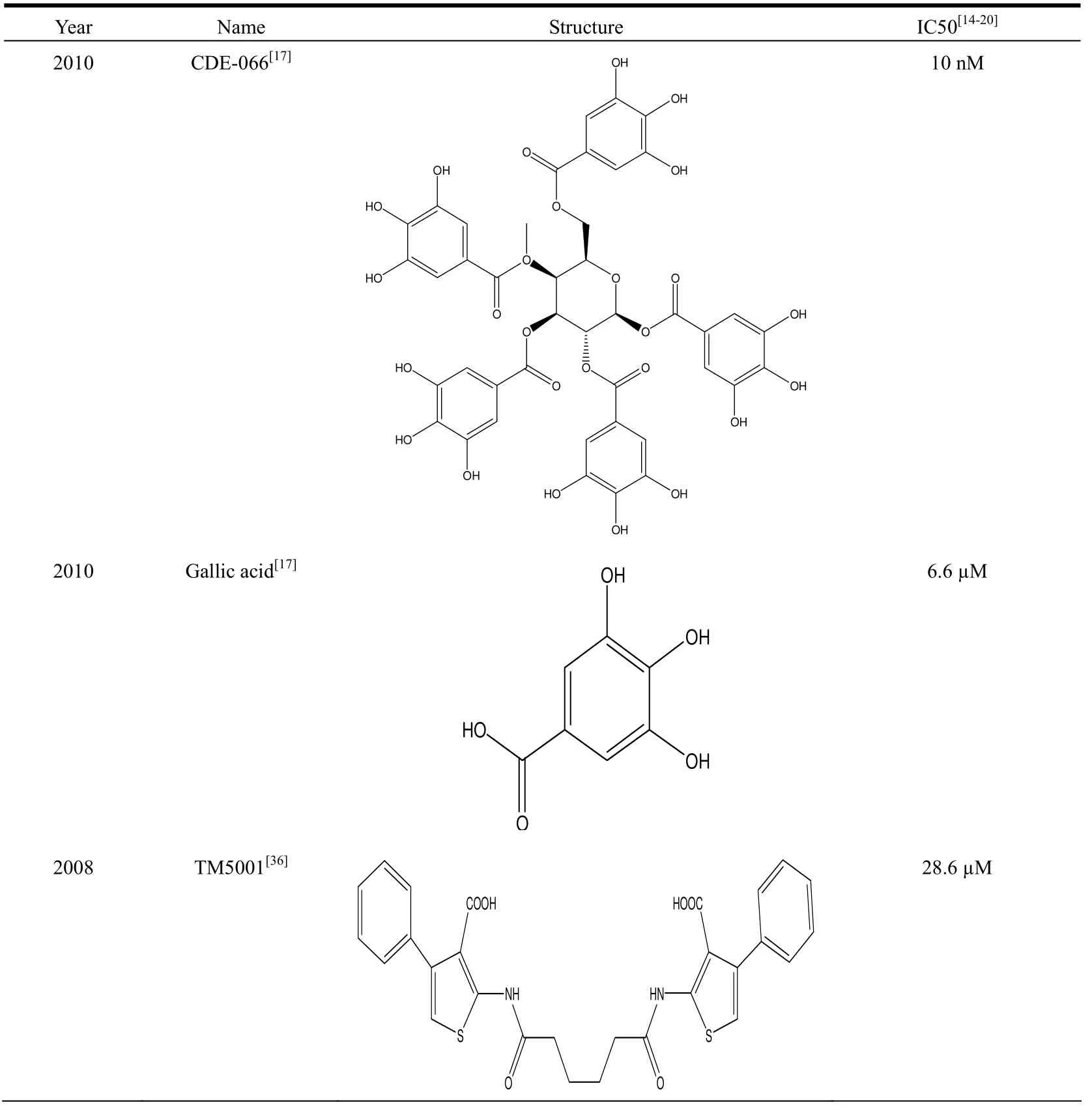
Table 1. Reported Small Molecular PAI-1 Inhibitors
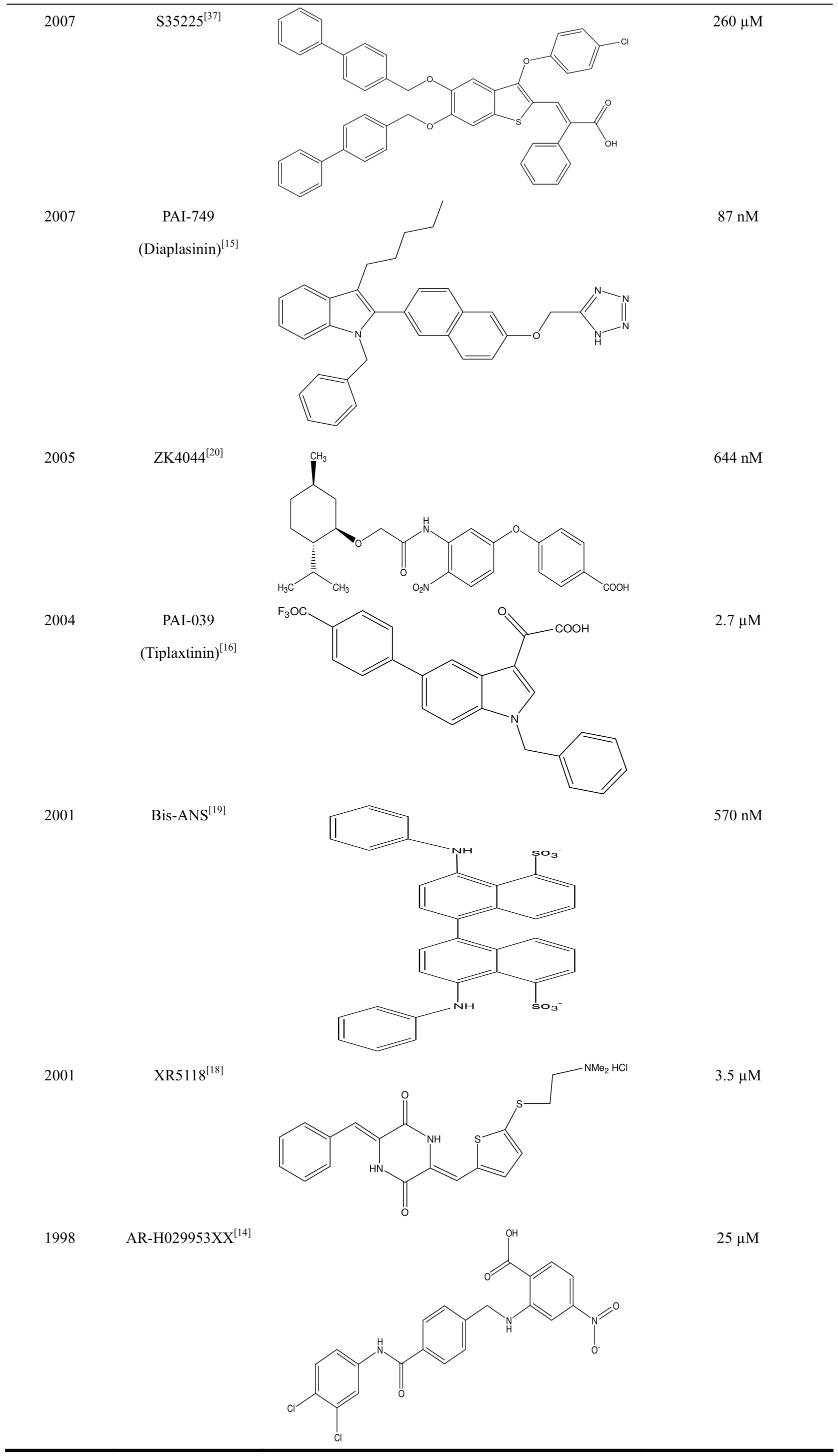
2007 S35225[37]2007 PAI-749(Diaplasinin)[15]2005 ZK4044[20]l N N CH3260 µM 87 nM 644 nM 2004 PAI-039(Tiplaxtinin)[16]2001 Bis-ANS[19] 2.7 µM 570 nM 2001 XR5118[18]l 3.5 µM O 1998 AR-H029953XX[14]OHO Cl 25 µM Cl

Fig. 1. (a) Gallate (green) binds at a cavity formed by α helix D, E, F (cyan) and β sheet A(red) of PAI-1. (b) Detailed interaction of gallate with the surrounding residues of PAI-1
It is interesting to note that the chemical structures of these small molecular inhibitors are quite diversified. This is in large contrast to other enzymatic inhibitors, e.g. inhibitors of serine protease, which very often contains basic chemical moieties (guanidine or amidine) targeting at a specific pocket (S1 pocket)0[27]. In this study, we report the crystal structure of PAI-1 in complex with gallic acid and propose an inhibitory mechanism of this inhibitor based on the crystal structure.
2 EXPERIMENTAL
2.1 Protein expression and purification
Purified pT7-PL/PAI-1(14-1B, with N152HK156T-Q321L-M356I mutations) vectors were transformed into competent BL21 (DE3) cells. The cells were cultured overnight at 37 ℃ in LB con-taining 100 μg/mL ampicillin. A 10 mL aliquot of this starter culture was added to 1 L LB containing 1 g/L glucose and grown for 6 h. Expression of PAI-1(14-1B) was induced by the addition of 0.5 mM IPTG and the culture incubated overnight. Cells were collected by centrifugation at 10,000 g for 10 min. and lysed by sonication, followed by centrifugation in buffer A (50 mM MES, pH 6.1, 1.0 M NaCl). The hexa-His-tagged PAI-1 in the supernatant was subsequently captured through a nickel column. PAI-1(14-1B) was collected and dialyzed for 2 h against buffer A.
2.2 Crystallization of PAI-1:gallate complex and data collection
PAI-1(14-1B) was concentrated to about 10 mg/mL and crystallized at room temperature using the sitting-drop technique with equal volumes (1 μL)of protein and reservoir solution (1.1 M ammonium sulfate, 0.1 M MES, pH 6.1). The crystals appeared as clustered thin plates. The single crystals with diffraction-quality were finally obtained by streak seeding method. These crystals were then soaked for 2 days in the crystallization mother liquid containing supersaturated gallic acid. At this pH value, the gallic acid is most likely to convert to the sodium gallate, thus we will use the term of gallate in the following discussion. For X-ray data collection, a single crystal was transferred to a cryoprotectant solution containing 20% glycerol, 1.6 M ammonium sulfate, and 0.1 M MES at pH = 6.1, and was flash-frozen in a liquid nitrogen stream. Diffraction data were collected at BL17U of Shanghai Synchrotron Radiation Facility (SSRF). All diffraction data were indexed and integrated by the HKL2000 program suite[28].
2.3 Crystal structure determination and refinement
The crystal structure of PAI-1:gallate complex was solved using the program MOLREP[29]and the structure of active PAI-1 (PDB code 1DVM) as a searching model. After refinement with REFMAC5 program[30], the sigma-weighted Fo–Fc map clearly showed the electron density of gallate. The resulting model was refined and manually built iteratively using REFMAC5 and COOT[31]. The final structures were analyzed by PROCHECK[32]and PYMOL[33].
3 RESULTS AND DISCUSSION
3.1 Crystal structure of PAI-1 in complex with gallic acid
PAI-1 contains a number of cavities in its structure, however, the binding sites of small molecular inhibitors, including gallic acid, on PAI-1 are not known. To study the inhibitory mechanism of gallic acid, we solved the crystal structure of PAI-1 in complex with gallic acid to 2.3 Å. The crystals are in space group P1 and have cell parameters of a =65.28, b = 74.99, c = 103.87 Å, α = 90.91, β = 93.28,and γ = 115.82°. The X-ray data were collected to high quality with the Rmerge of 0.053, the completeness of 94.23%, and the redundancy of 3.5.The structure was refined to the R and Rfreevalues of 0.0205 and 0.0258 and tight stereochemistry with root mean square deviations of bond lengths and bond angle of 0.016 Å and 2.00°, respectively. The structure was determined at pH 6.1, and at this pH value, the gallic acid is most likely to convert to the sodium gallate, so we will use the term of gallate in the following discussion. A major challenge for the structural study of active PAI-1 is its short half-life time and aggregation at high concentration. The active PAI-1 converts spontaneously to the inactive latent form with a half-life of 1~2 h at 37 ℃. To overcome these difficulties, we used the stable PAI-1 variant (N152H-K156T-Q321L-M356I, named 14-1B)[34], which maintains its activity but has a much longer half-life (140 h) and thus is suitable for structural studies.
The crystal structure of the complex showed that PAI-1 has the typical serpin fold with three β-sheet strands, nine α-helixes and a flexible reactive center loop (RCL). The current crystal form contains 4 PAI-1 molecules in the crystallographic asymmetric unit. Gallate molecules were found in two PAI-1 molecules (A and C). However, these two gallate molecules have identical binding conformations.Gallate locates in a cavity formed by PAI-1 helix D,helix E, helix F and β-strand 2A. Gallate is at the edge of β-strand 2A, forming hydrogen bonds to the PAI-1 residues Thr94 (2.7 Å) and Asp95 (3.0 Å) of β-strand 2A. Gallate also makes direct contacts to residues Tyr79, Ser119 and Trp139 from helix D,helix E, and helix F, respectively. Taking together,the presence of gallate locks together the secondary structural elements of helix D, helix E, helix F and β-strand 2A.
3.2 Structural comparison of PAI-1:gallate with active form PAI-1 and latent form PAI-1
We observed that PAI-1 conformation in its complex with gallate is quite similar to the active form PAI-1 (PDB code 1dvm[35]) with root mean square deviations (RMSD) of 0.3~0.4 Å when all atoms were compared. This confirms that the PAI-1 in the PAI-1:gallate is in an active form, and also shows that gallate does not perturb the conformation of PAI-1.
On the contrary, comparison of PAI-1 in its complex with gallate with the latent form PAI-1 (PDB code 1dvn[35]) shows large difference (RMSD of 9.0 Å). This is mainly due to the insertion of N-terminal side of RCL into the beta sheet A of PAI-1, leading to the movement of beta strand 2A. This movement also eliminates the pocket that accommodates gallate(Fig. 2).
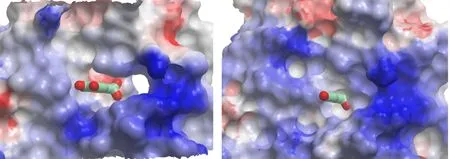
Fig. 2. Gallate locating in a pocket of PAI-1 (left, in charge surface representation).This pocket does not exist in the inactive latent form of PAI-1 (right)
3.3 Inhibitory mechanism of the active PAI-1 by gallate
Under physiological conditions, active PAI-1 was recognized by uPA, forming a reversible Michaelis complex. The RCL loop of PAI-1 is cleaved by uPA at the P1-P1΄ site followed by inserting the N-terminal side of RCL into the beta strand A of PAI-1 as a new beta strand (s4A). This formation of new s4A requires the expansion of beta sheet A and the movement of s2A strand. The uPA protease was then dragged to the other pole of PAI-1 and was inactivated due to the distortion of its active site, forming a covalent inactivated protease complex. The presence of gallate fixes β-strand 2A to the neighboring α-helixes D and F and thus delays the expansion of β-sheet A during RCL insertion (Fig. 3). This gives enough time for the uPA protease to complete the catalytic cycle, resulting in the cleavage of PAI-1 into the inactive cleaved form.
In conclusion, we determined the crystal structure of PAI-1:gallate complex and found that gallate inserts into the cavity formed by helix D, helix E,helix F and β-strand 2A of PAI-1. This work provides structural insights into the PAI-1 inhibitory mechanism of gallic acid, which may be applicable to other PAI-1 inhibitors.
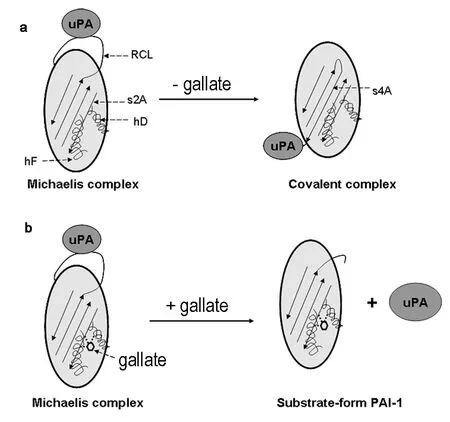
Fig. 3. Structural mechanism of PAI-1 inhibition by gallate. (a) Physiologically, PAI-1 recognizes uPA and forms a reversible Michaelis complex. The RCL loop of PAI-1 is then cleaved by uPA at the P1-P1΄ site followed by inserting the N-terminal side of RCL into the beta sheet A of PAI-1, forming a new s4A. The protease was then dragged to the other pole of PAI-1 to form a SDS-stable inactivated protease complex. (b) Gallate binds to residues on the βl strand s2A and α-helix D and F of PAI-1, bonding together this region. Thus gallate hinders the movement of s2A and α-helix F and delay the insertion of RCL as s4A, leading to the cleavage of PAI-1 as a substrate and the conversion to the cleaved form
(1) Andreasen, P. A.; Kjoller, L.; Christensen. L.; Duffy, M. J. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int. J.Cancer 1997, 72, 1–22.
(2) Vaughan, D. E. Plasminogen activator inhibitor-1: a common denominator in cardiovascular disease. J. Investig. Med. 1998, 46, 370-6.
(3) (Eren, M.; Painter, C. A.; Atkinson, J. B.; Declerck, P. J.; Vaughan, D. E. Age-dependent spontaneous coronary arterial thrombosis in transgenic mice that express a stable form of human plasminogen activator inhibitor-1. Circulation 2002, 106, 491–6.
(4) Erickson, L. A.; Fici, G. J.; Lund, J. E.; Boyle, T. P.; Polites, H. G.; Marotti, K. R. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nature 1990, 346, 74–6.
(5) Carmeliet, P.; Stassen, J. M.; Schoonjans, L.; Ream, B.; van den Oord, J. J.; De Mol, M.; Mulligan, R. C.; Collen, D. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J. Clin. Invest. 1993, 92, 2756–60.
(6) Abrahamsson, T.; Nerme, V.; Stromqvist, M.; Akerblom, B.; Legnehed, A.; Pettersson, K.; Westin, E. A. Anti-thrombotic effect of a PAI-1 inhibitor in rats given endotoxin. Thromb. Haemost. 1996, 75, 118–26.
(7) Van Giezen, J. J.; Wahlund, G.; Abrahamsson, T. The Fab-fragment of a PAI-1 inhibiting antibody reduces thrombus size and restores blood flow in a rat model of arterial thrombosis. Thromb. Haemost. 1997, 77, 964–9.
(8) Deng, G.; Curriden, S. A.; Wang, S.; Rosenberg, S.; Loskutoff, D. J. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J. Cell Biol. 1996, 134, 1563–71.
(9) Stefansson, S.; Lawrence, D. A. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature 1996,383, 441–3.
(10) Bajou, K.; Noel, A.; Gerard, R. D.; Masson, V.; Brunner, N.; Holst-Hansen, C.; Skobe, M.; Fusenig, N. E.; Carmeliet, P.; Collen, D. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998, 4, 923–8.
(11) Webb, D. J.; Thomas, K. S.; Gonias, S. L. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes MCF-7 cell growth. J. Cell Biol. 2001, 152, 741–52.
(12) Look, M. P.; Van Putten, W. L.; Duffy, M. J.; Harbeck, N.; Christensen, I. J.; Thomssen, C.; Kates, R.; Spyratos, F.; Ferno, M.;Eppenberger-Castori, S. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J. Natl. Cancer Inst. 2002, 94, 116–28.
(13) Duffy, M. J. Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: from pilot to level 1 evidence studies.Clin. Chem. 2002, 48, 1194–7.
(14) Gils, A.; Stassen, J. M.; Nar, H.; Kley, J. T.; Wienen, W.; Ries, U. J.; Declerck, P. J. Characterization and comparative evaluation of a novel PAI-1 inhibitor. Thromb. Haemost. 2002, 88, 137–43.
(15) Gardell, S. J.; Krueger, J. A.; Antrilli, T. A.; Elokdah, H.; Mayer, S.; Orcutt, S. J.; Crandall, D. L.; Vlasuk, G. P. Neutralization of plasminogen activator inhibitor I (PAI-1) by the synthetic antagonist PAI-749 via a dual mechanism of action. Mol. Pharmacol. 2007, 72, 897–906.
(16) Elokdah, H.; Abou-Gharbia, M.; Hennan, J. K.; McFarlane, G.; Mugford, C. P.; Krishnamurthy, G.; Crandall, D. L. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. J. Med. Chem. 2004, 47, 3491–4.
(17) Cale, J. M.; Li, S. H.; Warnock, M.; Su, E. J.; North, P. R.; Sanders, K. L.; Puscau, M. M.; Emal, C. D.; Lawrence, D. A. Characterization of a novel class of polyphenolic inhibitors of plasminogen activator inhibitor-1. J. Biol. Chem. 2010, 285, 7892–902.
(18) Friederich, P. W.; Levi, M.; Biemond, B. J.; Charlton, P.; Templeton, D.; Van Zonneveld, A. J.; Bevan, P.; Pannekoek, H.; ten Cate, J. W. Novel low-molecular-weight inhibitor of PAI-1 (XR5118) promotes endogenous fibrinolysis and reduces postthrombolysis thrombus growth in rabbits.Circulation 1997, 96, 916–21.
(19) Pedersen, K. E.; Einholm, A. P.; Christensen, A.; Schack, L.; Wind, T.; Kenney, J. M.; Andreasen, P. A. Plasminogen activator inhibitor-1 polymers,induced by inactivating amphipathic organochemical ligands. Biochem. J. 2003, 372, 747–55.
(20) Liang, A.; Wu, F.; Tran, K.; Jones, S. W.; Deng, G.; Ye, B.; Zhao, Z.; Snider, R. M.; Dole, W. P.; Morser, J. Characterization of a small molecule PAI-1 inhibitor, ZK4044. Thromb. Res. 2005, 115, 341–50.
(21) Komissarov, A. A.; Andreasen, P. A.; Bodker, J. S.; Declerck, P. J.; Anagli, J. Y.; Shore, J. D. Additivity in effects of vitronectin and monoclonal antibodies against alpha-helix F of plasminogen activator inhibitor-1 on its reactions with target proteinases. J. Biol. Chem. 2005, 280, 1482–9.
(22) Mathiasen, L.; Dupont, D. M.; Christensen, A.; Blouse, G. E.; Jensen, J. K.; Gils, A.; Declerck, P. J.; Wind, T.; Andreasen, P. A. A peptide accelerating the conversion of plasminogen activator inhibitor-1 to an inactive latent state. Mol. Pharmacol. 2008, 74, 641–53.
(23) Blake, C. M.; Sullenger, B. A.; Lawrence, D. A.; Fortenberry, Y. M. Antimetastatic potential of PAI-1-specific RNA aptamers. Oligonucleotides 2009, 19, 117–28.
(24) Dupont, D. M.; Madsen, J. B.; Kristensen, T.; Bodker, J. S.; Blouse, G. E.; Wind, T.; Andreasen, P. A. Biochemical properties of plasminogen activator inhibitor-1. Front. Biosci. 2009, 14, 1337–61.
(25) Liu, Z.; Schwimer, J.; Liu, D.; Lewis, J.; Greenway, F. L.; York, D. A.; Woltering, E. A. Gallic acid is partially responsible for the antiangiogenic activities of Rubus leaf extract. Phytother. Res. 2006, 20, 806–13.
(26) Garcia Mediero, J. M.; Ferruelo Alonso, A.; Paez Borda, A.; Lujan Galan, M.; Angulo Cuesta, J.; Chiva Robles, V.; Berenguer Sanchez, A. Effect of polyphenols from the Mediterranean diet on proliferation and mediators of in vitro invasiveness of the MB-49 murine bladder cancer cell line.Actas Urol. Esp. 2005, 29, 743–9.
(27) Ngo, J. C.; Jiang, L.; Lin, Z.; Yuan, C.; Chen, Z.; Zhang, X.; Yu, H.; Wang, J.; Lin, L.; Huang, M. Structural basis for therapeutic intervention of uPA/uPAR system. Curr. Drug Targets. 2011, 12, 1729–43.
(28) Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol, Academic Press, New York,1997, pp. 307–326.
(29) Vagin, A.; Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Cryst. 1997, 30, 1022–1025.
(30) Murshudov, G. N.; Vagin, A. A.; Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D.Biol. Crystallogr. 1997, 53, 240–55.
(31) Emsley, P.; Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–32.
(32) Laskowski, R. A.; MacArthur, M. W.; Mass, D. S.; Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallograph. 1993, 26, 283–291.
(33) DeLano, W. L. The PyMol Molecular Graphics System. DeLano Scientific, San Carlos, CA 2004
(34) Lawrence, D. A.; Ginsburg, D.; Day, D. E.; Berkenpas, M. B.; Verhamme, I. M.; Kvassman, J. O.; Shore, J. D. Serpin-protease complexes are trapped as stable acyl-enzyme intermediates. J. Biol. Chem. 1995, 270, 25309–12.
(35) Stout, T. J.; Graham, H.; Buckley, D. I.; Matthews, D. J. Structures of active and latent PAI-1: a possible stabilizing role for chloride ions.Biochemistry 2000, 39, 8460–9.
(36) Izuhara, Y.; Yamaoka, N.; Kodama, H.; Dan, T.; Takizawa, S.; Hirayama, N.; Meguro, K.; Strihou, C. Y.; Miyata, T. A novel inhibitor of plasminogen activator inhibitor-1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. Journal of Cerebral. Blood Flow & Metabolism 2010, 30, 904–912.
(37) Rupin, A.; Gaertner, R.; Mennecier, P.; Richard, I.; Benoist, A.; Nanteuil, G. D.; Verbeuren, T. J. S35225 is a direct inhibitor of Plasminogen Activator Inhibitor type-1 activity in the blood. Thrombosis Research. 2008, 122, 265–270.
- 结构化学的其它文章
- Synthesis of a Weak Basic uPA Inhibitor and Crystal Structure of a Complex with uPA①
- Study on the Structure and Characteristics of Recyclable Copper Removal Adsorbent①
- Interesting Weak Interactions in Three Silver Coordination Polymers Based on Tetrafluorobenzenedicarboxylate and Benzonitrile Ligands①
- Synthesis and Crystal Structure of (Z)-N-(2-(diethylamino)ethyl)-7-(5-fluoro-2-oxoindolin-3-ylidene)-2-methyl-4,5,6,7-tetrahydro-1H-indole-3-carboxamide Methanol Solvate①
- Synthesis, Crystal Structure, and Biological Activity of 4-Chlorobenzaldehyde(2-trifluoromethylTrifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]- thieno[2,3-d]pyrimidin-4-yl)hydrazone Monohydrate①
- DFT Study on the Electronic and Structural Properties of MoS6-/0 Clusters①

