Interesting Weak Interactions in Three Silver Coordination Polymers Based on Tetrafluorobenzenedicarboxylate and Benzonitrile Ligands①
LIU Shu-Qin LI Xiao-Juan DI Ling DING Bao-Jun ZHANG Jian-Jun
(Chemistry College, Dalian University of Technology, Dalian 116024, China)
1 INTRODUCTION
Recently, much progress has been made in the development of coordination polymers (CPs) due to their intriguing structural diversities and many interesting properties[1-2]. Among them, Ag+based CPs occupy an important position as Ag+is an extremely soft Lewis acid, which causes the metal ion to adopt various coordination geometries[3].Furthermore, Ag(I) also has a good affinity for many functional groups. Ag(I) CPs based on polycarboxylates and benzonitrile derivatives have been well developed. However, similar compounds derived from polycarboxylates/benzonitrile mixed ligands were less concerned[4].
Weak interactions such as hydrogen bonds and π-π stacking interactions play an important role in the supramolecular solid-state architectures[5].Although many reports about these interactions have been published, their strength and geometrical preferences are still not very clear and a detailed understanding of these interactions is still required for rational supramolecular design.
Herein, we report three CPs based upon mixed ligands (tetrafluorobenzenedicarboxylic acid and benzonitrile): 1, 2 and 3. A variety of weak interactions are observed in the compounds and play an important role in affecting the final overall structures of them.
2 EXPERIMENTAL
2.1 Synthesis
[Ag2(PhCN)2(o-HL)2]n (1)A mixture of AgCF3CO2(0.1 mmol) and o-H2L (0.2 mmol) in 3 mL benzonitrile was stirred for 30 min before being transferred to a glass tube and layered with hexane as a diffusion solvent. After 1 week, colorless block crystals were obtained, washed with EtOH and dried in air (51% yield). Elemental analysis (%) calcd. for C30H12Ag2F8N2O8: C, 40.21; H, 1.35; N, 3.13.Found (%): C, 40.21; H, 1.35; N, 3.53.
[Ag2(PhCN)2(m-HL)2]n (2)The above mentioned synthesis procedure was repeated except m-H2L was used. The colorless block crystals were obtained, washed with EtOH and dried in air (61%yield). Elemental analysis (%) calcd. for C15H6Ag-F4NO4: C, 40.21; H, 1.35; N, 3.13. Found (%): C,40.40; H, 1.39; N, 3.62.
[Ag2(PhCN)2(p-L)]n (3)The above mentioned synthesis procedure was repeated except o-H2L was replaced by p-H2L. The colorless block crystals were obtained, washed with EtOH and dried in air (67%yield). Elemental analysis (%) calcd. for C44H20Ag4F8N4O8: C, 40.15; H, 1.53; N, 4.26.Found (%): C, 40.55; H, 1.53; N, 4.66.
2.2 Structure determination
Intensity data were measured at 150.2 K on a Rigaku/MSC Mercury CCD area detector system with a graphite-monochromated MoKα radiation (λ= 0.07107 nm) by using an ω scan mode. A symmetry-related absorption correction was applied. All intensity data were corrected for Lorentz and polarization effects. The structure was solved by direct methods and refined by full-matrix least-squares methods using SHELXL-97[6]. All the full-occupancy non-hydrogen atoms were refined anisotropically. Hydrogen atoms on C atoms were generated and refined isotropically. Hydrogen atoms related to carboxylate group of 2 were located from Fourier map. Details of X-ray experiment and crystal data are summarized in Table 1. Selected bond lengths and bond angles are listed in Table 2.
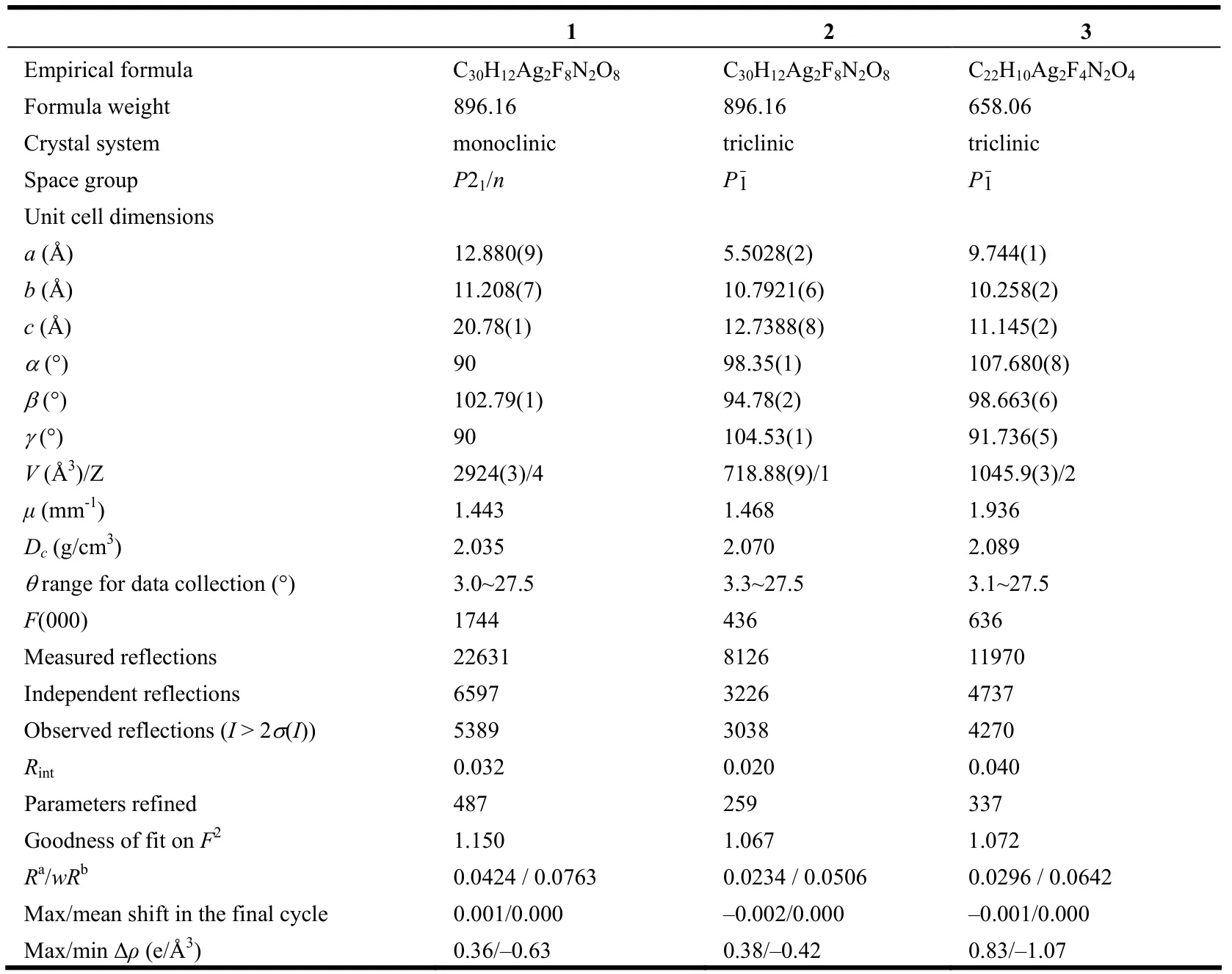
Table 1. Crystal Data Collection and Structure Refinement Parameters for Compounds
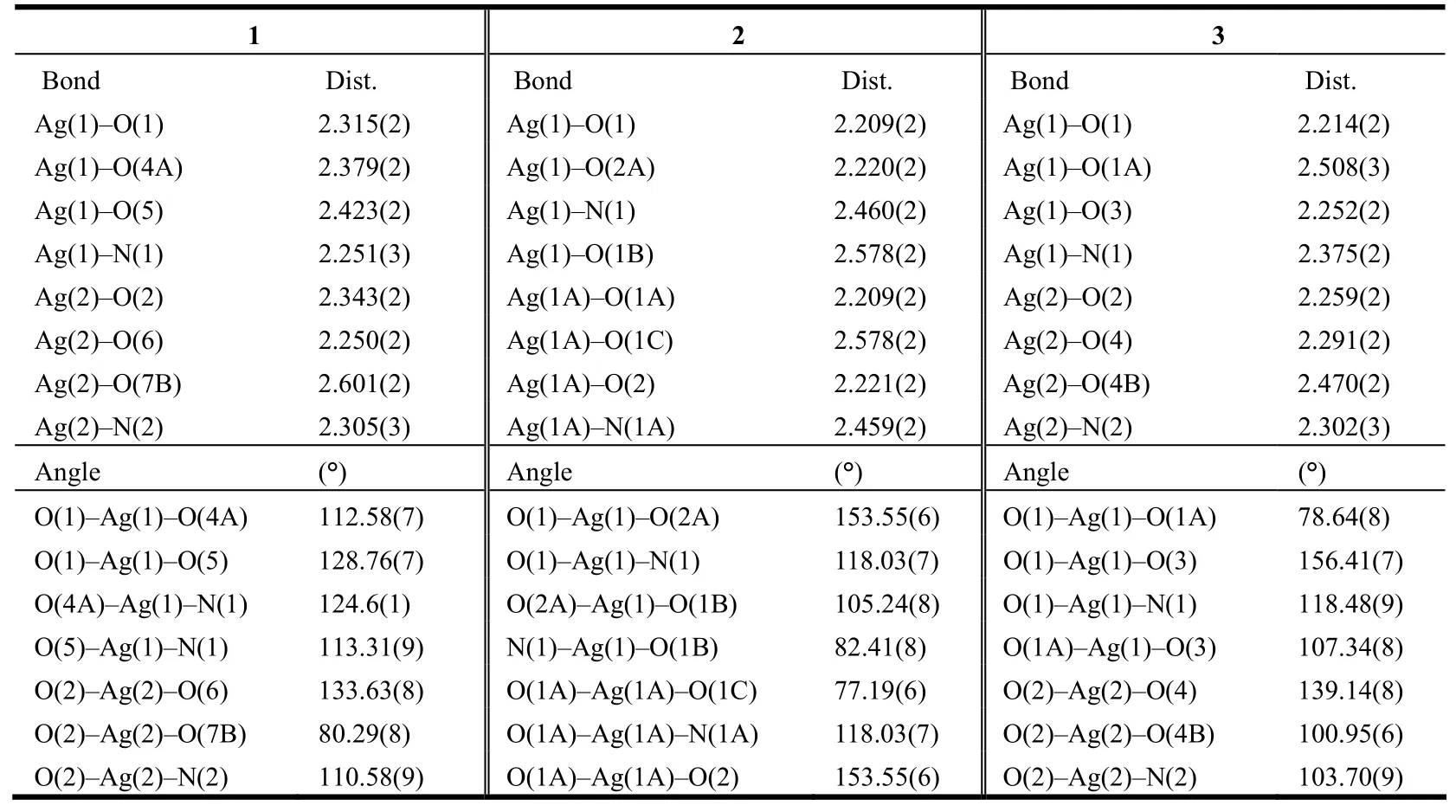
Table 2. Selected Bond Lengths (Å) and Bond Angles (°) for the Compounds
3 RESULTS AND DISCUSSION
3.1 Crystal structure of 1
1 features 1D CPs based on 4-coordinated Ag+ions connected by o-HL ligands. Fig. 1(a) shows the coordination environment of the two independent Ag+ions in the compound. Both Ag+ions have a distorted tetrahedral {NO3} donor set. The bond angles around Ag+ions range from 80.29(8) to 133.63(8)°. The Ag–O and Ag–N bond distances are in the ranges of 2.250(2)~2.601(2) and 2.251(3)~2.305(3) Å, respectively. All are consistent with the values reported for Ag-carboxylate and Ag-benzonitrile complexes[4]. Each o-HL ligand is coordinated to three Ag ions and bears a μ3-ηO1:ηO1:ηO1coordination mode. Two neighboring Ag ions (Ag1 and Ag2) are bridged by two carboxylate groups and the separation is 3.173(2) Å, shorter than that of the vander Waals Ag–Ag contact (3.40 Å) and longer than that in the metallic silver (2.89 Å), suggesting the existence of weak argentophilic interaction[3].

Fig. 1. Structure of 1. (a) Coordination environment of the Ag+ ions. Symmetry codes: A: 1–x, –y, –z;B: 2–x, –y, –z; (b) Structure of the 1D chain. π-π interaction is represented as the dotted lines
With the help of o-HL ligands, the {Ag2} subunits are connected into a 1 D wave-like chain decorated with benzonitrile, running along the a direction.Inside the chain, the neighboring benzonitrile and o-HL are packed in such a close mode that strong offset face-to-face π-π interaction is formed between contiguous benzene rings with ring-ring separation of 3.770 Å (Fig. 1(b)). Besides, the afore-mentioned two benzene rings further form strong offset face-to-face π-π interactions with the benzene rings of o-HL ligand from neighboring chain (ring-ring separations are 3.815 and 3.634 Å respectively).Thus an interesting π-π interaction chain running along the b direction is formed. Furthermore, edgeto-face π-π interactions between C(26)–H(9) and C(29)–H(12) of one benzonitrile to the benzene ring of another independent benzonitrile (C(26) to C(29))are also observed in the structure. The correspondence separations are 3.260 and 3.449 Å, respectively. All these π-π interactions connect the 1D coordination chains into a 3 D supramolecular network (Fig. 2).
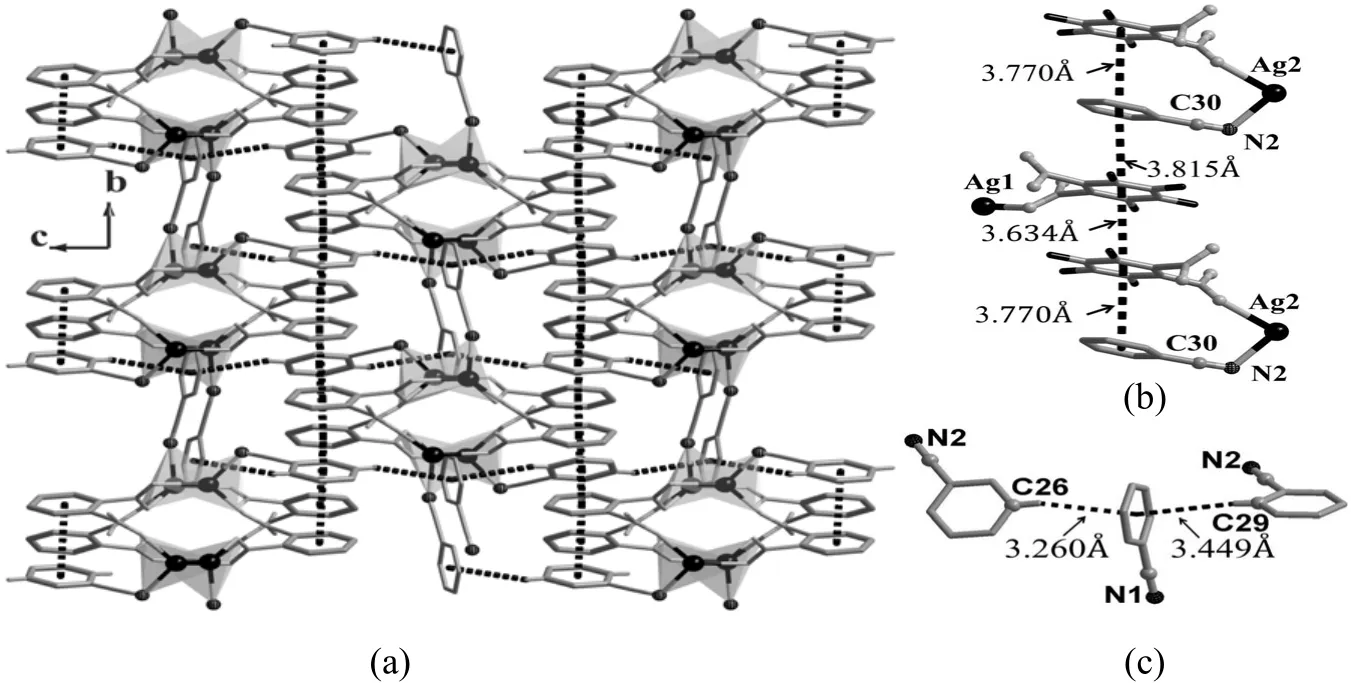
Fig. 2. Structure of 1. (a) π-π interaction nets inside the structure; (b) Offset face-to-face π-π interactions extended along the b direction; (c) Edge-to-face π-π interactions extended along the c direction. π-π interaction is represented as dotted lines and hydrogen atoms are omitted for clarity

Fig. 3. Structure of 2. (a) Coordination environment of the Ag+ ion. Symmetry codes: A: 1–x, 1–y, 1–z;B: –x, 1–y, 1–z; C: 1+x, y, z; (b) 1D coordination polymer extended along the a direction; (c) Overall 2D layered structure viewed along the a direction. Hydrogen bond is represented as the dotted lines
3.2 Crystal structure of 2
The structure of 2 may be described as 1D CPs based on Ag+nodes, m-HL and benzonitrile ligands(Fig. 3). In the structure, each Ag+ion also has a distorted tetrahedral {NO3} donor set. Neighboring Ag ions are bridged either by two carboxylate groups or just two carboxylate oxygen atoms. The corresponding Ag··Ag distances are 2.980(2) and 3.748 Å respectively, also suggesting the existence of weak argentophilic interaction[3]. Each dicarboxylate ligand only uses one carboxylate group to coordinate to three Ag ions. Each PhCN ligand acts as a terminal ligand and only uses nitrogen atom to coordinate to the Ag+ion. Ag+ions are bridged by the ligands to form a 1D coordination polymer, running along the a direction. Hydrogen bonds between the free disordered carboxylate groups (O··O distances are in the range of 2.62(1)–2.73(1) Å)connect the chains into layers, as shown in Fig. 3(c).
3.3 Crystal structure of 3
3 possesses 2D nets built of Ag+ions, p-L and benzonitrile ligands. Both of the two independent Ag+ions have a distorted tetrahedral {NO3} donor set (Fig. 4). Each p-L ligand uses two carboxylate groups to coordinate to six Ag+ions. The two neighboring Ag ions (Ag(1) and Ag(2)) are bridged by two carboxylate groups and their separation is 2.897(1) Å.

Fig. 4. Structure of 3. (a) Coordination environment of the Ag ion.Symmetry codes: A: 1–x, 1–y, 1–z; B: 1–x, –y, 1–z; (b) Structure of the 2D net
Ag+ions are bridged by the dicarboxylate ligands to form a 2D net decorated with terminal PhCN ligands and extended along the ab plane. Three different π-π interactions are observed between the PhCN ligands in 3. The first is the offset face-to-face π-π interaction between the PhCN ligands (N(1) and N(1B)) from two neighboring layers respectively.The two benzene rings are almost parallel with the center distance of 3.835 Å. The second is also an offset face-to-face π-π interaction but is between the PhCN ligands of N(2A) and N(2C). The two benzene rings are also almost parallel with a center distance of 4.609 Å. However, the cyanide groups and the benzene rings are packed in such a close mode that strong double offset face-to-face π-π interactions are formed with the corresponding separation of 3.593 Å. The third is a π-π interaction between the cyanide group of one PhCN ligand(N(1)) and the benzene ring of another PhCN ligand(N(2C)). They are not parallel and the separation of the center of cyanide to the benzene ring is 3.460 Å.These π-π interactions connect the 2D layers into a 3D supramolecular structure (Fig. 5).
3.4 Structural comparison of 1ˉ3 and the influencing factors
Depending on the sites of dicarboxylate groups on the benzene ring, the three dicarboxylate ligands can adopt different coordination modes to the Ag+ions,which play an important role in determining the structures of the products (either coordination chain or net). Furthermore, the compounds also provide a way of understanding a variety of weak interactions.The Ag··Ag argentophilic interactions in the three compounds help to strengthen the coordination chain or net. Strong offset face-to-face and edge-to-face π-π interactions connect the 1D coordination chains into a 3D supramolecular network in 1. Hydrogen bonds connect the chains into layers in 2. Three types of interesting π-π interactions also assemble the 2D layers into a 3D supramolecular structure in 3.These weak interactions contribute significantly to the stabilities of these three compounds in the crystalline state.
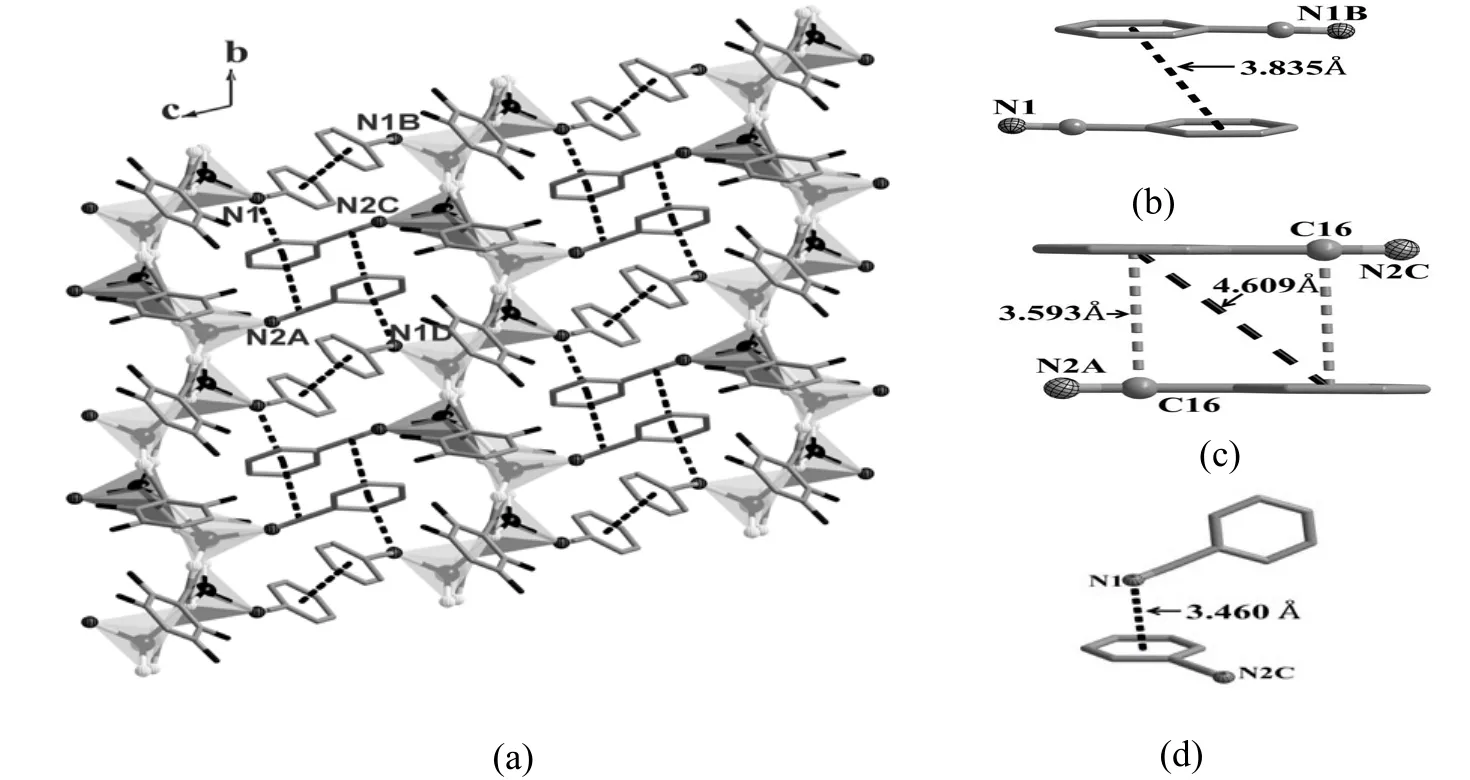
Fig. 5. Structure of 3. (a) π-π interaction nets inside the structure; (b) Offset face-to-face π-π interactions between the PhCN ligands (N1 and N1B); (c) Offset face-to-face π-π interactions between the PhCN ligands (N2A and N2C); (d) Edge-to-face π-π interactions between the PhCN ligands (N1 and N2C). π-π interaction is represented as the dotted lines.Symmetry codes: A: x, 1+y, z; B: 2–x,1–y, 2–z; C: 1–x, 1–y, 2–z; D: 1–x, 2–y, 2–z
(1) Murray, L. J.; Dinca, M.; Long, J. R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314.
(2) Zhang, J. J.; Wojtas, L.; Larsen, R. W.; Eddaoudi, M.; Zaworotko, M. J. Temperature and concentration control over interpenetration in a polymorphic metal-organic material. J. Am. Chem. Soc. 2009, 131, 17040–17041.
(3) (a) Liu, S. Q.; Ning, G. L.; Zhang, X. F.; Konaka, H.; Munakata, M. Crystal structure of an octanuclear sandwich cluster of silver(I) and 4,4΄-bis(2,5-dimethylstyryl)biphenyl. Chin. J Chem. 2006, 24, 1259–1262. (b) Wang, C. C.; Wang, P. Syntheses and crystal structures of two silver(I)complexes with organic carboxylic acid and bidentate N-donor ligands. Chin. J. Struc. Chem. 2011, 30, 811–818.
(4) Sun, D.; Liu, F. J.; Hao, H. J.; Li, Y. H.; Huang, R. B.; Zheng, L. S. Six low-dimensional silver(I) coordination complexes derived from 2-aminobenzonitrile and carboxylates. Inorg. Chim. Acta 2012, 387, 271–276.
(5) Loots, L.; Barbour, L. J. A simple and robust method for the identification of π-π packing motifs of aromatic compounds. CryEngCommun. 2012, 14, 300–304.
(6) (a) Sheldrick, G. M. SHELXS-97, Program for X-ray Crystal Structure Solution. University of Göttingen: Göttingen, Germany 1997.(b) Sheldrick, G. M. SHELXL-97, Program for X-ray Crystal Structure Refinement. University of Göttingen: Göttingen, Germany 1997.
- 结构化学的其它文章
- Synthesis of a Weak Basic uPA Inhibitor and Crystal Structure of a Complex with uPA①
- Study on the Structure and Characteristics of Recyclable Copper Removal Adsorbent①
- Crystal Structure of PAI-1 in Complex with Gallate①
- Synthesis and Crystal Structure of (Z)-N-(2-(diethylamino)ethyl)-7-(5-fluoro-2-oxoindolin-3-ylidene)-2-methyl-4,5,6,7-tetrahydro-1H-indole-3-carboxamide Methanol Solvate①
- Synthesis, Crystal Structure, and Biological Activity of 4-Chlorobenzaldehyde(2-trifluoromethylTrifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]- thieno[2,3-d]pyrimidin-4-yl)hydrazone Monohydrate①
- DFT Study on the Electronic and Structural Properties of MoS6-/0 Clusters①

