Synthesis, Crystal Structure, and Biological Activity of 4-Chlorobenzaldehyde(2-trifluoromethylTrifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]- thieno[2,3-d]pyrimidin-4-yl)hydrazone Monohydrate①
YANG Ping WANG Yan DUAN Zheng-Chao SHAO Yu NIE Guang-Hua SONG Xin-Jian ② TIAN Da-Ting a (Key Laoratory of Biologic Resources Protection and Utilization of Huei Province, Huei University for Nationalities, Enshi 445000, China) (School of Chemical and Environmental Engineering,Huei University for Nationalities, Enshi 445000, China)
1 INTRODUCTION
Pyrimidines and their fused heterocycles have found wide applications in the design and discovery of novel bioactive molecules and drugs[1]. For example, thieno[2,3-d]pyrimidine derivatives have provoked much interest due to their structural flexibility and biological importance. They are known to exhibit remarkable biological activities, such as fungicidal[2], insecticidal[3], herbicidal[4], and pharmacological properties[5-8]. It has been also reported that hydrazones possess a multitude of bioactivities in the agrochemical and medicinal fields, such as fungicidal[9], insecticidal[10], anticancer[11], anti-inflammmatory[12]and antimicrobial[13-14]activities. The hydrazone group is a highly efficient pharmacophore that is widely used in molecular design. In addition,hydrazones are very useful starting materials in many reactions in the synthesis of various bioactive molecules. Generally speaking, strategically positioned fluorine substituents, the trifluoromethyl group in particular, in heterocyclic compounds play a fundamental role in medicine and agrochemicals because of their enhanced activities and decreased toxicities[15-16]. These observations prompted us to search for novel biologically active thieno[2,3-d]pyrimidine compounds incorporating both hydrazone and trifluoromethyl groups. In the present work, we report the preparation, crystal structure, DFT studies as well as fungicidal activity of 4-chloroben- zaldehyde (2-trifluoromethyl-5,6,7,8-tetrahydroben zo-[4,5]thieno[2,3-d]pyrimidin-4-yl) hydrazone.
2 EXPERIMENTAL
2.1 Materials and instruments
All chemicals used for preparation were of analytical grade. Reaction solvents were dried by standard methods and distilled prior to use.1H NMR spectra were obtained on a BRUKER Avance-400 MHz spectrometer with TMS as internal standard and DMSO-d6as the solvent. IR spectra were recorded in the range of 4000–400 cm-1on a Nicolet NEXUS 470 FT-IR spectrophotometer, using KBr pellets. MS spectra were performed by a Finnigan Trace MS 2000 organic mass spectrometer using the electron ionization (EI) method. Elemental analysis was carried out on a Vario EL III CHNSO analyzer.X-ray diffraction data were collected on a Bruker Smart APEX-II CCD diffractometer equipped with a graphite-monochromatized MoKα (λ = 0.71073 Å)radiation. Melting points were measured with an X-4 digital melting-point apparatus and uncorrected.
2.2 Synthesis of the title compound
The synthetic route of the title compound 5 is outlined in Scheme 1. 2-Amino-4,5,6,7-tetrahydrobenzo- [b]thiophene-3-carbonitrile 1 was prepared according to the modified Gewald procedures[17-18].
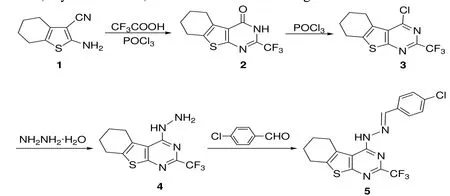
Scheme 1. Procedure of preparing the title compound 5
Preparation of 4-chloro-2-trifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine 3: A mixture of compound 1 (1.78 g, 10 mmol), trifluororoacetic acid (28 mL) and phosphorus oxychloride(2 mL) was refluxed for 2 h in a round-bottomed flask. The remaining trifluoroacetic acid was removed by distillation at reduced pressure. To the reaction mixture at room temperature, ice water was added and neutralized with potassium carbonate till no bubble occurred. The resulting yellow precipitate was filtered, washed with cold water, and dried to give 2-trifluoromethyl-5,6,7,8-tetrahydrobenzo-[4,5]thieno[2,3-d]pyrimidin-4(3H)-one 2, which was then added directly to a round-bottomed flask without further purification and refluxed with phosphorus oxychloride (15 mL) for 5 h. The excess of phosphorus oxychloride was removed under reduced pressure. The residue was poured over crushed ice,followed by neutralization with sodium bicarbonate solution and then filtrated. The solid precipitate was filtered off and recrystallized from n-hexane to deliver 4-chloro-2-trifluoromethyl- 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine 3 in 71%yield as yellowish solid. m.p. 118–119 ℃;1H NMR(DMSO-d6, 400 MHz): δ 2.95–3.04 (m, 4H, CH2at 5 and 8), 1.83–1.86 (m, 4H, CH2at 6 and 7); EI-MS(%): m/z 292 (M+).
Preparation of (2-trifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)hydrazi ne 4: To a solution of 3 (1.46 g, 5 mmol) in EtOH(10 mL) was added hydrazine monohydrate (3 mL).The mixture was stirred under reflux for 3 h and cooled to room temperature. The precipitate was collected by filtration and washed with n-hexane to afford (2-trifluoromethyl-5,6,7,8-tetrahydrobenzo-[4,5]thieno[2,3-d]pyrimidin-4-yl)- hydrazine 4 in 91% yield as yellowish solid. m.p.: 230–231 ℃.
Preparation of the title compound 5: A mixture of compound 4 (0.58 g, 2 mmol) and 4-chlorobenzaldehyde (0.30 g, 2.1 mmol) in EtOH (10 mL) was stirred under reflux for 1 h and cooled to room temperature. The reaction mixture was concentrated under reduced pressure. The residue was recrystallized from ethanol to afford 4-chlorobenzaldehyde(2-trifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]thienoo[2,3-d]pyrimidin-4-yl)- hydrazone 5 in 82% yield as white solid. m.p.: 228~–229 ℃.1H NMR(DMSO-d6, 400 MHz): 10.66 (s, 1H, NH), 8.45 (s,1H, N=CH), δ 7.55~–7.77 (m, 4H, Ar–H), δ 2.89~–3.11 (m, 4H, CH2at 5 and 8), 1.82~–1.88 (m,4H, CH2at 6 and 7). FT-IR (KBr, cm-1): 3368 (N–H),1607, 1573, 1506 (C=N, C=C), 1350, 1137 (CF3);EI-MS (%): m/z 410 (M+), 125 (100). Anal. Calcd.(%) for C18H14ClF3N4S: C, 52.62; H, 3.43; N, 13.64.Found (%): C, 52.81; H, 3.58; N, 13.83.
2.3 Crystal data and structure determination
Block-shaped colorless crystals of compound 5 for X-ray analysis were obtained by vapour diffusion from aqueous tetrahydrofuran (THF) at room temperature. A single crystal with dimensions of 0.10mm × 0.06mm × 0.05mm was mounted on a Bruker SMART APEX-II CCD diffractometer equipped with a graphite-monochromated MoKα (λ= 0.71073 Å) radiation. The intensity data were collected by using a Ψ-ω scan mode in the range of 2.23≤θ≤25.00° at 296(2) K. A total of 12924 reflections were collected and 3331 were independent with Rint= 0.0403, of which 2343 were observed with I > 2σ(I) and used in the succeeding refinements. Absorption correction was not applied.The structure was solved by direct methods with SHELXS-97[19]and expanded using Fourier difference techniques. The non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were added according to theoretical models. Structural refinement was carried out by full-matrix leastsquares techniques on F2with SHELXL-97[19]. The final refinement gave R = 0.0564 and wR = 0.1681 (w= 1/[σ2(Fo2) + (0.1142P)2+ 0.2638P], where P = (Fo2+2Fc2)/3). S = 1.058, (Δ/σ)max= 0.000, (Δρ)max= 0.401 and (Δρ)min= ˉ0.388 e/Å3.
3 RESULTS AND DISCUSSION
The structure of the title compound 5 was confirmed by IR,1H NMR, EI-MS and elemental analysis. These spectroscopic data are in accordance with the assumed structure. The selected bond lengths and bond angles are listed in Table 1, and the selected torsion angles in Table 2. Table 3 enumerates the significant hydrogen bonds. The molecular structure, packing diagram and intermolecular interactions of 5 are depicted in Figs. 1, 2 and 3, respectively.
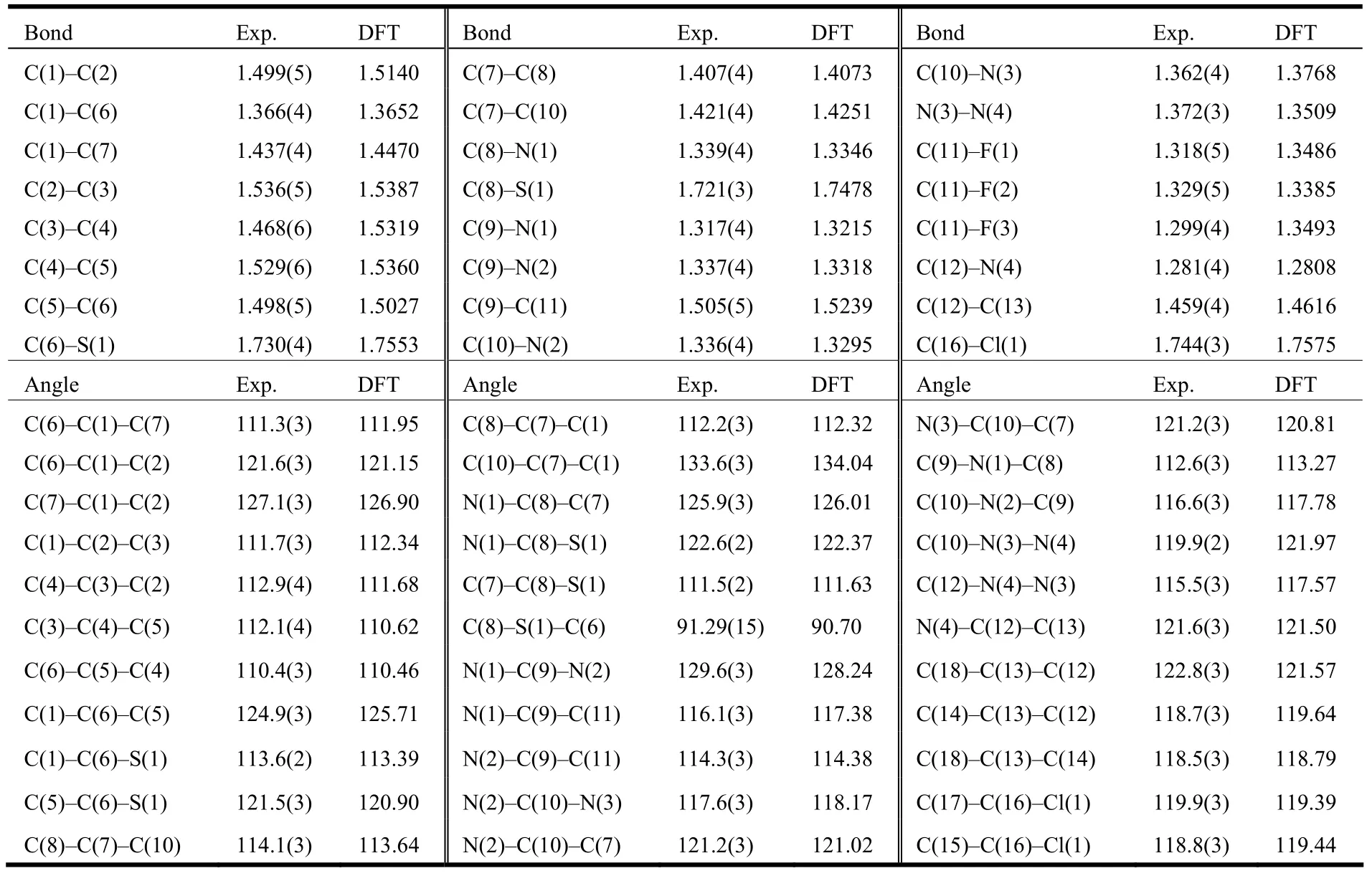
Table 1. Comparison of Selected Bond Lengths (Å) and Bond Angles (°) for Compound 5 as Determined by X-ray Diffraction and from DFT Geometry Optimization (Å, °)
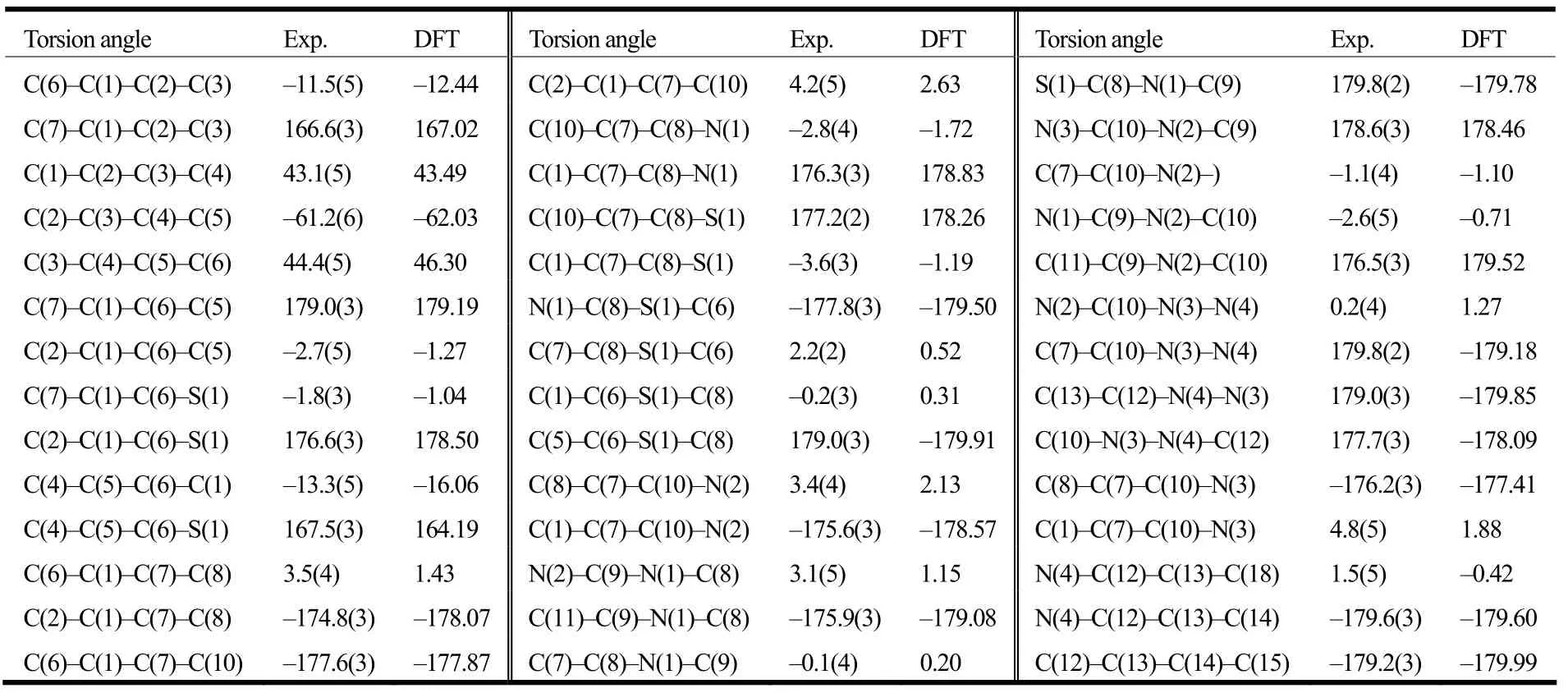
Table 2. Comparison of Selected Torsion Angles (°) for Compound 5 as Determined by X-ray Diffraction and from DFT Geometry Optimization (°)
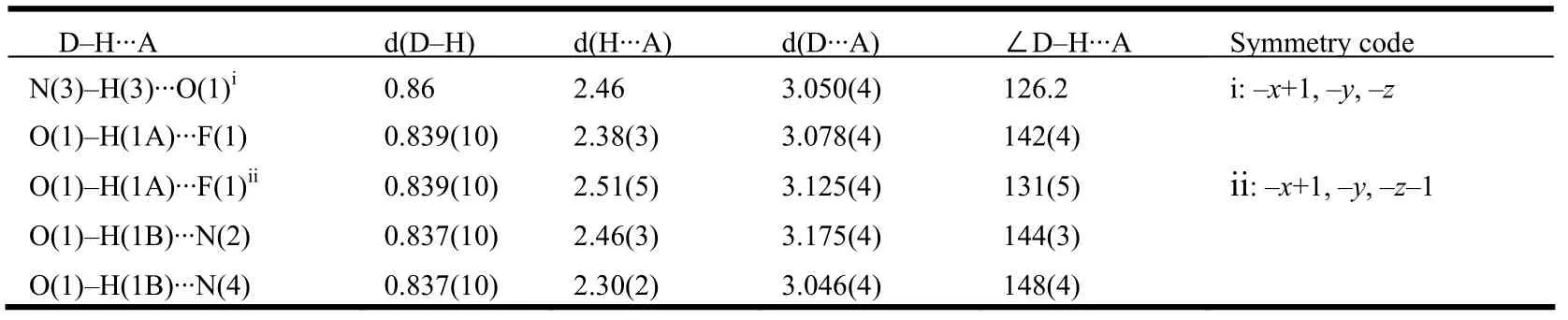
Table 3. Hydrogen-bonding Geometry (Å, °) for Compound 5
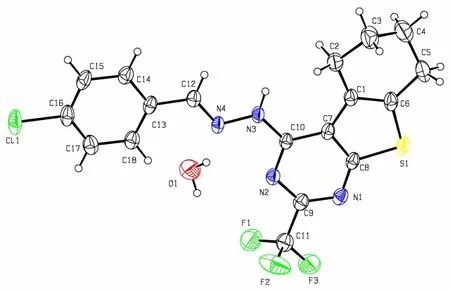
Fig. 1. Molecular structure of compound 5
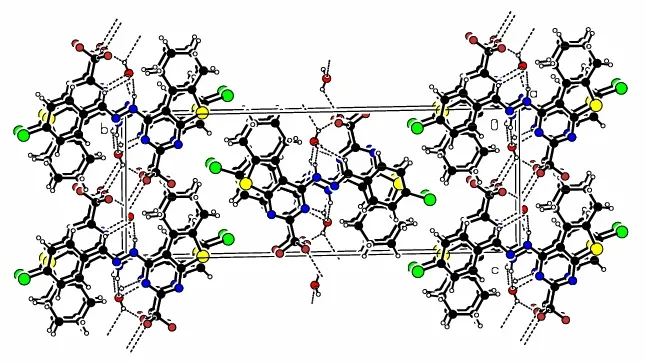
Fig. 2. Packing diagram of compound 5 approximately along the a axis
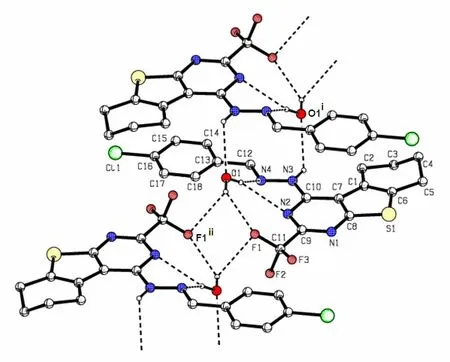
Fig. 3. Intermolecular interactions, including hydrogen bonds and π-π stacking interactions in the crystal(Symmetry codes: (i) –x+1, –y, –z; (ii) –x+1, –y, –z–1). Some H atoms are omitted for clarity
The asymmetric unit of compound 5 (Fig. 1)consists of one 4-chlorobenzaldehyde (2-trifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3- d]pyrimidin-4-yl)hydrazone molecule and one water molecule. The hydrazone molecule assumes an E configuration with the benzene ring and thieno[2,3-d]pyrimidine ring located on opposite sides of the C(12)=N(4) bond. The thieno[2,3-d]pyrimidine ring,which is approximately coplanar with a maximum deviation of 0.048(3) Å at atom C(1), forms a dihedral angle of 1.76(14)° with the benzene ring.The cyclohexene ring adopts a half-chair conformation with calculated puckering parameters of: QT= 0.466(5) Å, θ = 51.9(5)°, φ = 152.3(7)°[20]. All the C–N distances (Table 1) fall between the normal C=N double bond (1.27 Å) and C–N single bond(1.47 Å), showing that all N atoms are partially characterized by sp2hybridization. Besides, the torsion angles (Table 2) of C(7)–C(10)–N(3)–N(4)(179.8(2)º), C(10)–N(3)–N(4)–C(12) (177.7(3)º),C(13)–C(12)–N(4)–N(3) (179.0(3)º) and N(4)–C(12)–C(13)–C(14) (179.6(3)º) are all close to 180º,indicating the hydrazone moiety (C(10)–N(3)–N(4)=C(12)–C(13)) is essentially coplanar with the adjacent thieno[2,3-d]pyrimidine and benzene planes.Obviously, the hydrazone molecule is nearly planar except for the cyclohexene and trifluoromethyl moieties, as can be ascribed to the existence of delocalized π conjugated system.
In the crystal packing (Fig. 2), the title hydrazone molecules are linked by water molecules via intermolecular N(3)–H(3)··O(1), O(1)–H(1B)··N(2),O(1)–H(1B)··N(4) and O(1)–H(1A)··F(1) hydrogen bonds (Fig. 3 and Table 3). Also present are π-π stacking interactions not only between the benzene and thiophene rings but also between the benzene and pyrimidine (symmetry code i: –x+1, –y, –z)rings in an offset fashion. The dihedral angle between benzene and thiophene rings is 3.14(17)°,and the centroid-to-centroid distance is 3.635(2) Å(the perpendicular distances of thiophene ring on the benzene and benzene rings on the thiophene ring are 3.5049(13) and 3.5367(15) Å, respectively), while the dihedral angle between the benzene and pyrimidine rings is 1.34(17)°, and the corresponding centroid-to-centroid distance is 3.866(2) Å (the perpendicular distances of pyrimidine ring on the benzene and benzene rings on the pyrimidine ring are 3.5297(13) and 3.5013(15) Å, respectively). The sum of these interactions results in the formation of a three-dimensional framework.
4 GEOMETRY OPTIMIZATION AND FRONTIER MOLECULAR ORBITALS
Gas phase geometries of the title compound 5 were optimized by the DFT/B3LYP method[21-23]combined with the 6-311G** basis set using Gaussian 09W package[24]. The optimized parameters are listed in Tables 1 and 2 along with the experimental values. As can be seen from Tables 1 and 2, the calculated values are in good agreement with the experimental values. Compared with the experiment,we can find that most of the optimized bond lengths are slightly larger than the experimental values because the experimental results were obtained at solid phase and the theoretical calculations belong to the gaseous phase. The largest difference between the experimental and calculated bond angles of C(10)–N(3)–N(4) and C(12)–N(4)–N(3) (Table 1) is 2.07°. The difference between the calculated and experimental torsion angles is smaller than 4.21°(Table 2).
Fig. 4 shows the distribution of HOMO (the highest occupied molecular orbital) and LUMO (the lowest unoccupied molecular orbital) orbitals computed at the B3LYP/6-311G** level for the title compound 5. Seen from Fig. 4, the HOMO and LUMO are principally delocalized among the benzene ring, thieno[2,3-d]pyrimidine ring, hydrazone moiety (C(10)–N(3)–N(4)=C(12)–C(13)) and atom Cl(1), while the LUMO electronic density shows that the electron cloud shifts more toward the benzene ring on the left and the electron density on C(1), S(1) and N(3) atoms disappears in LUMO compared to that in HOMO. Note that the trifluoromethyl moieties and C(2), C(3) and C(4) atoms of the cyclohexene do not play any role in the HOMO nor in the LUMO. These results suggest the presence of a π-electron delocalization system in most part of the molecules, which are consistent well with the X-ray diffraction analysis.

Fig. 4. Frontier molecular orbitals of compound 5
5 BIOLOGICAL ACTIVITIES
The fungicidal activities of compound 5 were screened against five kinds of fungi (Fusarium oxysporium f.sp.vasinfectum, Dothiorella gregaria,Gibberella zeae, Botrytis cinereapers and Rhizoctonia solani) at the concentration of 50 ppm according to the reported method[25]. The results of preliminary bioassay indicated that the title compound exhibits relatively good fungicidal activity against Fusarium oxysporium f.sp.vasinfectum and Dothio--rella gregaria. The inhibition rates against the selec- ted five kinds of fungi are 80.6%, 71.3%,32.7%, 30.6% and 45.0%, respectively. The good fungicidal effect could be attributed to the introduction of thieno[2,3-d]pyrimidine ring and the trifluoromethyl group into the hydrazone molecule.
(1) Adepu, R.; Rambabu, D.; Prasad, B.; Meda, C. L. T.; Kandale, A.; Krishna, G. R.; Reddy, C. M.; Chennuru, L. N.; Parsa, K. V. L.; Pal, M. Novel thieno[2,3-d]pyrimidines: their design, synthesis, crystal structure analysis and pharmacological evaluation. Org. Biomol. Chem. 2012, 10, 5554–5569.
(2) Sun, Y.; Wu, J.; Feng, L. L.; Ding, M. W. Efficient synthesis and fungicidal activities of 2-alkylamino-3-aryl-6-(1H-1,2,4-triazol-1-yl)-thieno[2,3-d]pyrimidin-4(3H)-ones. Arkivoc. 2009, 111–118.
(3) Aly, A. S.; El-Gazzar, A. B. A.; Hussein, H. A. R. The synthesis of some new derivatives derived from 1,2,3,4-tetrahydrocyclohepteno[4,5]thieno[2,3-d]pyrimidine. Phosphorus Sulfur Silicon Relat. Elem. 2007, 1820, 35–56.
(4) Ren, Q. Y.; Tan, X. S.; He, H. W. Efficient synthesis of fused pyrimidine derivatives with biological activity via aza-wittig reaction.Curr. Org. Syn. 2011, 8, 752–763.
(5) Deng, Y. J.; Zhou, X. L.; Desmoulin, S. K.; Wu, J. M.; Cherian, C.; Hou, Z. J.; Matherly, L. H.; Gangjee, A. Synthesis and biological activity of a novel series of 6-substituted thieno[2,3-d]pyrimidine antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors over the reduced folate carrier and proton-coupled folate transporter for cellular entry. J. Med. Chem. 2009, 52, 2940–2951.
(6) Kotaiah, Y.; Harikrishna, N.; Nagaraju, K.; Rao, C. V. Synthesis and antioxidant activity of 1,3,4-oxadiazole tagged thieno[2,3-d] pyrimidine derivatives. Eur. J. Med. Chem. 2012, 58, 340–345.
(7) Miwa, K.; Hitaka, T.; Imada, T.; Sasaki, S.; Yoshimatsu, M.; Kusaka, M.; Tanaka, A.; Nakata, D.; Furuya, S.; Endo, S.; Hamamura, K.; Kitazaki, T.Discovery of 1-{4[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3--3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl]-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor. J. Med. Chem. 2011, 54, 4998–5012.
(8) Song, X. J.; Duan, Z. C.; Shao, Y.; Dong, X. G. Facile synthesis of novel fluorinated thieno[2,3-d]pyrimidine derivatives containing 1,3,4-thiadiazole. Chin. Chem. Lett. 2012, 23, 549–552.
(9) Weng, J. Q.; Tan, C. X.; Liu, X. H. Synthesis and fungicidal activity of hydrazones containing 4-methylbenzo[d]thiazole moiety. J. Pestic. Sci. 2012, 37, 164–168.
(10) Wu, J.; Song, B. A.; Hu, D. Y.; Yue, M.; Yang, S. Design, synthesis and insecticidal activities of novel pyrazole amides containing hydrazone substructures. Pest Manag. Sci. 2012, 68, 801–810.
(11) Chimenti, F.; Bizzarri, B.; Maccioni, E.; Secci, D.; Bolasco, A .; Chimenti, P.; Fioravanti, R.; Granese, A.; Carradori, S.; Tosi, F.; Ballario, P.;Vernarecci, S.; Filetici, P. A novel histone acetyltransferase inhibitor modulating Gcn5 network:cyclopentylidene-[4-(4'-chlorophenyl)thiazol-2-yl]hydrazone. J. Med. Chem. 2009, 52, 530–536.
(12) El-Sayed, M. A. A.; Abdel-Aziz, N. I.; Abdel-Aziz, A. A. M.; El-Azab, A. S.; Asiri, Y. A.; ElTahir, K. E. H. Design, synthesis, and biological evaluation of substituted hydrazone and pyrazole derivatives as selective COX-2 inhibitors:Molecular docking study. Bioorg. Med. Chem. 2011, 19, 3416–3424.
(13) Ajani, O. O.; Obafemi, C. A.; Nwinyi, O. C.; Akinpelu, D. A. Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioorg. Med. Chem. 2010, 18, 214–221.
(14) Pan, X. Q.; Huang, S. S.; Wang, F.; Fan, H.; Leng, H.; Zhang, H. L.; Diao, Y. P. Synthesis, crystal structure and antibacterial activity of two hydrazones derived from 2-fluorobenzhydrazide. Chin. J. Struct. Chem. 2013, 32, 142–148.
(15) Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369.
(16) Shao, Y.; Yang, P.; Li, S. R.; Wang, F. S.; Hu, W. B. Synthesis, crystal structure and biological activity of N-(2,6-difluorobenzoyl)-N'-[5-(4-trifluoromethyl-phenyl)-1,3,4-thiadiazol-2-yl]urea. Chin. J. Struct. Chem. 2011, 30, 848–852.
(17) Wang, T.; Huang, X. G.; Liu, J.; Li, B.; Wu, J. J.; Chen, K. X.; Zhu, W. L.; Xu, X. Y.; Zeng, B. B. An efficient one-pot synthesis of substituted 2-aminothiophenes via three-component Gewald reaction catalyzed by L-Proline. Synlett. 2010, 1351–1354.
(18) Huang, X. G.; Liu, J.; Ren, J. M.; Wang, T.; Chen, W. D.; Zeng, B. B. A facile and practical one-pot synthesis of multisubstituted 2-aminothiophenes via imidazole-catalyzed Gewald reaction. Tetrahedron 2011, 67, 6202–-6205.
(19) Sheldrick, G. M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122.
(20) Cremer, D.; Pople, J. A. A general definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358.
(21) Becke, A. D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377.
(22) Johnson, B. G.; Gill, P. M. W.; Pople, J. A. The performance of a family of density functional methods. J. Chem. Phys. 1993, 98, 5612–5626.
(23) Kohn, W.; Becke, A. D.; Parr, R. G. Density functional theory of electronic structure. J. Phys. Chem. 1996, 100, 12974–12980.
(24) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.;Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda,R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.;Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.;Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.;Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.1,Gaussian, Inc., Wallingford CT, 2009.
(25) Li, G. H.; Yang, H. Synthesis and antifungal bioactivity of 2-{2-[3-(subsititued phenyl)-3-oxo-2-1,2,4-triazol-1-ylpropyl]-phenyl}-2-methoxyimino-acetic acid methyl ester and N-methyl-acetamide derivatives. Chin. J. Org. Chem. 2008, 30, 1918–1924.
- 结构化学的其它文章
- Synthesis and Crystal Structure of 5,5΄-Bis(4-hydroxylphenyl)diazo-dipyrromethane①
- A Novel Cu-based Metallosalan Complex:Synthesis, Structure and Chiral Sensor Study①
- Syntheses, Crystal Structures and Properties of Two Metal-organic Complexes Based on 5-(4-Pyridyl)-methoxyl Isophthalic Acid①
- DFT Study on the Electronic and Structural Properties of MoS6-/0 Clusters①
- Synthesis and Crystal Structure of (Z)-N-(2-(diethylamino)ethyl)-7-(5-fluoro-2-oxoindolin-3-ylidene)-2-methyl-4,5,6,7-tetrahydro-1H-indole-3-carboxamide Methanol Solvate①
- Interesting Weak Interactions in Three Silver Coordination Polymers Based on Tetrafluorobenzenedicarboxylate and Benzonitrile Ligands①

