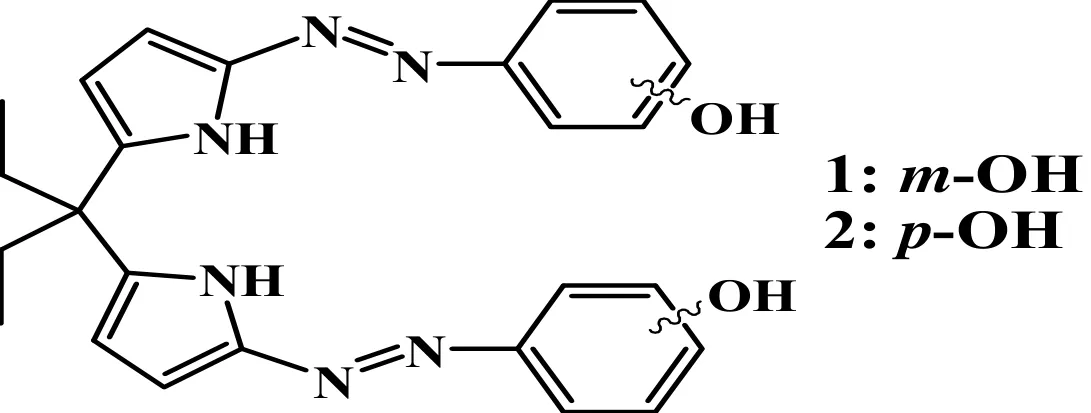
Synthesis and Crystal Structure of 5,5΄-Bis(4-hydroxylphenyl)diazo-dipyrromethane①
SHI Hong-Mei YIN Zhen-Ming
(Tianjin Key Laboratory of Structure and Performance for Functional Molecules;Key Laboratory of Inorganic-organic Hybrid Functional Material Chemistry, Ministry of Education; College of Chemistry, Tianjin Normal University, Tianjin 300387, China)
1 INTRODUCTION
Non-covalent interactions, especially the hydrogen bonding interactions, play a crucial role in crystal engineering for the construction of supramolecular assemblies with predefined architectures.Studies on the self-assembled ensembles of pyrrole based compounds through hydrogen bonds recently have attracted much attention in the field of crystal engineering[1-3]. Various structures, from 0D to 3D,have been generated by α-carbonyl-functionalized pyrrole compounds through hydrogen bonds[4-10].Over the past decade, azopyrrole dyes have been attracting much attention because of their rapidly increasing role in the design of advanced materials and devices. For examples, Dolphin and coworker found that a series of symmetrical 2,5-bisazopyrroles and BF2-azopyrrole complexes show near infrared absorption[11]. Mikroyannidis and coworkers have designed some bulk heterojunction organic solar cells and photovoltaic devices based on azopyrrole compounds[12-14]. Raposo and coworkers have synthesized many azopyrrole based dyes which can act as nonlinear optical materials and photoswitch[15-18].Many pyrrole azocrown ethers, which are good lead(II) chemosensors, were designed and synthesized by Wangner-Wysiecka and Biernat[19-21]. On the other hand, the self-assembly of azopyrrole through hydrogen bonds between pyrrole NH and azo nitrogen atom drew our attention. We have already demonstrated the interlocked type selfassemblies of some 5,5΄-bisdiazo-dipyrromethane compounds in both solid state and solution via quadruple N–H··N hydrogen bonds[22]. In this context, we anticipate that changing the attached methyl on the phenyl rings to other group that can be involved in weak interactions may result in interesting supramolecular arrays. Hydroxyl group is well known for its ability to form hydrogen bonds.So, continuing our study, we decided to study the crystal engineering of hydroxyl substituted 5,5΄-bisdiazo-dipyrromethane compounds. In the previous paper, we have reported the crystal structure of 5,5΄-bis(3-hydroxylphenyl)diazo-dipyrromethane (1)with methanol, THF and acetone, from which[n]cantane, rotaxane and broken [n]cantane like supramolecular structures were obtained[23]. Herein,the crystal structure of compound 2 on the basis of N–H··N, O–H··O and C–H··N interactions has been discussed.
2 EXPERIMENTAL
2.1 Materials and apparatuses
1H NMR spectra were recorded in DMSO-d6, with TMS as the internal standard, on a BRUKER AV-400 MHz spectrometer. Mass spectra were recorded on an AEIMS-50/PS 30 mass spectrometer. Analyses of C, H and N were determined on a Perkin-Elmer 240 elemental analyzer. UV-Vis spectra were record on a SHIMADZU UV-2550 spectrometer. Melting points (m.p.) were recorded on an electro-thermal digital melting point apparatus and uncorrected.Meso-diethyl-2,2΄-pyrromethane was prepared according to literature procedures[24]. Other reagents were commercially available and used without further purification.
2.2 Preparation of compound 2
(i) A 0 ℃ solution of 4-aminophenol 545 mg (5 mmol) and 12 M aqueous HCl (4 mL) in water 4(mL) was treated with a 0 ℃ solution of 350 mg NaNO2(5 mmol) in water (10 mL), and the mixture was stirred at 0℃ for 0.5 h.
(ii) The diazonium salt solution previously prepared (5.0 mmol) was added dropwise to the solution of 500 mg dipyrromethane (2.5 mmol) in acetonitrile (25 mL) and three drops of acetic acid.The combined solution was maintained at 0 ℃ for 2 h with stirring. After this, EtOAc (25 mL) and water (25 mL) were added. The organic layer was separated and washed with water (20 mL) and dried with anhydrous MgSO4. The dried solution was evaporated and the residue was purified by column chromatography on silica, eluted by a 1:2(v/v) ethyl acetate and petroleum ether solution,giving compound 2: 465 mg, brick red powder,yield 42%, m.p.: 80–81 ℃.1H NMR (400MHz,DMSO-d6): δ 0.70 (t, 6H, J = 7.2 Hz, -CH3), 2.21(q, 4H, J = 7.2 Hz, -CH2-), 6.16 (s, 2H, PyCH),6.64 (s, 2H, PyCH), 6.84(d, 4H, J = 8.4 Hz,ArCH), 7.58 (d, 4H, J = 8.4 Hz, ArCH), 9.89 (s,2H, -OH), 11.11 (s, 2H, PyNH).13C NMR(100MHz, DMSO-d6): δ 8.2, 26.2, 43.4, 109.2,115.7, 123.0, 141.5, 145.5, 145.8, 158.7. ESI-MS:443(M+1+). Elemental analysis: C29H34N6O2:Calcd. (%): C, 67.86; H, 5.92; N, 18.99. Found(%): C, 67.75; H, 6.12; N, 19.01.
2.3 X-ray crystallography
The single crystal of compound 2 suitable for X-ray crystallography studies was grown by slowly evaporating its MeOH solution. The crystal (0.18mm× 0.17mm × 0.16mm) was selected and mounted on a glass capillary. The diffraction data were measured on a BRUKER SMART APEX II CCD diffractometer equipped with a graphite-monochromatic Mo-Kα radiation (λ = 0.71073 Å) using an ω scan mode at 173(2) K. Of the 29208 total reflections collected in the range of 1.37≤θ≤25.01º, 10119 (Rint=0.0434) were independent. All data were corrected by semi-empirical method using the SADABS program. The program SAINT[25]was used for integration of the diffraction profiles. The structure was solved by direct methods using SHELXS program and refined with SHELXL[26]. The final refinement was performed by full-matrix least-squares methods with anisotropic thermal parameters for all nonhydrogen atoms on F2. The hydrogen atoms were added according to the theoretical models. The final refinement gave R = 0.0469, wR = 0.1143 (w =1/[σ2(Fo2) + (0.0614P)2+ 0.0000P], where P = (Fo2+ 2Fc2)/3), S = 1.001, (Δρ)max= 0.204 and (Δρ)min=–0.237 e/Å3. The selected bond distances and bond angles are listed in Table 1.
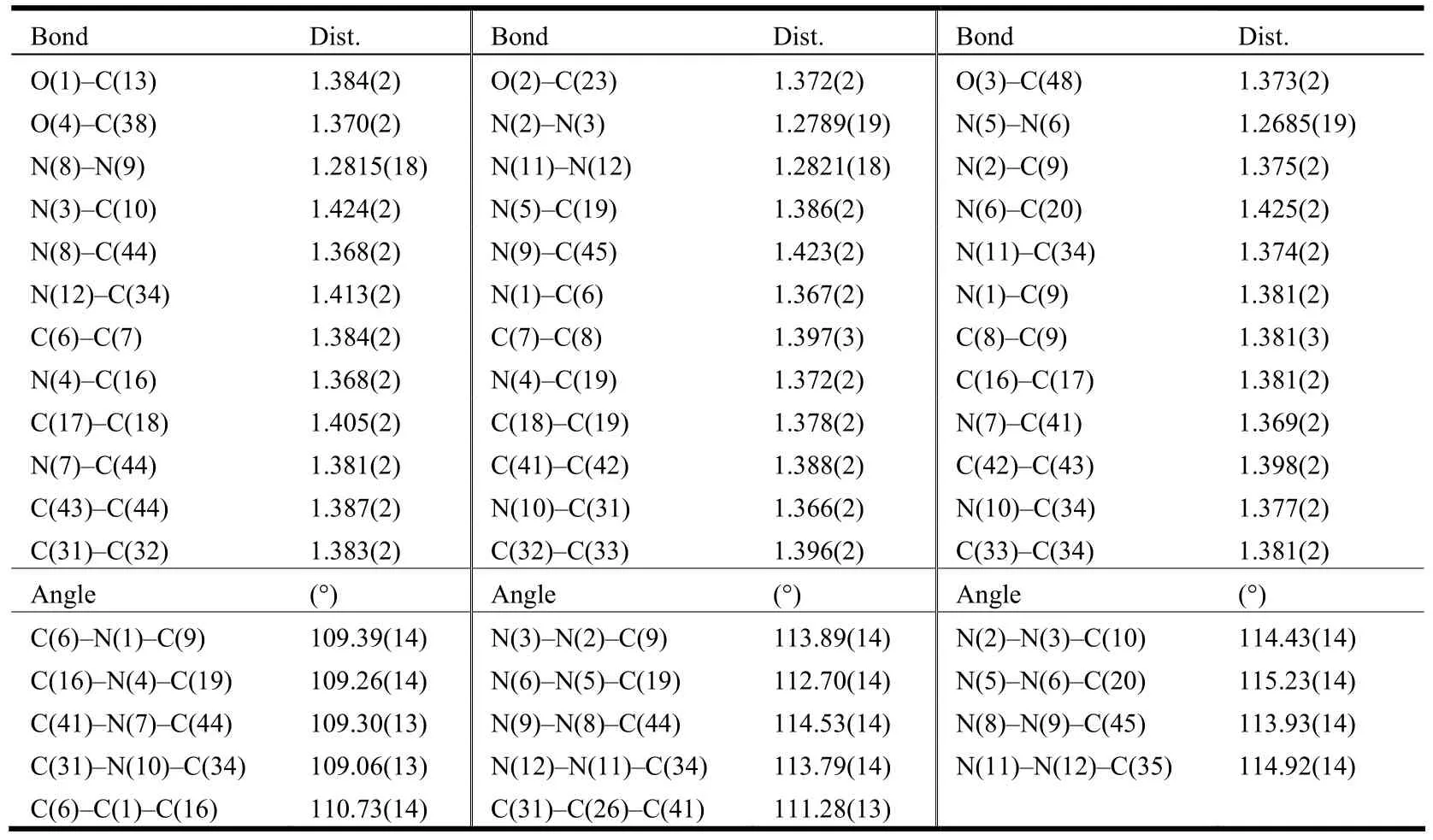
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
3 RESULTS AND DISCUSSION
The crystal structure of compound 2 is shown in Fig. 1. The asymmetric unit contains two molecules of compound 2 which are held together by four N–H··N hydrogen bonds and consequently forms interlocked type 2·2 dimer, similar to our previous observations[22-23,27-28]. The parameters of N–H··N hydrogen bonds are listed in Table 2, which are also consistent with the early results[22-23,27-28]. The diazo groups adopt trans conformation and distances varied in the range of 1.269(2)–1.282(2) Å. The dihedral angles phenyl(1)(C(10)–C(15))/pyrrole(C(6)C(9), N(1)), phenyl(2)(C(20)–C(25))/pyrrole(C(16)C(19), N(4)), phenyl(3)(C(35)–C(40))/pyrrole(C(31)C(34), N(10)) and phenyl(4)(C(45)–C(50))/pyrrole(C(41) C(44), N(7)) are 19.9, 11.8, 6.3 and 12.5°,respectively.
Neighboring two 2·2 dimers, which are enantiomers, are held together through a pair of O–H··O hydrogen bonds between hydroxyl groups (O(3)–H(3)··O(1) and O(4)–H(4)··O(2)) and consequently form zipper like double-strand chains rather than double-stand helical as expected (Fig. 2). The double-strand chains are further assembled into a 2-D layer structure by the connection of O(2)–H(2)··O(3) hydrogen bonds (Fig. 2). It is worthy to note that the adjacent two chains in the layer are pointing to the opposite directions. The layers are held together by C(43)–H(43)··N(11) hydrogen bonds to generate a 3-D structure (Fig. 2). The parameters of hydrogen bonds are also listed in Table 2.When the 2·2 dimers self-assembled into a layer structure, there leave some square voids. Interestingly, the voids are not eclipsed but form square tubes along the c axis when the layers are packed with each other (Fig. 3). Some solvent molecules should be included in the tubes and interact with the hydroxyl groups from compound 2. Unfortunately,the structure can not be exactly refined. The cavity is about 27.3% of the total volume of the crystal as calculated using the pro- gram PLATON[29].

Fig. 1. ORTEP diagram for compound 2, with the displacement ellipsoids drawn at the 30% probability level
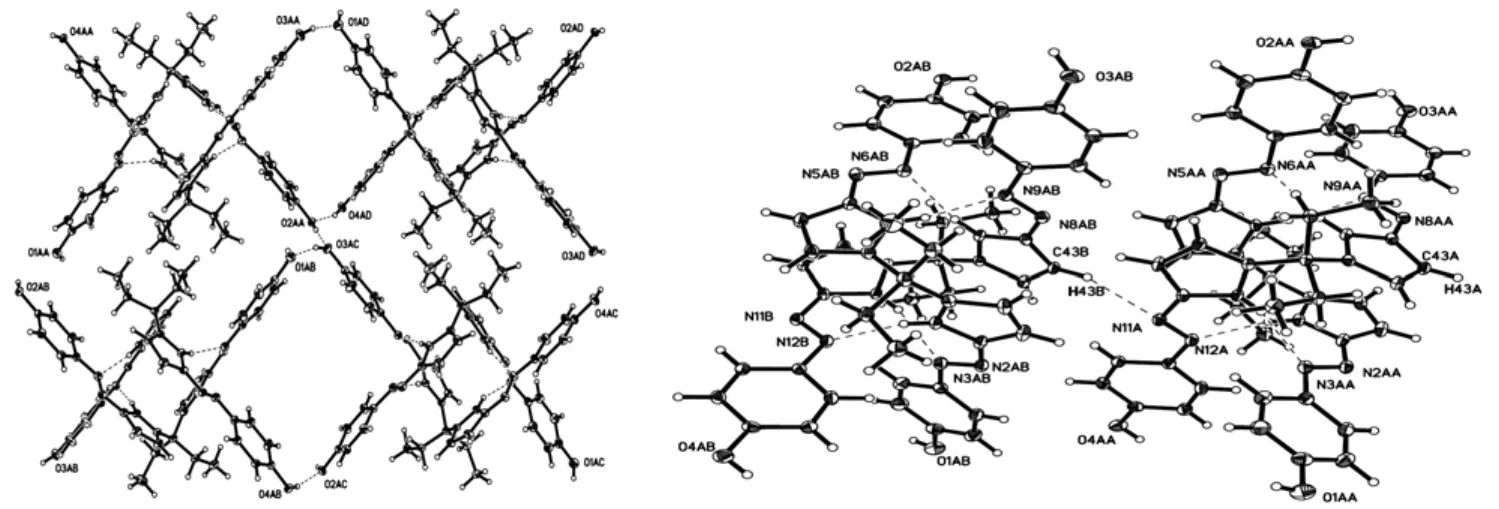
Fig. 2. O–H··O hydrogen-bonded layer structure (left)and C–H··N hydrogen bonds in the crystal of compound 2 (right)
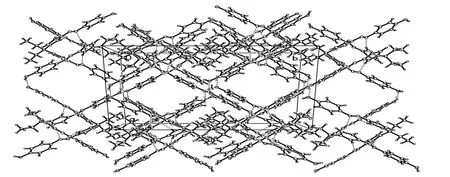
Fig. 3. Crystal packing of compound 2
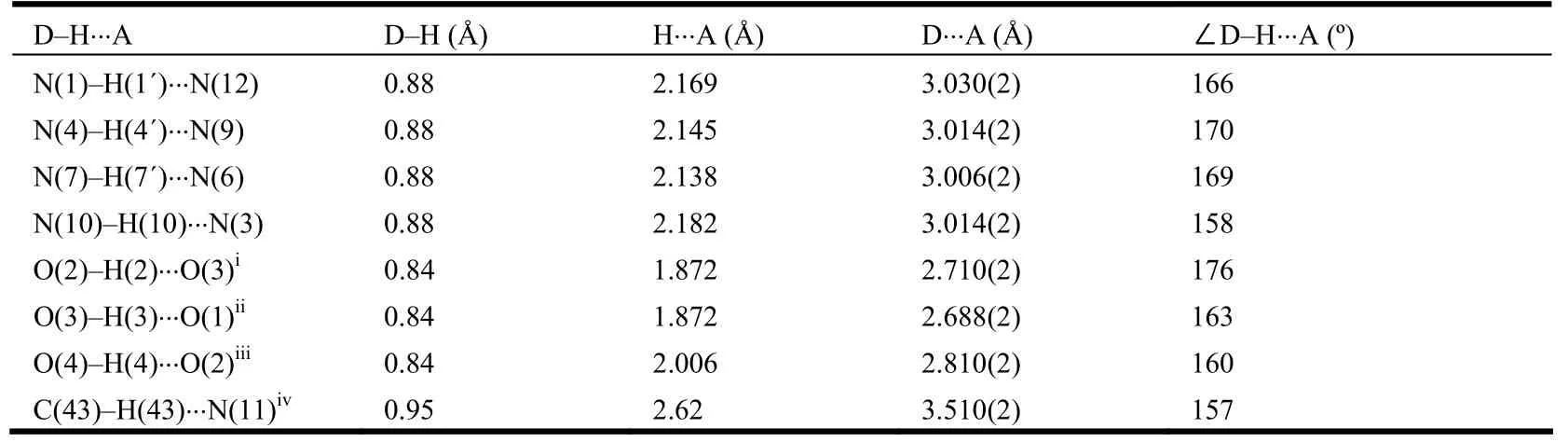
Table 2. Hydrogen Bond Parameters in the Crystal of Compound 2
A brief comparison between the crystals of compounds 2 and 1 reveals that in both crystals the 5,5΄-bisdiazo-dipyrromethane unit prefers to self-assemble into an interlocked type dimer structure through quadruple N–H··N hydrogen bonds[23]. It further demonstrates the robustness and application perspective of quadruple hydrogen-bonded dimer as a useful and reliable building block for crystal engineering. In the crystals of compound 1, the dimers self-assembled into a rotaxane and cantane structure, whereas into double-strand chains in those of 2. The difference indicates that both the type and position of the substituent can influence the supramolecular structure greatly. It provides an approach to change the supramolecular structure of 5,5΄-bisdiazo-dipyrromethane compounds.
In the previous paper, we have also reported the crystal structure of 3,3΄-bis(5-(N-(4-hydroxylphenyl)imidomethyl)pyrrol-2-yl)pentane, an imine analogue to compound 1, which forms an interpenetrated grid structure through N–H··O and O–H··O hydrogen bonds under the mediation of included methanol molecules[30]. The supramolecular structure difference between the two crystals should be ascribed to the different ability to form hydrogen bonds of 2-azopyrrole and 2-iminepyrrole.
The UV/Vis spectra of compound 2 in methanol,Et2O and CH3CN are shown in Fig. 4, which have two peaks at 389 and 429 nm. The peak at 385 nm is similar to that in other 5,5΄-bisdiazo-dipyrromethane compounds and should belong to the absorption of azopyrrole group. The peak at 429 nm is absent in the spectra of other 5,5΄-bisdiazo-dipyrromethane compounds[31]. The UV-Vis spectra are similar for some hydroxyl azo dyes[32], which means the existence of azo-hydrazone tautomerization of compound 2 in solution. The UV/Vis spectra of compound 1 in methanol only have the peak at 389 nm, which further proves the appearance of azo-hydrazone tautomerization of compound 2 in solution. The spectra difference in three solvents suggests that compound 2 prefers to the hydrazone tautomer in protic solvent (methanol) and azo tautomer in aprotic solvents (Et2O and CH3CN).

Fig. 4. UV-Vis spectra of compound 1 (1 × 10-5 M)
EFERENCES
(1) Desiraju. G. R. Crystal Engineering∶ The Design of Organic solids. Elsevier, Amsterdan 1989.
(2) Sessler, J. L.; Berthon-Gelloz, G.; Gale, P. A.; Camiolo, S.; Anslyn, E. V.; Anzenbacher, P. Jr.; Furuta, H.; Kirkovits, G. J.; Lynch, V. M.; Maeda, H.;Morosini, P.; Schere, M.; Shriver, J.; Zimmerman, R. S. Oligopyrrole-based solid state self-assemblies. Polyhedron 2003, 22, 2963-2983.
(3) Munro, O. Q.; Joubert, S. D.; Grimmer, C. D. Molecular recognition: preorganization of a bis(pyrrole) Schiff base derivative for tight dimerization by hydrogen bonding. Chem. Eur. J. 2006, 12, 7987-7999.
(4) Maeda, H.; Kusunose, Y.; Terasaki, M.; Ito, Y.; Fujimoto, C.; Fujii, R.; Nakanishi, T. Micro- and nanometer-scale porous, fibrous, and sheet architectures constructed by supramolecular assemblies of dipyrrolyldiketones. Chem. Asian J. 2007, 2, 350-357.
(5) Scherer, M.; Sessler, J. L.; Moini, M.; Gebauer, A.; Lynch, V. Self-assembly of pyrrole-ferrocene hybrids, determined inter alia by a new chemically induced electrospray mass spectrometry technique. Chem. Eur. J. 1998, 4, 152-158.
(6) Senge, M. O.; Smith, K. M. Hydrogen-bonding patterns in six derivatives of 2,4-dimethylpyrrole. Acta Crystallogr. 2005, C61, o537-o541.
(7) Norsten, T. B.; McDonald, R.; Branda, N. R. The self-assembly and spontaneous resolution of a hydrogen-bonded helix. Chem. Commun. 1999, 719-720.
(8) Silva, M. R.; Sobral, A. J. F. N.; Silva, J. A.; Santos, A. C.; Melo, S. M.; Beija, A. M. Dimer formation in 4-benzyl-5-methoxymethyl-3-methyl-1-H-pyrrole-2-carboxylic acid benzyl ester. J. Chem. Crystallogr. 2007, 37, 695-698.
(9) Yin, Z.; Li, Z. Pyrrole-2-carboxylate dimer: a robust supramolecular synthon for crystal engineering. Tetra. Lett. 2006, 47, 7875-7879.
(10) Cui, Y.; Yin, Z.; Dong, L.; He, J. Q. Self-assembly of pyrrole-2-carboxylates: substituent effect on the synthon conversion. J. Mol. Struct. 2009, 938, 322-327.
(11) Li. Y.; Patrick, B. O.; Dolphin, D. Near-infrared absorbing azo dyes: synthesis and X-ray crystallographic and spectral characterization of monoazopyrroles, bisazopyrroles, and a boron-azopyrrole complex. J. Org. Chem. 2009, 74, 5237-5243.
(12) Mikroyannidis, J. A.; Sharma, G. D.; Sharma, S. S.; Vijay, Y. K. Novel low band gap phenylenevinylene copolymer with BF2-azopyrrole complex units: synthesis and use for efficient bulk heterojunction solar cells. J. Phys. Chem. C 2010, 114, 1520-1527.
(13) Mikroyannidis, J. A.; Kabanakis, A. N.; Tsagkournos, D. V.; Balraju, P.; Sharma, G. D. Bulk heterojunctin solar cells based on a low band gap soluble bisazopyrrole and the corresponding BF2-aopyrrole complex. J. Mater. Chem. 2010, 20, 6464-6471.
(14) Sharma, G. D.; Mikroyannidis, J. A.; Sharma, S. S.; Thomas, K. R. J. Bulk heterojunction organic photovoltaic devices based on small molecules featuring pyrrole and carbazole and 2-(4-nitrophenyl)acrylonitrile acceptor segments as donor and fullerene derivatives as acceptor. Dyes Pigm. 2012, 94, 320-329.
(15) Raposo, M. M. M.; Castro, M. C. R.; Fonseca, A. M. C.; Schellenberg, P.; Belsley, M. Design, synthesis, and characterization of electrochemical,nonlinear optical properties, and theoretical studies of novel thienylpyrrole azo dyes bearing benzothiazole acceptor groups.Tetrahedron 2011, 67, 5189-5198.
(16) Raposo, M. M. M.; Fonseca, A. M. C.; Castro, M. C. R.; Belsley, M.; Cardoso, M. F. S.; Carvalho, L. M.; Coelho, P. J. Synthesis and characterization of novel diazenes bearing pyrrole, thiophene and thiazole heterocycles as efficient photochromic and nonlinear optical (NLO)materials. Dyes Pigm. 2011, 91, 62-73.
(17) Raposo, M. M. M.; Castro, M. C. R.; Belsley, M.; Fonseca, A. M. C. Push-pull bithiophene azo-chromophores bearing thiazole and benzothiazole acceptor moieties: synthesis and evaluation of their redox and nonlinear optical properties. Dyes Pigm. 2011, 91, 454-465.
(18) Coelho P. J.; Castro, M. C. R.; Fonseca, A. M. C.; Raposo M. M. M. Photoswitching in azo dyes bearing thienylpyrrole and benzothiazole heterocyclic systems. Dyes Pigm. 2011, 92, 745-748.
(19) Wagner-Wysiecka, E.; Rzymowski, T.; Fonari, M. S.; Kulmaczewshi, R.; Luboch, E. Pyrrole azocrywn ethers-synthesis, crystal structures, and fluorescence. Tetrahedron 2011, 67, 1862-1872.
(20) Szczygelska-Tao, J.; Fonari, M. S.; Biernat, J. F. Chromogenic azole diaothiacrown ethers. Supramol. Chem. 2008, 20, 651-658.
(21) Wagner-Wysiecka, E.; Luboch, E.; Kowalczyk, M.; Biernat, J. F. Chromogenic macrocyclic derivatives of azoles-synthesis and properties.Tetrahedron 2003, 59, 4415-4420.
(22) Yin, Z.; Wang, W.; Guo, J.; Wang, J.; He, J.; Cheng, J. P. Quadruple hydrogen bonded self-assemblies of 5,5΄-bisdiazo-dipyrromethane.CrystEngComm. 2008, 10, 957-959.
(23) Yin, Z.; Wang, M.; Wang, W.; Wang, X. Solvent effects on the self-assemblies of 5,5-bis(3-hydroxyphenyldiazo)-dipyrromethane.J. Mol. Struct. 2013, 1031, 85-90.
(24) Sobral, A. J. F. N.; Rebanda, N. G. C. L.; Silva, M. D.; Lampreia, S. H.; Silva, M. R.; Beja, A. M.; Paixao, J. A.; d΄a Rocha Gonsalves, A. M.One-step synthesis of dipyrromethanes in water. Tetrahedron Lett. 2003, 44, 3971-3973.
(25) Bruker, A. X. S. SHELXTL. Version 5.1, Bruker AXS, Madison, WI, USA 1998.
(26) Sheldrick, G. M. SHELX 97, Program for X-ray Crystal Structure Solution and Refinement. Göttingen University, Germany 1997.
(27) Yin, Z.; Wang, W.; Du, M.; Wang, X.; Guo, J. Crystal engineering of 5,5΄-bisdiazo-dipyrromethane with halogen··π synthons.CrystEngComm. 2009, 11, 2441-2446.
(28) Li, C.; Li, B. Y.; Sun, S. P.; Yin, Z. Synthesis and hydrogen-bonded interpenetrating grid structure of 5,5΄-bis[4-(2-hydroxyethyl)phenyl]diazo-dipyrromethane. Chin. J. Struct. Chem. 2012, 31, 797-802.
(29) Spek, A. L. PLATON. A Multipurpose Crystallographic Tool. Utrecht University: The Netherlands 2002.
(30) Xu, L.; Liu, S. Y.; Yin, Z. Synthesis and crystal structure of 3,3΄-bis(5-(N-(4-hydroxylphenyl)imidomethyl)-pyrrol-2-yl)pentane·2CH3OH. Chin. J.Struct. Chem. 2010, 29, 613-617.
(31) Yin, Z.; Yan, Y.; Sun, S.; Wang, W. Synthesis, structures and properties of Ni(II) complexes with 5,5΄-bis(4-halogenphenyl)diazo-dipyrromethane. J.Coord. Chem. 2012, 65, 865-874.
(32) Isak, S. J.; Eyring, E. M.; Spikes, J. D.; Meekins, P. A. Direct blue dye solutions: photo properties. J. Photochem. Photobiol. A 2000, 134, 77.
- 结构化学的其它文章
- A Novel Cu-based Metallosalan Complex:Synthesis, Structure and Chiral Sensor Study①
- Syntheses, Crystal Structures and Properties of Two Metal-organic Complexes Based on 5-(4-Pyridyl)-methoxyl Isophthalic Acid①
- DFT Study on the Electronic and Structural Properties of MoS6-/0 Clusters①
- Synthesis, Crystal Structure, and Biological Activity of 4-Chlorobenzaldehyde(2-trifluoromethylTrifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]- thieno[2,3-d]pyrimidin-4-yl)hydrazone Monohydrate①
- Synthesis and Crystal Structure of (Z)-N-(2-(diethylamino)ethyl)-7-(5-fluoro-2-oxoindolin-3-ylidene)-2-methyl-4,5,6,7-tetrahydro-1H-indole-3-carboxamide Methanol Solvate①
- Interesting Weak Interactions in Three Silver Coordination Polymers Based on Tetrafluorobenzenedicarboxylate and Benzonitrile Ligands①

