A Novel Cu-based Metallosalan Complex:Synthesis, Structure and Chiral Sensor Study①
YE Cheng-Cheng ZHU Cheng-Feng GONG Teng-Fei SHENG En-Hong② XUAN Wei-Min CUI Yong② LIU Bi-Zhn② (College of Chemistry nd Mterils Siene,Anhui Norml University, Wuhu, Anhui 241000, Chin) (Shool of Chemistry nd Chemil Tehnology nd Stte Key Lortory of Metl Mtrix Composites, Shnghi Jiotong University, Shnghi 200240, Chin) (Chin Too, Shnghi 200240, Chin)
1 INTRODUCTION
Coordination-driven self-assembly provides unique opportunities to design and prepare discrete ordered metal-organic materials, including metallocycles[1], cages[2], helicates[3]and so on, which have exhibited attractive promising applications in diverse areas such as catalysis, separation and sensor[1-5].Metallosalen has been proved to be very effective building block to construct functional metal-organic materials[6]. However, its derivative salan[7], a reduced form of salen, is rather poorly explored in spite of the fact that it also bears the N2O2 coordination pocket and could exhibit more flexibilities and additional functionalities due to the presence of unique asymmetric NH recognition sites.
Metallosalan complexes have manifested themselves for considerable applications in homogenous catalysis and sensor, and could exhibit diverse coordination modes compared with its salen counterpart[7-8]. For example, Katsuki et al. have incorporated a series of transition metal centers into the scaffold of chiral salan ligands and demonstrated their excellent performance for many asymmetric reactions[9-11]. Meanwhile, Pu et al. have established a chiral BINOL-based salan system for discriminating a wide range of chiral substrates, which showed extremely high enantioselectivity[12]. Recently, we have reported the self-assembly of porous chiral helicate cages based on Zn(salan) and illustrated their enantioselective recognition and separation ability of small organic molecules[13]. The coordination unsaturated Zn centers and exposed NH-groups are believed to offer the primary active sites to interact with chiral amino acids. Inspired by the successful utility of such chiral metallosalan based assembly for asymmetric processes, we want to extend our synthetic library by choosing other metal centers that could afford various coordination configurations similar to Zn to assemble with a dipyridyl-functionalized salan ligand so as to construct novel chiral sensor systems with built-in and predesigned functionalities. Herein we report the synthesis of a novel Cu(salan) complex and its chiral senor property for tartaric acid.
2 EXPERIMENTAL
2.1 Materials and apparatus
The ligand N,N΄-bis(3-(E)-4-vinylpyridy-5-tertbutyl-2-hydroxybenzyl)-1,2-phenylendiamine (H2L)was synthesized according to the procedure of literature by replacing chiral 1,2-diamino-cyclohexane with 1,2-phenylendiamine[13]. All of the chemicals are commercially available, and used without further purification. Elemental analyses of C,H and N were performed with an EA1110 CHNS-0 CE elemental analyzer. The IR (KBr pellet) spectra were recorded (400–4000 cm-1region) on a Nicolet Magna 750 FT-IR spectrometer. Thermogravimetric analyses (TGA) were carried out in a N2atmosphere at a heating rate of 10 ℃ min-1on a STA449C integration thermal analyzer. CD spectra were recorded on a J-815 spectropolarimeter (Jasco, Japan).Titration experiments were carried out by adding 50 μL solution of substrates (1× 10-3M) in water to a solution of 1 (1 × 10-5M) in 2.5 mL MeOH every 5 minutes. The CD spectra were then recorded after adding the substrates.
2.2 Crystallographic measurements and structure determination
A dark single crystal of the title compound with dimensions of 0.12mm × 0.10mm × 0.10mm was mounted on a glass fiber in a random orientation.The data for the compounds were collected on a Bruker SMART Apex II CCD-based X-ray diffractometer equipped with a graphite-monochromatized Mo-Kα radiation (λ = 0.71073 Å) using an ω-2θ scan mode in the range of 1.67<θ<25.00° (–18≤h≤21, –22≤k≤22, –19≤l≤19) at 296(2) K. The structure was solved by direct methods with SHELXS-97 and refined with SHELXL-97[14-15]. All the non-hydrogen atoms were refined by full-matrix least-squares techniques with anisotropic displacement parameters. The hydrogen atoms were geometrically fixed at the calculated positions attached to their parent atoms, and treated as riding atoms. The final refinement converged to R = 0.0499 and wR =0.1395 (w = 1/[σ2(Fo2) + (0.0857P)2+ 4.8123P],where P = (Fo2+ 2Fc2)/3), S = 1.073, (Δ/σ)max=0.000, (Δρ)max= 0.774 and (Δρ)min= –0.444 e·Å-3.The selected bond lengths and bond angles as well as H bonds are given in Tables 1 and Table 2,respectively.
2.3 Synthesis of H2L
The salen (370 mg, 0.5 mmol) precursor was dissolved in methanol (25 mL), and 6 equivalent of NaBH4(220 mg, 0.3 mmol) was added portion-wise with stirring. The reaction mixture was stirred overnight at r.t., during which its color turned light orange. The reaction mixture was poured into 100 mL of water, and the desired H2L was isolated by vacuum filtration (0.316 g, 85% yield). Calcd. for C50H54N4O2: C, 80.83; H, 7.33; N, 7.54. Found: C,79.46; H, 7.87; N, 6.89.1H NMR (CDCl3) δ:8.49–8.50 (d, 4H, pyridylH), 7.63~7.67 (d, 2H,vinylH), 7.45~7.46 (d, 2H, ArH), 7.34~7.36 (d, 4H,pyridylH), 7.27~7.28 (m, 6H, ArH), 7.10~7.11 (d,2H, vinylH), 6.97~6.99 (m, 4H, ArH), 6.77~6.78 (d,2H, ArH), 4.06 (s, 2H, methenylH), 3.61~3.90 (dd,4H, methyleneH), 1.27 (s, 18H, CMe3).13C NMR(CDCl3) δ: 154.28, 150.09, 145.84, 142.08, 137.43,129.17, 128.81, 128.35, 126.60, 125.88, 123.43,123.26, 121.08, 66.81, 50.78, 34.28, 31.70. FTIR(KBr pellet, cm-1): 3419(m), 3064(m), 2959(s),2866(s), 1630(m), 1595(s), 1481(s), 1456(s),1417(w), 1363(w), 1286(w), 1209(w), 1114(w),989(w), 972(m), 970(m), 870(w), 805(w), 766(w),701(w). ESI-MS: m/z 743.4 (Calcd. m/z 743.42 for[L+H]+) .
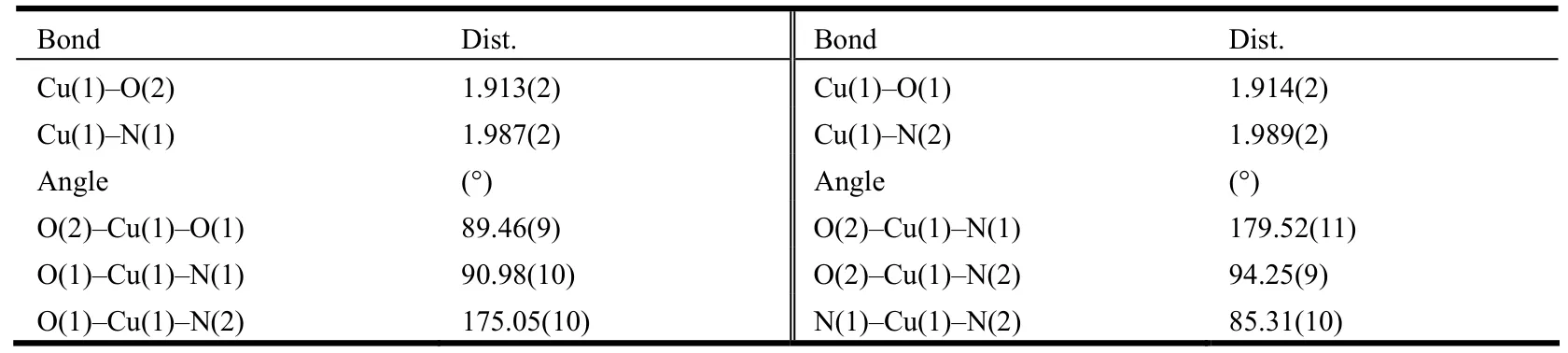
Table 1. Selected Bond Lengths (Å) and Bond Angles (º)

Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°) for 1
2.4 Synthesis of complex 1
The ligand H2L (7.4 mg, 0.01 mmol) was dissolved in CH3CN (1 mL). The solution was then carefully layered onto the aqueous solution of Cu(OAc)2·H2O (3.2 mg, 0016 mmol) in a long tube.Black block-like crystals of 1 were obtained from the wall of tube after three days. Anal. Calcd. (%)for C54H70N6O8Cu: C, 65.20; H, 7.09; N, 8.45.Found: C, 65.90; H, 7.04; N, 8.26. IR (KBr, cm-1):3427(m), 3064(w), 3030(w), 2957(m), 2866(w),1621(m), 1597(s), 1550(w), 1448(s), 1419(w),1362(w), 1314(w), 1288(w), 1210(w), 1123(w),995(m), 874(w), 828(w), 764(w), 701(w), 632(w),529(w). ESI-MS: m/z 804.45 (Calcd. m/z 804.44 for[L+H]+) .
3 RESULTS AND DISCUSSION
3.1 Synthesis and characterization
As outlined in Scheme 1, the dipyridyl-functionalized salan ligand N,N΄-bis(3-(E)-4-vinylpyridy-5-tert-butyl-2-hydroxybenzyl)-1,2-phenylendiamine (H2L) was synthesized in 85% yield by simple reduction of the salen precursor with NaBH4according to literatures. Layering the CH3CN solution of H2L onto the aqueous solution of Cu(OAc)2·H2O afforded black block-like crystals [(CuL)]·2CH3CN·6H2O (1) in good yield.
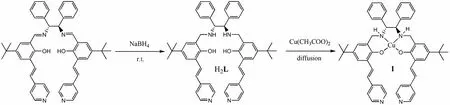
Scheme 1. Synthesis of the ligand H2L and 1
The absorption peaks at 3064 and 3030 cm-1in IR spectrum for 1 could be assigned to vas(C–H)stretching vibrations arising from the benzene and pyridine rings, while peaks at 2957 and 2866 cm-1could be ascribed to the vasand vsstretching vibration of -CH3, respectively. The bands in the range from 1550 to 1621 cm-1mainly derive from the C=C and C=N stretching vibrations of benzene and pyridine skeletons. Furthermore, the characteristic peaks for C–C, C–N and C–O stretching vibration could be easily found in the relatively lower bands (1123–1288 cm-1). The phase purity of 1 was supported by the powder X-ray diffraction(XRD) pattern of the bulk sample, which is consistent with the calculated pattern (Fig. 1). The thermal stability of 1 was investigated on crystalline samples under a N2atmosphere from 40 to 800 ℃.TGA curve shows that 1 loses 4% of its total weight before it begins to decompose at about 238 ℃,whereas the guest molecules account for about 17%weight percent. The inconsistency of experimental and calculated weight loss may arise from the partial loss of guest molecules during the washing process before the TGA test (Fig. 2).
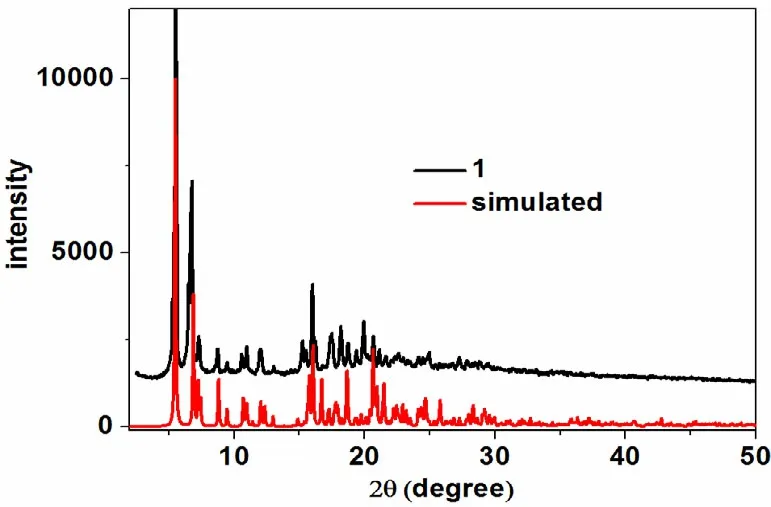
Fig. 1. Powder XRD patterns of 1
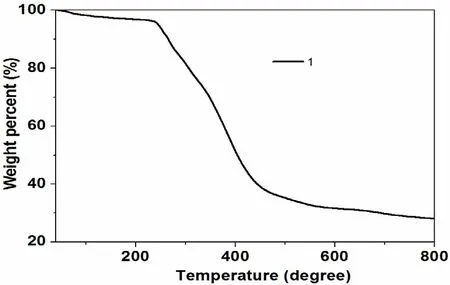
Fig. 2. Thermal analysis curve of 1
3.2 Structural description
Single-crystal structure analysis reveals that 1 crystallizes in the orthorhombic space group P21212 with one formula in the asymmetric unit, which contains one CuL unit, two CH3CN and six H2O molecules. In the CuL unit, the Cu center adopts a square geometry with the equatorial plane occupied by the N2O2 donors of one L ligand (Fig. 3a). The Cu–N bond lengths are 1.987(2) and 1.989(2) Å while Cu–O range from 1.913(2) to 1.914(2) Å,which are comparable with the reported values for the Cu(salan) complexes[16–17]. Salan typically binds to the metal centers in a non-planar arrangement such as cis-a and cis-b coordination modes owing to the intrinsic flexibility[7,18–20]. Interestingly, the Cu center takes the uncommon trans coordination fashion and is located –0.021(1) Å above the plane defined by the N2O2 coordination pocket, indicating the metal center and N2O2 are nearly coplanar (Fig.3a)[16]. As for the coordination units consisting of Cu(1), O(1), N(1), C(15)–C(17) or Cu(1), O(2), N(2),C(33)–C(35), they take distorted arrangements instead of rigid 6-membered rings in which the methylene groups (–0.3196(2) Å for C(15) and 0.3580(2) Å for C(33)) either situate above or below the corresponding planes. As a consequence, the two conjugated branches composed of phenyl ring and vinyl pyridine arrange in a staggered way with one terminal pyridine ring pointing to another in the edge-to-face fashion (Fig. 3a).
As shown in Fig. 3b, the basic building unit of complex 1 consists of two mononuclear Cu(salan)which orient in opposite directions and are locked together via weak intermolecular C(2)–H(2)··π(green, 3.6900(3) Å, 146.703(5)°) and C(33)–H(33B)··π (cyan, 3.4663(3) Å, 139.685(5)°) interactions. Four identical weak H bonds (C(6)–H(6)··O(1)) could be found between one building unit and adjacent four building units with the bond length and angle of 3.195(4) Å and 146.0°, respectively (Table 2 and Fig. 4). Besides, the C–H··π interaction (cyan, 3.4317(3) Å, 121.852(4)°) formed between C(15)–H(15b) and the pyridine ring(C(46)–C(50), N(4)) also contributes to the interconnection of adjoining building units. Consequently,one 21helix chain thus forms along the b axis with the helical pitch of 18.6750 Å (Fig. 4). The adjacent helices could be further linked by H bond to extend into a 4-connected 2D network paralleled to the ab plane (Fig. 4) and the neighboring H bond network will then layer one by one along the c axis to produce the final supramolecular structure, between which reside the guest acetonitrile and water molecules. Although some weak H bonds such as N(2)–H(2A)··O(10) and C(31)–H(31)··O(4) could be found between the salan skeleton and guest water molecules, they are too far to further link the 2D network together (Table 2).
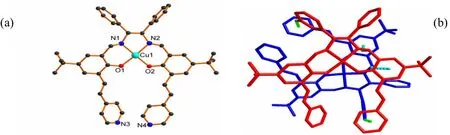
Fig. 3. Molecular structure (a) and basic building unit (b) of 1. Hydrogen atoms and guest molecules are omitted for clarity. The C–H··π interactions are highlighted in green and cyan dotted lines
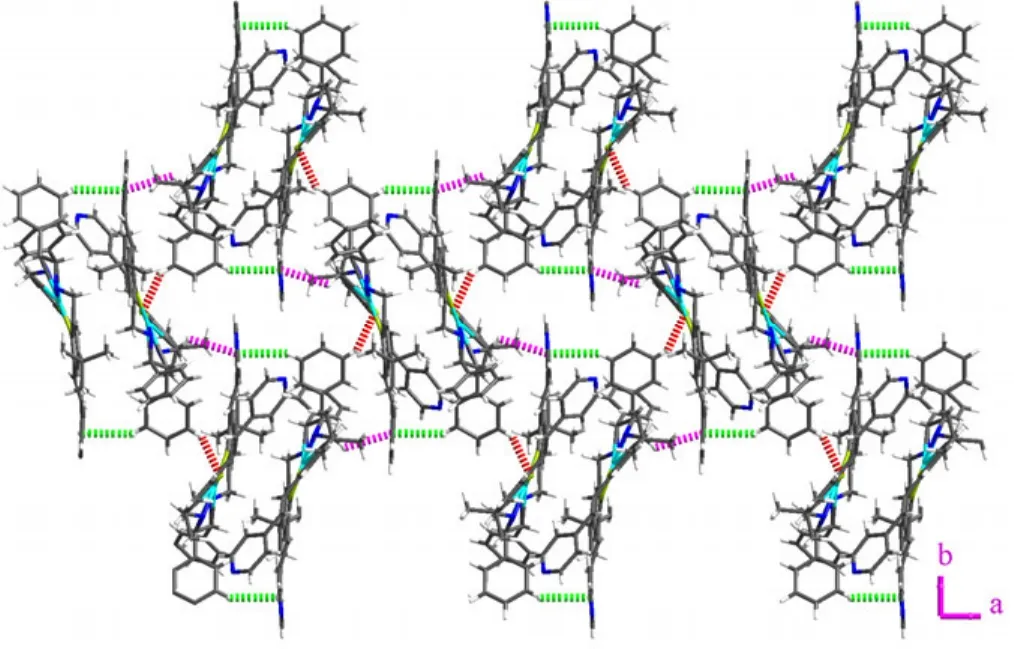
Fig. 4. 2D supramolecular network constructed by H bonds (red dotted line) and C–H··π interactions (purple and green dotted line) formed between adjacent four units
3.3 Chiral sensor property
Compared with the free ligand H2L, complex 1 exhibits significant enhancement of the CD signal(Fig. 5a). The distinguished increase of optical rotation may arise from the fixation and rigidification of the flexible backbone of ligand upon coordination with the metal center[13,21]. As mentioned above, metallosalan offers excellent platform to recognize chiral compounds. Based on the pioneering work of Pu[12], we tested the chiral senor ability of 1 towards some chiral substrates including chiral 1-phenylamine, mandelic acid, L-alanine and L-tartaric acid. 1 displayed high selectivity towards L-tartaric acid while no obvious change of CD signals of 1 could be observed upon titration with other substrates. As shown in Fig. 5b, the intensity of the high energy bands gradually decreased and a new band centered at 380 nm appeared with the addition of L-tartaric acid. The clear isobestic points indicated a new compound had formed during the titration process, accountable for the change of CD spectra. It should be noted that the color of 1 changed from orange to pale yellow before and after titration and could be easily detected by naked eyes(Fig. 5c), making 1 have great potential for practical applications[22].

Fig. 5. CD spectra of H2L and 1 (a), and CD titration of 1 with L-tartaric acid in methanol (1× 10-5 mol/L)(b) and the corresponding color change before and after titration (c)
4 CONCLUSION
In summary, we have synthesized one novel Cubased metallosalan complex 1 from a dipyridylfunctionalized salan ligand by a simple diffusion method. As expected, complex 1 has exhibited high chiral sensor ability towards L-tartaric acid due to the predesigned asymmetric NH-groups and unsaturated coordination metal center.
(1) Seidel, S. R.; Stang, P. J. High symmetry coordination cages via self-assembly. Acc. Chem. Res. 2002, 35, 972–983.
(2) Fujita, M.; Tominaga, M.; Hori, A.; Therrien, B. Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 2005, 38,369–378.
(3) Alrecht, M. "Let's twist again" - double-stranded, triple-stranded, and circular helicates. Chem. Rev. 2001, 101, 3457–3497.
(4) Liu, T.; Liu, Y.; Xuan, W.; Cui, Y. Chiral nanoscale metal-organic tetrahedral cages: diastereoselective self-assembly and enantioselective separation.Angew. Chem., Int. Ed. 2010, 49, 4121–4124.
(5) Li, G.; Yu, W.; Cui, Y. A homochiral nanotubular crystalline framework of metallomacrocycles for enantioselective recognition and separation. J. Am.Chem. Soc. 2008, 130, 4582–4583.
(6) Li, G.; Yu, W.; Ni, J.; Liu, T.; Liu, Y.; Sheng, E.; Cui, Y. Self-assembly of a homochiral nanoscale metallacycle from a metallosalen complex for enantioselective separation. Angew. Chem., Int. Ed. 2008, 47, 1245–1249.
(7) Atwood, D. Salan complexes of the group 12, 13, and 14 elements. Coord. Chem. Rev. 1997, 165, 267–296.
(8) Matsumoto, K.; Saito, B.; Katsuki, T. Asymmetric catalysis of metal complexes with non-planar ONNO ligands: salen, salalen and salan. Chem.Commun. 2007, 3619–3627.
(9) Kondo, S.; Saruhashi, K.; Seki, K.; Matsubara, K.; Miyaji, K.; Kubo, T.; Matsumoto, K.; Katsuki, T. A mu-oxo-mu-eta2:eta2-peroxo titanium complex as a reservoir of active species in asymmetric epoxidation using hydrogen peroxide. Angew. Chem., Int. Ed. 2008, 47, 10195–10198.
(10) Egami, H.; Oguma, T.; Katsuki, T. J. Oxidation catalysis of Nb(salan) complexes: asymmetric epoxidation of allylic alcohols using aqueous hydrogen peroxide as an oxidant. Am. Chem. Soc. 2010, 132, 5886–5895.
(11) Egami, H.; Katsuki, T. Iron-catalyzed asymmetric aerobic oxidation: oxidative coupling of 2-naphthols. J. Am. Chem. Soc. 2009, 131, 6082–6083.
(12) Lin, P. Enantioselective fluorescent sensors: a tale of BINOL. Acc. Chem. Res. 2012, 45, 150–163.
(13) Xuan, W.; Zhang, M.; Liu, Y.; Chen, Z.; Cui, Y. A chiral quadruple-stranded helicate cage for enantioselective recognition and separation. J. Am.Chem. Soc. 2012, 134, 6904–6907.
(14) Sheldrick, G. M. SHELXS-97, Program for X-ray crystal structure solution. University of Göttingen, Germany 1997.
(15) Sheldrick, G. M. SHELXL-97, Program for X-ray crystal structure refinement. University of Göttingen, Germany 1997.
(16) Constable, E.; Zhang, G.; Housecroft, C.; Neuburger, M.; Schaffner, S.; Woggon, W.; Zampese, J. Enantioselective catalysts for the henry reaction:fine-tuning the catalytic components. New J. Chem. 2009, 33, 2166–2173.
(17) Shi, Y.; Mao, Z.; Xue, Q.; Zhu, C.; Hu, H.; Cheng, Y. An international journal dedicated to rapid publications in inorganic and organometallic chemistry. Inorg. Chem. Commun. 2012, 20, 259–260.
(18) Adão, P.; Pessoa, J.; Henriques, R.; Kuznetsov, M.; Avecilla, F.; Maurya, M.; Kumar, U.; Correia, I. Synthesis, characterization, and application of vanadium-salan complexes in oxygen transfer reactions. Inorg. Chem. 2009, 48, 3542–3561.
(19) Yeori, A.; Goldberg, I.; Shuster, M.; Kol, M. Diastereomerically-specific zirconium complexes of chiral salan ligands: isospecific polymerization of 1-hexene and 4-methyl-1-pentene and cyclopolymerization of 1,5-hexadiene. J. Am. Chem. Soc. 2006, 128, 13062–13063.
(20) Yeori, A.; Groysman, S.; Goldberg, I.; Kol, M. Diastereoisomerically selective enantiomerically pure titanium complexes of salan ligands: synthesis,structure, and preliminary activity studies. Inorg. Chem. 2005, 44, 4466–4468.
(21) Argent, S.; Riis-Johannessen, T.; Jeffery, J.; Harding, L.; Ward, M. Diastereoselective formation and optical activity of an M4L6cage complex. Chem.Commun. 2005, 4647–4649.
(22) Song, F.; Ma, X.; Hou, J.; Huang, X.; Cheng, Y.; Zhu, C. (R,R)salen/salan-based polymer fluorescence sensors for Zn2+detection. Polymer. 2011, 52,6029–6036.
- 结构化学的其它文章
- Synthesis and Crystal Structure of 5,5΄-Bis(4-hydroxylphenyl)diazo-dipyrromethane①
- Syntheses, Crystal Structures and Properties of Two Metal-organic Complexes Based on 5-(4-Pyridyl)-methoxyl Isophthalic Acid①
- DFT Study on the Electronic and Structural Properties of MoS6-/0 Clusters①
- Synthesis, Crystal Structure, and Biological Activity of 4-Chlorobenzaldehyde(2-trifluoromethylTrifluoromethyl-5,6,7,8-tetrahydrobenzo[4,5]- thieno[2,3-d]pyrimidin-4-yl)hydrazone Monohydrate①
- Synthesis and Crystal Structure of (Z)-N-(2-(diethylamino)ethyl)-7-(5-fluoro-2-oxoindolin-3-ylidene)-2-methyl-4,5,6,7-tetrahydro-1H-indole-3-carboxamide Methanol Solvate①
- Interesting Weak Interactions in Three Silver Coordination Polymers Based on Tetrafluorobenzenedicarboxylate and Benzonitrile Ligands①

